Abstract
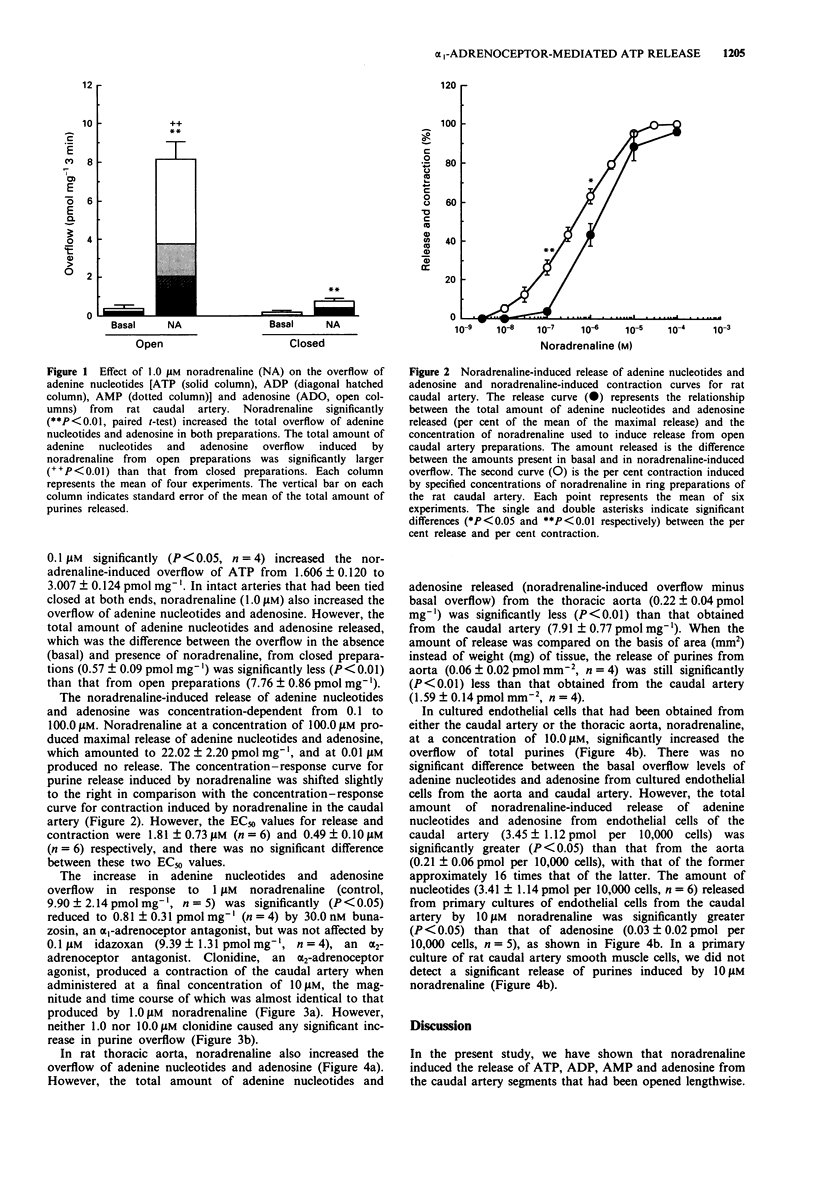
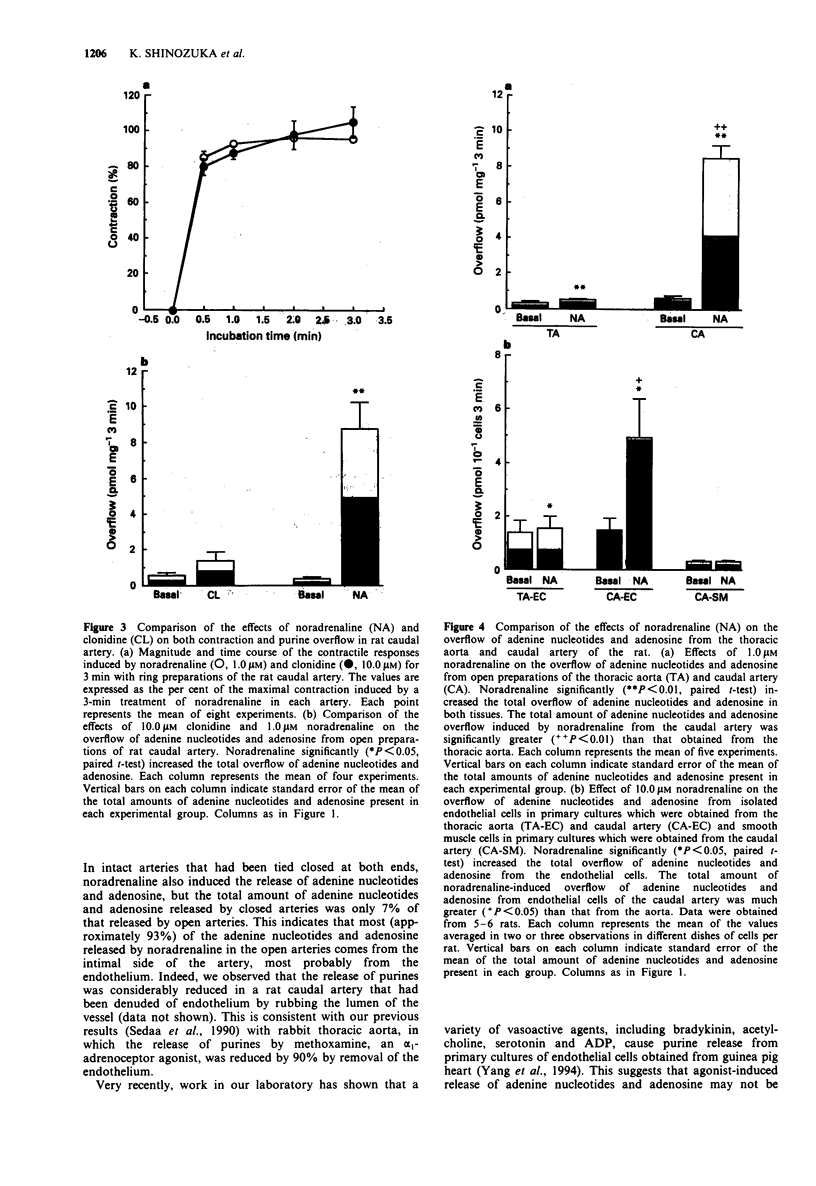
1. Noradrenaline-induced release of endogenous adenine nucleotides (ATP, ADP, AMP) and adenosine from both rat caudal artery and thoracic aorta was characterized, using high-performance liquid chromatography with fluorescence detection. 2. Noradrenaline, in a concentration-dependent manner, increased the overflow of ATP and its metabolites from the caudal artery. The noradrenaline-induced release of adenine nucleotides and adenosine from the caudal artery was abolished by bunazosin, an alpha 1-adrenoceptor antagonist, but not by idazoxan, an alpha 2-adrenoceptor antagonist. Clonidine, an alpha 2-adrenoceptor agonist, contracted caudal artery smooth muscle but did not induce release of adenine nucleotides or adenosine. 3. Noradrenaline also significantly increased the overflow of ATP and its metabolites from the thoracic aorta in the rat; however, the amount of adenine nucleotides and adenosine released from the aorta was considerably less than that released from the caudal artery. 4. Noradrenaline significantly increased the overflow of ATP and its metabolites from cultured endothelial cells from the thoracic aorta and caudal artery. The amount released from the cultured endothelial cells from the thoracic aorta and caudal artery. The amount released from the cultured endothelial cells from the aorta was also much less than that from cultured endothelial cells from the caudal artery. In cultured smooth muscle cells from the caudal artery, a significant release of ATP or its metabolites was not observed. 5. These results suggest that there are vascular endothelial cells that are able to release ATP by an alpha 1-adrenoceptor-mediated mechanism, but that these cells are not homogeneously distributed in the vasculature.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando J., Ohtsuka A., Korenaga R., Kamiya A. Effect of extracellular ATP level on flow-induced Ca++ response in cultured vascular endothelial cells. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1192–1199. doi: 10.1016/0006-291x(91)91698-c. [DOI] [PubMed] [Google Scholar]
- Bodin P., Bailey D., Burnstock G. Increased flow-induced ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br J Pharmacol. 1991 May;103(1):1203–1205. doi: 10.1111/j.1476-5381.1991.tb12324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Local control of blood pressure by purines. Blood Vessels. 1987;24(3):156–160. doi: 10.1159/000158691. [DOI] [PubMed] [Google Scholar]
- Chamley J. H., Campbell G. R., Burnstock G. Dedifferentiation, redifferentiation and bundle formation of smooth muscle cells in tissue culture: the influence of cell number and nerve fibres. J Embryol Exp Morphol. 1974 Oct;32(2):297–323. [PubMed] [Google Scholar]
- Gordon E. L., Pearson J. D., Dickinson E. S., Moreau D., Slakey L. L. The hydrolysis of extracellular adenine nucleotides by arterial smooth muscle cells. Regulation of adenosine production at the cell surface. J Biol Chem. 1989 Nov 15;264(32):18986–18995. [PubMed] [Google Scholar]
- Gordon E. L., Pearson J. D., Slakey L. L. The hydrolysis of extracellular adenine nucleotides by cultured endothelial cells from pig aorta. Feed-forward inhibition of adenosine production at the cell surface. J Biol Chem. 1986 Nov 25;261(33):15496–15507. [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M., Hara T., Honda M., Ishinaga Y., Moriyama K., Masumura S. Metabolic aspects of endothelial cells cultured from rat aortae. Artery. 1992;19(5):284–296. [PubMed] [Google Scholar]
- Ishii R., Shinozuka K., Kobayashi Y., Hattori K., Hashimoto T., Takeuchi K. Methoxamine enhances the release of endogenous noradrenaline from rabbit ear artery: possible involvement of ATP. Naunyn Schmiedebergs Arch Pharmacol. 1993 Jul;348(1):46–52. doi: 10.1007/BF00168535. [DOI] [PubMed] [Google Scholar]
- Milner P., Bodin P., Loesch A., Burnstock G. Rapid release of endothelin and ATP from isolated aortic endothelial cells exposed to increased flow. Biochem Biophys Res Commun. 1990 Jul 31;170(2):649–656. doi: 10.1016/0006-291x(90)92141-l. [DOI] [PubMed] [Google Scholar]
- Mohri K., Takeuchi K., Shinozuka K., Bjur R. A., Westfall D. P. Simultaneous determination of nerve-induced adenine nucleotides and nucleosides released from rabbit pulmonary artery. Anal Biochem. 1993 May 1;210(2):262–267. doi: 10.1006/abio.1993.1194. [DOI] [PubMed] [Google Scholar]
- Ohno M., Ochiai M., Taguchi J., Hara K., Akatsuka N., Kurokawa K. Stretch may enhance the release of endothelium-derived relaxing factor in rabbit aorta. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1038–1042. doi: 10.1016/s0006-291x(05)80890-3. [DOI] [PubMed] [Google Scholar]
- Ohoka M., Honda M., Morioka S., Ishikawa S., Nakayama K., Yamori Y., Moriyama K. Effects of E-1020, a new cyclic AMP-specific phosphodiesterase inhibitor, on cyclic AMP and cytosolic free calcium of cultured vascular smooth muscle cells. Jpn Circ J. 1990 Jun;54(6):679–687. doi: 10.1253/jcj.54.679. [DOI] [PubMed] [Google Scholar]
- Pearson J. D., Gordon J. L. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature. 1979 Oct 4;281(5730):384–386. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Romero J. C., Vanhoutte P. M. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986 Jun;250(6 Pt 2):H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- Sedaa K. O., Bjur R. A., Shinozuka K., Westfall D. P. Nerve and drug-induced release of adenine nucleosides and nucleotides from rabbit aorta. J Pharmacol Exp Ther. 1990 Mar;252(3):1060–1067. [PubMed] [Google Scholar]
- Shinozuka K., Bjur R. A., Westfall D. P. Characterization of prejunctional purinoceptors on adrenergic nerves of the rat caudal artery. Naunyn Schmiedebergs Arch Pharmacol. 1988 Sep;338(3):221–227. doi: 10.1007/BF00173391. [DOI] [PubMed] [Google Scholar]
- Shinozuka K., Kobayashi Y., Shimoura K., Hattori K. Role of nitric oxide from the endothelium on the neurogenic contractile responses of rabbit pulmonary artery. Eur J Pharmacol. 1992 Nov 3;222(1):113–120. doi: 10.1016/0014-2999(92)90470-o. [DOI] [PubMed] [Google Scholar]
- Shinozuka K., Sedaa K. O., Bjur R. A., Westfall D. P. Participation by purines in the modulation of norepinephrine release by methoxamine. Eur J Pharmacol. 1991 Jan 17;192(3):431–434. doi: 10.1016/0014-2999(91)90236-j. [DOI] [PubMed] [Google Scholar]
- Shinozuka K., Tanabe Y., Kobayashi Y., Shimoura K., Takaori S., Takeuchi K., Bjur R. A., Westfall D. P., Hattori K. Comparison of endogenous adenyl purine release from aorta and caudal artery of rat. Proc West Pharmacol Soc. 1993;36:89–93. [PubMed] [Google Scholar]
- Shoji T. Comparison of pre- and postsynaptic alpha-adrenoceptor blocking effects of E-643 in the isolated vas deferens of the rat. Jpn J Pharmacol. 1981 Jun;31(3):361–368. doi: 10.1254/jjp.31.361. [DOI] [PubMed] [Google Scholar]
- Vials A., Burnstock G. A2-purinoceptor-mediated relaxation in the guinea-pig coronary vasculature: a role for nitric oxide. Br J Pharmacol. 1993 Jun;109(2):424–429. doi: 10.1111/j.1476-5381.1993.tb13586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. D. Role of adenine compounds in autonomic neurotransmission. Pharmacol Ther. 1988;38(2):129–168. doi: 10.1016/0163-7258(88)90095-2. [DOI] [PubMed] [Google Scholar]
- Yang S., Cheek D. J., Westfall D. P., Buxton I. L. Purinergic axis in cardiac blood vessels. Agonist-mediated release of ATP from cardiac endothelial cells. Circ Res. 1994 Mar;74(3):401–407. doi: 10.1161/01.res.74.3.401. [DOI] [PubMed] [Google Scholar]
- Yokotani K., Okuma Y., Osumi Y. Release of endogenous noradrenaline from the vascularly perfused rat stomach in vitro: modulation by pre- and postsynaptic adrenoceptors. J Pharmacol Exp Ther. 1992 Feb;260(2):728–733. [PubMed] [Google Scholar]


