Abstract
1 We have investigated the effects of RP 73401, a novel, potent and highly selective cyclic nucleotide phosphodiesterase (PDE) type IV inhibitor, in guinea-pig and rat models of bronchoconstriction and allergic inflammation. In some models, the effects of RP 73401 have been compared with those of the standard PDE type IV inhibitor, rolipram.
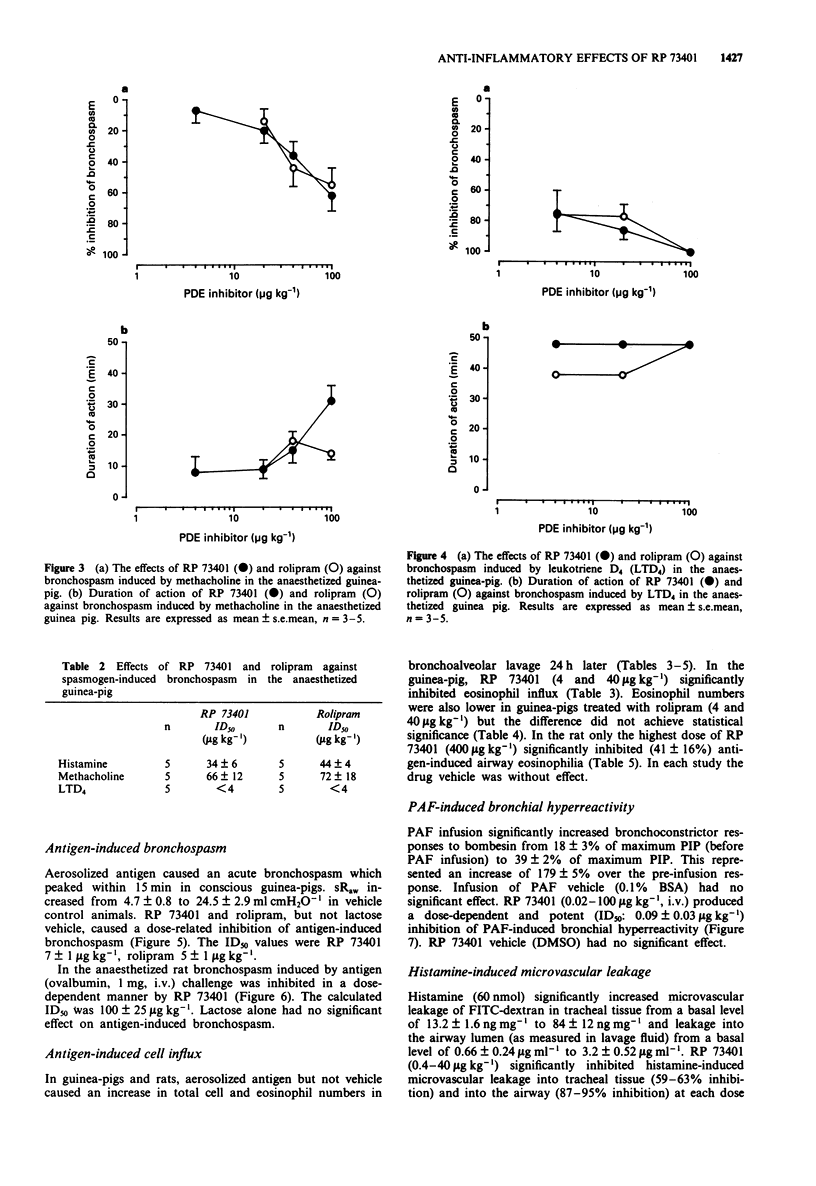
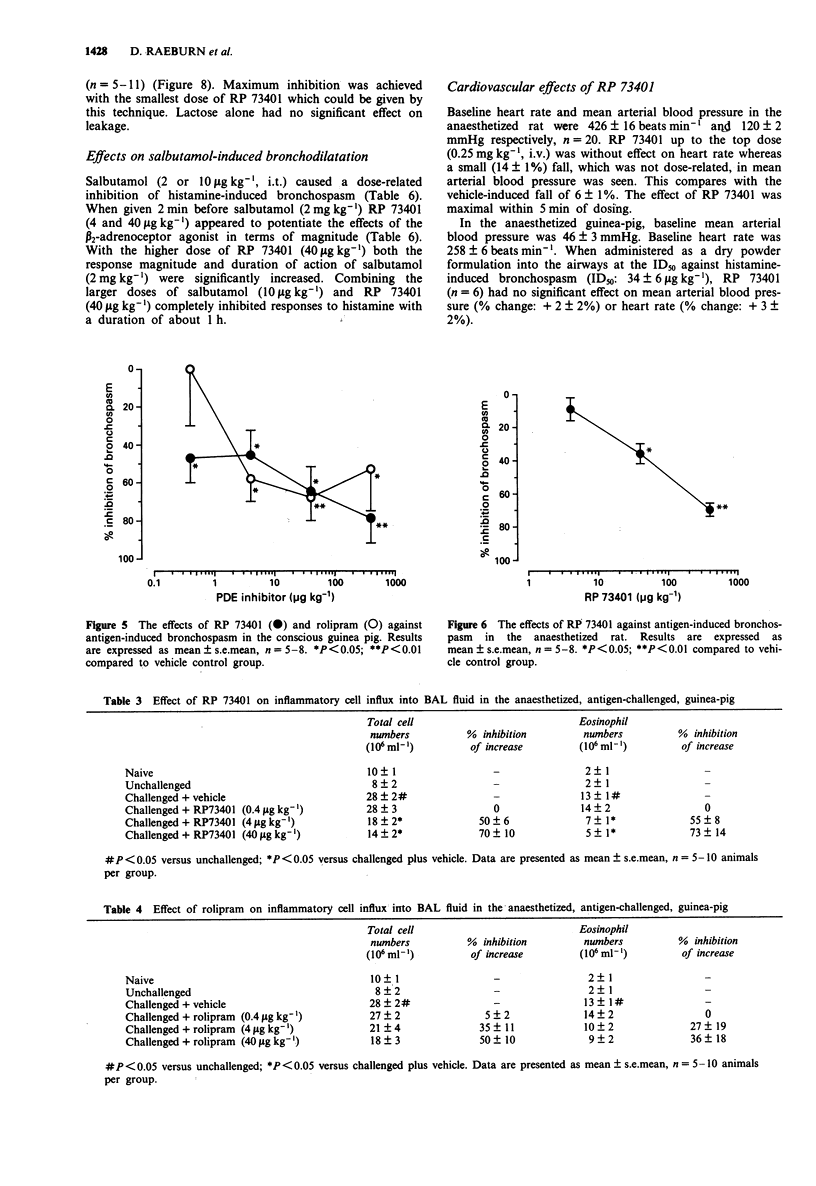
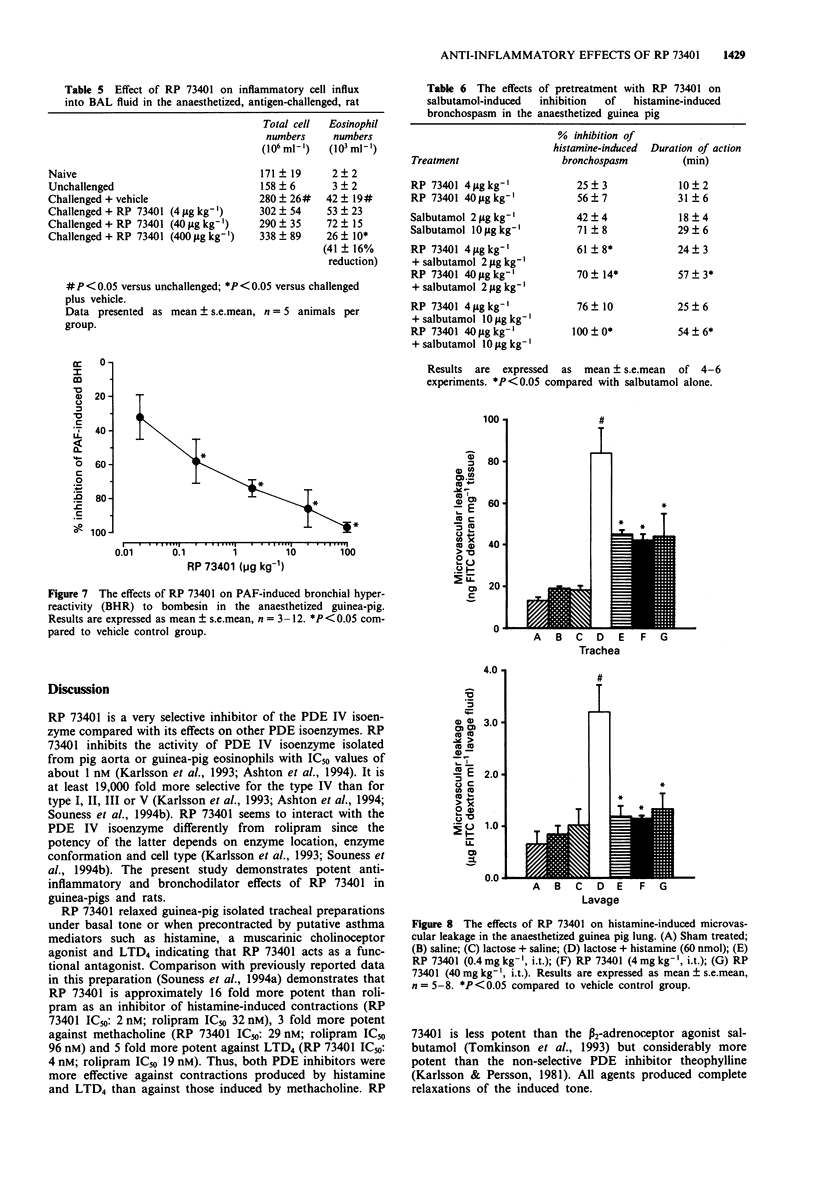
2 RP 73401 (0.4-400 μg kg-1, intratracheally (i.t.) on lactose) inhibited antigen-induced bronchospasm in previously sensitized conscious guinea-pigs (ID50: 7±1 μg kg-1) and in anaesthetized rats (ID50: 100±25 μg kg-1). Rolipram inhibited the antigen-induced bronchospasm in guinea-pigs with an ID50 of 5±1 μg kg-1. In guinea-pig bronchoalveolar lavage (BAL) fluid, total inflammatory cell and eosinophil numbers were reduced by RP 73401 (ID50S: 3.9±0.8 μg kg-1 and 3.2±0.7 μg kg-1, respectively). In the rat, inflammatory cell numbers are less affected. Only the highest dose of RP 73401 (400 μg kg-1) significantly inhibited eosinophil influx (41±16% inhibition).
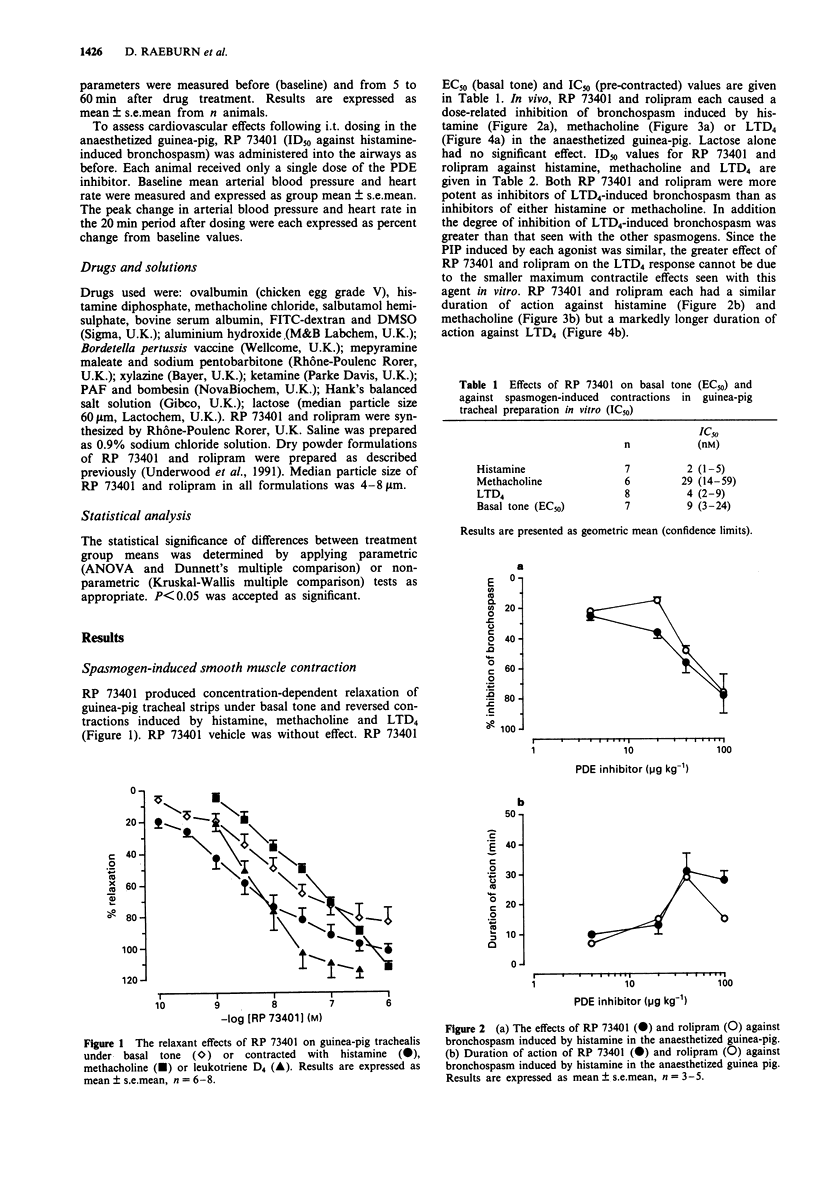
3 RP 73401 (0.02-100 μg kg-1, i.v.) inhibited PAF-induced bronchial hyperreactivity to bombesin in the anaesthetized guinea-pig (ID50: 0.09±0.03 μg kg-1) and inhibited (0.4-40 μg kg-1, i.t.) histamine-induced airway microvascular leakage in the anaesthetized guinea-pig by approximately 60% at all doses.
4 RP 73401 relaxed guinea-pig isolated trachea under basal tone (EC50: 9 nM) and when precontracted with histamine (IC50: 2 nM), methacholine (IC50: 29 nM) or leukotriene D4 (LTD4, IC50: 4 nM).
5 RP 73401 (0.4-100 μg kg-1, i.t.) inhibited bronchospasm induced by histamine (ID50: 34±6 μg kg-1), methacholine (ID50: 66±12 μg kg-1) and LTD4 (ID50: <4 μg kg-1) in the anaesthetized guinea-pig. Against these same bronchoconstrictors, rolipram (i.t.) had ID50 values of 44±4, 72±18 and <4 μg kg-1 respectively. RP 73401 (4 and 40 μg kg-1, i.t.) increased the magnitude and duration of bronchodilatation produced by salbutamol in the anaesthetized guinea-pig. At doses producing significant bronchodilatation, RP 73401 was without effect on heart rate or blood pressure in the anaesthetized guinea-pig. RP 73401 (0.01-0.25 mg kg-1, i.v.) did not affect heart rate and produced only a small fall in blood pressure in the anaesthetized rat.
6 These data demonstrate that RP 73401 and rolipram inhibit antigen- and mediator-induced bronchospasm in guinea-pigs with the same potency. Furthermore, RP 73401 administered directly into the airways, protects against allergic airway inflammation. These results indicate the importance of PDE IV in regulating smooth muscle and inflammatory cell activity. At doses suppressing the inflammatory response in the lung, RP 73401 had little effect in the cardiovascular system. RP 73401 may have a role as a bronchodilator and, more importantly, as a prophylactic anti-inflammatory agent in the treatment of asthma.
Keywords: Phosphodiesterase inhibitor, RP 73401, bronchospasm, bronchodilator, inflammation, anti-inflammatory, anti-asthmatic, salbutamol, rolipam
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton M. J., Cook D. C., Fenton G., Karlsson J. A., Palfreyman M. N., Raeburn D., Ratcliffe A. J., Souness J. E., Thurairatnam S., Vicker N. Selective type IV phosphodiesterase inhibitors as antiasthmatic agents. The syntheses and biological activities of 3-(cyclopentyloxy)-4-methoxybenzamides and analogues. J Med Chem. 1994 May 27;37(11):1696–1703. doi: 10.1021/jm00037a021. [DOI] [PubMed] [Google Scholar]
- Barnes P. J., Pedersen S. Efficacy and safety of inhaled corticosteroids in asthma. Report of a workshop held in Eze, France, October 1992. Am Rev Respir Dis. 1993 Oct;148(4 Pt 2):S1–26. doi: 10.1164/ajrccm/148.4_Pt_2.S1. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Djukanović R., Roche W. R., Wilson J. W., Beasley C. R., Twentyman O. P., Howarth R. H., Holgate S. T. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990 Aug;142(2):434–457. doi: 10.1164/ajrccm/142.2.434. [DOI] [PubMed] [Google Scholar]
- Giembycz M. A., Dent G. Prospects for selective cyclic nucleotide phosphodiesterase inhibitors in the treatment of bronchial asthma. Clin Exp Allergy. 1992 Mar;22(3):337–344. doi: 10.1111/j.1365-2222.1992.tb03095.x. [DOI] [PubMed] [Google Scholar]
- Heaslip R. J., Buckley S. K., Sickels B. D., Grimes D. Bronchial vs. cardiovascular activities of selective phosphodiesterase inhibitors in the anesthetized beta-blocked dog. J Pharmacol Exp Ther. 1991 May;257(2):741–747. [PubMed] [Google Scholar]
- Karlsson J. A., Persson C. G. Influence of tracheal contraction on relaxant effects in vitro of theophylline and isoprenaline. Br J Pharmacol. 1981 Sep;74(1):73–79. doi: 10.1111/j.1476-5381.1981.tb09956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C. D., Shahid M. Inhibitors of cyclic nucleotide phosphodiesterase isoenzymes--their potential utility in the therapy of asthma. Pulm Pharmacol. 1994 Feb;7(1):1–17. doi: 10.1006/pulp.1994.1001. [DOI] [PubMed] [Google Scholar]
- Pennock B. E., Cox C. P., Rogers R. M., Cain W. A., Wells J. H. A noninvasive technique for measurement of changes in specific airway resistance. J Appl Physiol Respir Environ Exerc Physiol. 1979 Feb;46(2):399–406. doi: 10.1152/jappl.1979.46.2.399. [DOI] [PubMed] [Google Scholar]
- Persson C. G. Plasma exudation and asthma. Lung. 1988;166(1):1–23. doi: 10.1007/BF02714025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Naline E., Karlsson J. A., Raeburn D., Advenier C. Effects of rolipram and siguazodan on the human isolated bronchus and their interaction with isoprenaline and sodium nitroprusside. Br J Pharmacol. 1993 Jul;109(3):774–778. doi: 10.1111/j.1476-5381.1993.tb13641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeburn D., Karlsson J. A. Effects of isoenzyme-selective inhibitors of cyclic nucleotide phosphodiesterase on microvascular leak in guinea pig airways in vivo. J Pharmacol Exp Ther. 1993 Dec;267(3):1147–1152. [PubMed] [Google Scholar]
- Raeburn D., Souness J. E., Tomkinson A., Karlsson J. A. Isozyme-selective cyclic nucleotide phosphodiesterase inhibitors: biochemistry, pharmacology and therapeutic potential in asthma. Prog Drug Res. 1993;40:9–32. doi: 10.1007/978-3-0348-7147-1_3. [DOI] [PubMed] [Google Scholar]
- Souness J. E., Villamil M. E., Scott L. C., Tomkinson A., Giembycz M. A., Raeburn D. Possible role of cyclic AMP phosphodiesterases in the actions of ibudilast on eosinophil thromboxane generation and airways smooth muscle tone. Br J Pharmacol. 1994 Apr;111(4):1081–1088. doi: 10.1111/j.1476-5381.1994.tb14855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkinson A., Karlsson J. A., Raeburn D. Comparison of the effects of selective inhibitors of phosphodiesterase types III and IV in airway smooth muscle with differing beta-adrenoceptor subtypes. Br J Pharmacol. 1993 Jan;108(1):57–61. doi: 10.1111/j.1476-5381.1993.tb13439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torphy T. J., Undem B. J. Phosphodiesterase inhibitors: new opportunities for the treatment of asthma. Thorax. 1991 Jul;46(7):512–523. doi: 10.1136/thx.46.7.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood S. L., Lingham D., Pearson J., Raeburn D. A novel technique for the administration of bronchodilator drugs formulated as dry powders to the anaesthetized guinea pig. J Pharmacol Methods. 1991 Nov;26(3):203–210. doi: 10.1016/0160-5402(91)90044-6. [DOI] [PubMed] [Google Scholar]
- de Boer J., Philpott A. J., van Amsterdam R. G., Shahid M., Zaagsma J., Nicholson C. D. Human bronchial cyclic nucleotide phosphodiesterase isoenzymes: biochemical and pharmacological analysis using selective inhibitors. Br J Pharmacol. 1992 Aug;106(4):1028–1034. doi: 10.1111/j.1476-5381.1992.tb14451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


