Abstract
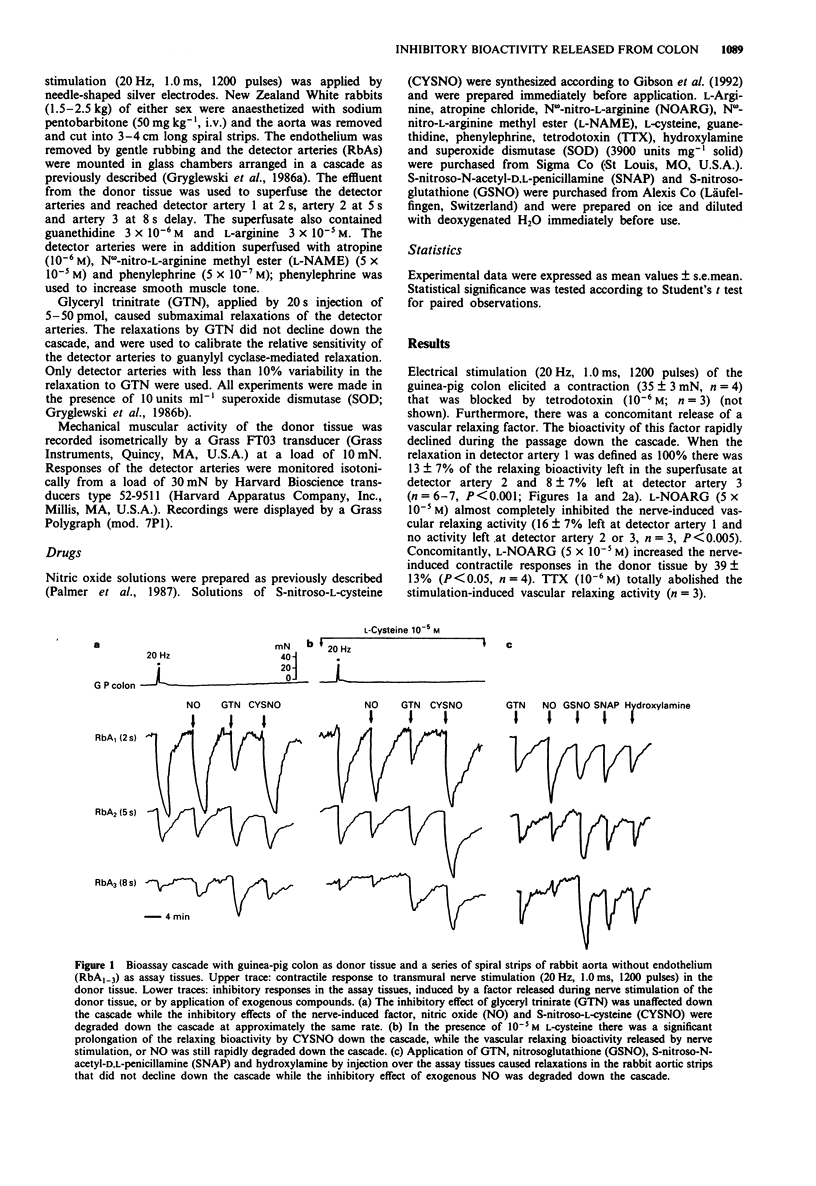
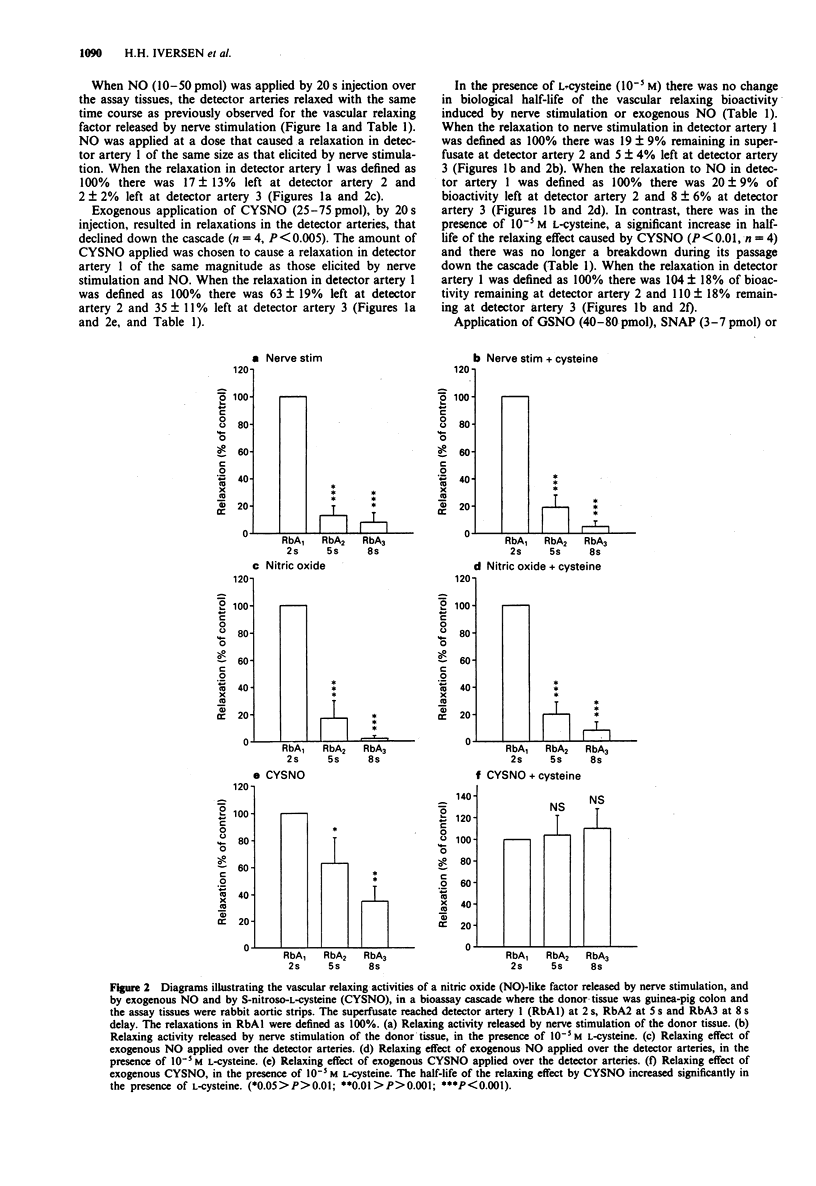
1. The aim of the present study was to compare the biological activity of S-nitroso-L-cysteine (CYSNO), S-nitrosoglutathione (GSNO), S-nitroso-N-acetyl-D,L-penicillamine (SNAP) and hydroxylamine to that of nitric oxide (NO) and a vascular relaxing factor released by nerve stimulation in the guinea-pig intestine. The biological activity was examined in a bioassay system with guinea-pig colon as donor tissue and a series of spiral strips of rabbit aorta without endothelium as detector tissues. 2. Electrical stimulation of the guinea-pig colon released a vascular relaxing factor. The half-life of the relaxing factor down the bioassay cascade was the same as exogenously applied NO. N omega-nitro-L-arginine (L-NOARG) inhibited the release of bioactivity. 3. The relaxations of the assay tissues caused by exogenous CYSNO also declined during the passage down the cascade. However, in the presence of L-cysteine (10(-5) M) the half-life of CYSNO increased and there was no significant breakdown through the cascade. In contrast, the half-life of applied NO and the vascular relaxing factor released by nerve stimulation was unaffected by the presence of L-cysteine. 4. Exogenously applied GSNO (20-50 nM), SNAP (2-4 nM) and hydroxylamine (300-600 nM) caused relaxations that did not decline during the passage down the cascade. 5. In summary, the relaxation of the bioassay tissues during nerve stimulation was indistinguishable from the relaxation induced by NO, whereas relaxations induced by CYSNO, GSNO, SNAP and hydroxylamine showed different pharmacological profiles. The released bioactivity is thus likely to be NO itself.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belai A., Schmidt H. H., Hoyle C. H., Hassall C. J., Saffrey M. J., Moss J., Förstermann U., Murad F., Burnstock G. Colocalization of nitric oxide synthase and NADPH-diaphorase in the myenteric plexus of the rat gut. Neurosci Lett. 1992 Aug 31;143(1-2):60–64. doi: 10.1016/0304-3940(92)90233-w. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Bult H., Boeckxstaens G. E., Pelckmans P. A., Jordaens F. H., Van Maercke Y. M., Herman A. G. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990 May 24;345(6273):346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- Desai K. M., Sessa W. C., Vane J. R. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991 Jun 6;351(6326):477–479. doi: 10.1038/351477a0. [DOI] [PubMed] [Google Scholar]
- Feelisch M., te Poel M., Zamora R., Deussen A., Moncada S. Understanding the controversy over the identity of EDRF. Nature. 1994 Mar 3;368(6466):62–65. doi: 10.1038/368062a0. [DOI] [PubMed] [Google Scholar]
- Gibson A., Babbedge R., Brave S. R., Hart S. L., Hobbs A. J., Tucker J. F., Wallace P., Moore P. K. An investigation of some S-nitrosothiols, and of hydroxy-arginine, on the mouse anococcygeus. Br J Pharmacol. 1992 Nov;107(3):715–721. doi: 10.1111/j.1476-5381.1992.tb14512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Liu X. R., Martin W. The effects of L-arginine and NG-monomethyl L-arginine on the response of the rat anococcygeus muscle to NANC nerve stimulation. Br J Pharmacol. 1989 Dec;98(4):1080–1082. doi: 10.1111/j.1476-5381.1989.tb12650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Moncada S., Palmer R. M. Bioassay of prostacyclin and endothelium-derived relaxing factor (EDRF) from porcine aortic endothelial cells. Br J Pharmacol. 1986 Apr;87(4):685–694. doi: 10.1111/j.1476-5381.1986.tb14586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Jia L., Furchgott R. F. Inhibition by sulfhydryl compounds of vascular relaxation induced by nitric oxide and endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1993 Oct;267(1):371–378. [PubMed] [Google Scholar]
- Kowaluk E. A., Fung H. L. Spontaneous liberation of nitric oxide cannot account for in vitro vascular relaxation by S-nitrosothiols. J Pharmacol Exp Ther. 1990 Dec;255(3):1256–1264. [PubMed] [Google Scholar]
- Li C. G., Rand M. J. Evidence for a role of nitric oxide in the neurotransmitter system mediating relaxation of the rat anococcygeus muscle. Clin Exp Pharmacol Physiol. 1989 Dec;16(12):933–938. doi: 10.1111/j.1440-1681.1989.tb02404.x. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Sanders K. M., Ward S. M. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am J Physiol. 1992 Mar;262(3 Pt 1):G379–G392. doi: 10.1152/ajpgi.1992.262.3.G379. [DOI] [PubMed] [Google Scholar]
- Shuttleworth C. W., Murphy R., Furness J. B. Evidence that nitric oxide participates in non-adrenergic inhibitory transmission to intestinal muscle in the guinea-pig. Neurosci Lett. 1991 Sep 2;130(1):77–80. doi: 10.1016/0304-3940(91)90231-h. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Simon D. I., Osborne J. A., Mullins M. E., Jaraki O., Michel T., Singel D. J., Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Thomas G., Ramwell P. W. Vascular relaxation mediated by hydroxylamines and oximes: their conversion to nitrites and mechanism of endothelium dependent vascular relaxation. Biochem Biophys Res Commun. 1989 Oct 31;164(2):889–893. doi: 10.1016/0006-291x(89)91542-8. [DOI] [PubMed] [Google Scholar]
- Thornbury K. D., Ward S. M., Dalziel H. H., Carl A., Westfall D. P., Sanders K. M. Nitric oxide and nitrosocysteine mimic nonadrenergic, noncholinergic hyperpolarization in canine proximal colon. Am J Physiol. 1991 Sep;261(3 Pt 1):G553–G557. doi: 10.1152/ajpgi.1991.261.3.G553. [DOI] [PubMed] [Google Scholar]
- Toda N., Baba H., Okamura T. Role of nitric oxide in non-adrenergic, non-cholinergic nerve-mediated relaxation in dog duodenal longitudinal muscle strips. Jpn J Pharmacol. 1990 Jun;53(2):281–284. doi: 10.1254/jjp.53.281. [DOI] [PubMed] [Google Scholar]
- Vanin A. F. Endothelium-derived relaxing factor is a nitrosyl iron complex with thiol ligands. FEBS Lett. 1991 Sep 2;289(1):1–3. doi: 10.1016/0014-5793(91)80894-9. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Xue C., Shuttleworth C. W., Bredt D. S., Snyder S. H., Sanders K. M. NADPH diaphorase and nitric oxide synthase colocalization in enteric neurons of canine proximal colon. Am J Physiol. 1992 Aug;263(2 Pt 1):G277–G284. doi: 10.1152/ajpgi.1992.263.2.G277. [DOI] [PubMed] [Google Scholar]
- Wiklund N. P., Leone A. M., Gustafsson L. E., Moncada S. Release of nitric oxide evoked by nerve stimulation in guinea-pig intestine. Neuroscience. 1993 Apr;53(3):607–611. doi: 10.1016/0306-4522(93)90609-j. [DOI] [PubMed] [Google Scholar]


