Abstract
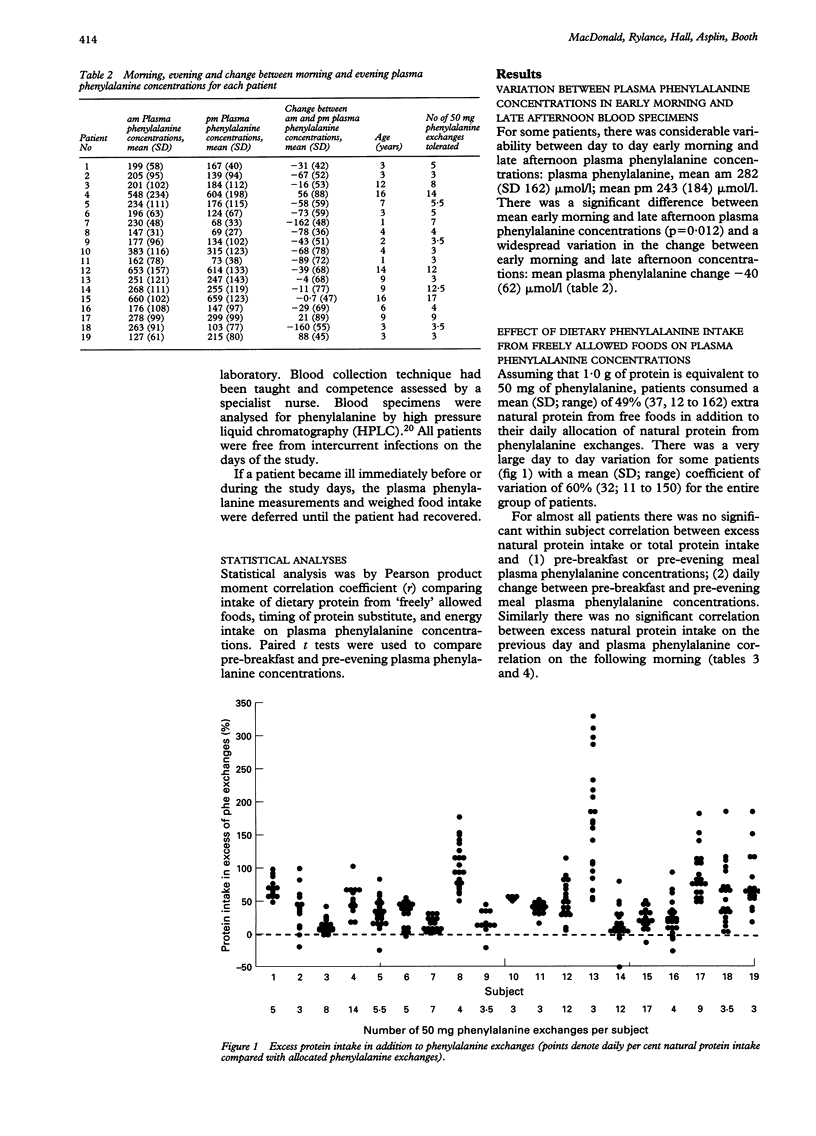
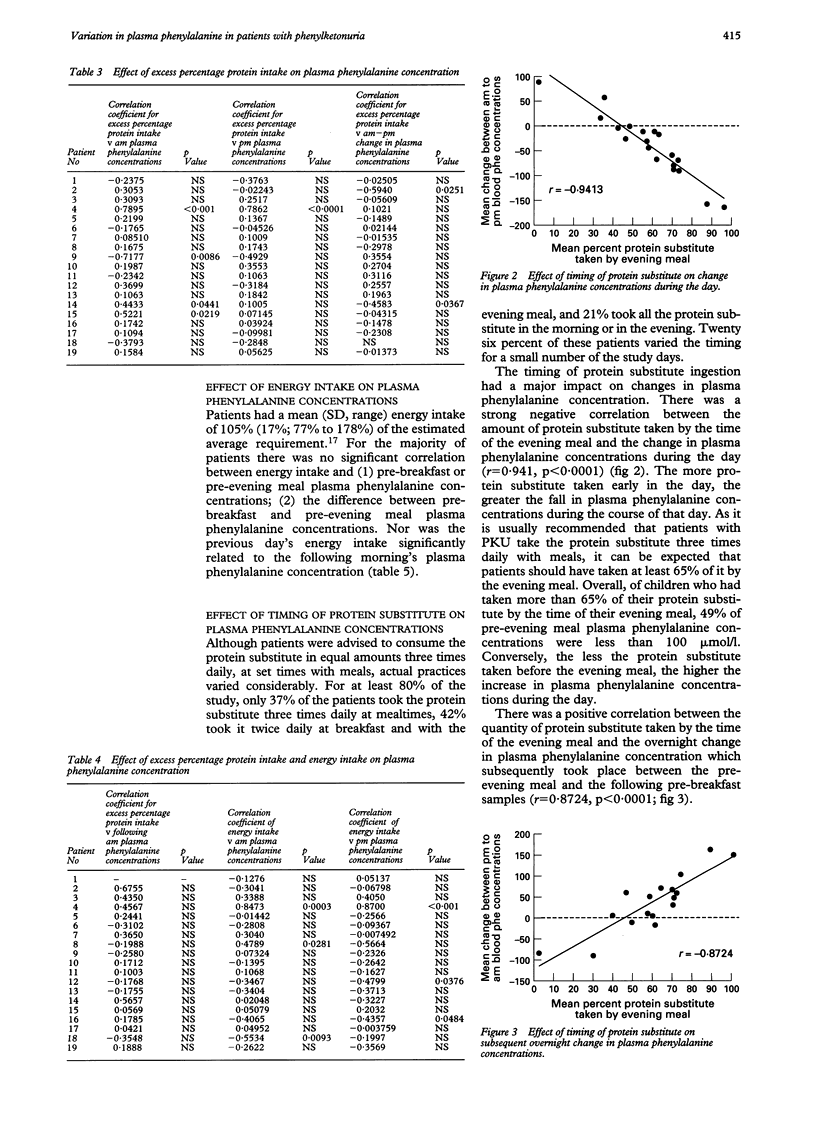
The optimal dietary management of children with phenylketonuria (PKU) has rarely been rigorously explored. The aim of this study was to assess longitudinally the effects of three factors thought to influence plasma phenylalanine concentrations in PKU: total energy intake; protein intake from natural foods allowed freely in addition to allocated phenylalanine exchanges; and the distribution of protein substitute throughout the day. Nineteen subjects, 15 girls and four boys aged 1-16 years, were enrolled. Food intake was weighed, and twice daily plasma phenylalanine concentrations measured during either 3-day or 4-day periods, for a total of 21 days throughout six months. There was a negative correlation between the percentage of protein substitute eaten by the time of the evening meal and the fall in plasma phenylalanine concentration during the day (r = -0.941; p < 0.0001). On average, 49% of pre-evening meal plasma phenylalanine concentrations were less than 100 mumol/l in children who had taken at least 65% of their protein substitute by the time of their evening meal. There was no correlation between excess natural protein intake from freely allowed foods and (a) pre-breakfast or pre-evening meal plasma phenylalanine concentrations or (b) the daily change between pre-breakfast and pre-evening meal concentrations. Nor was there any correlation between excess natural protein intake on the previous day and plasma phenylalanine concentration on the following morning. Energy intake was not correlated with plasma phenylalanine concentrations. It is therefore preferable to distribute the protein substitute evenly through the day in order to achieve stable phenylalanine concentrations, rather than to carry out further fine manipulation of the phenylalanine intake, which would make management of the diet even more difficult.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton N. D., Green A. HPLC measurement of phenylalanine in plasma. Clin Chem. 1988 Nov;34(11):2241–2244. [PubMed] [Google Scholar]
- Bick U., Fahrendorf G., Ludolph A. C., Vassallo P., Weglage J., Ullrich K. Disturbed myelination in patients with treated hyperphenylalaninaemia: evaluation with magnetic resonance imaging. Eur J Pediatr. 1991 Jan;150(3):185–189. doi: 10.1007/BF01963563. [DOI] [PubMed] [Google Scholar]
- Farquhar D. L., Steven F., Westwood A. Preliminary report on inverse diurnal variation of phenylalanine: implications in maternal phenylketonuria. Hum Nutr Appl Nutr. 1985 Jun;39(3):224–226. [PubMed] [Google Scholar]
- Gropper S. S., Gropper D. M., Acosta P. B. Plasma amino acid response to ingestion of L-amino acids and whole protein. J Pediatr Gastroenterol Nutr. 1993 Feb;16(2):143–150. doi: 10.1097/00005176-199302000-00008. [DOI] [PubMed] [Google Scholar]
- Hanley W. B., Linsao L., Davidson W., Moes C. A. Malnutrition with early treatment of phenylketonuria. Pediatr Res. 1970 Jul;4(4):318–327. doi: 10.1203/00006450-197007000-00002. [DOI] [PubMed] [Google Scholar]
- Lou H. C., Güttler F., Lykkelund C., Bruhn P., Niederwieser A. Decreased vigilance and neurotransmitter synthesis after discontinuation of dietary treatment for phenylketonuria in adolescents. Eur J Pediatr. 1985 May;144(1):17–20. doi: 10.1007/BF00491918. [DOI] [PubMed] [Google Scholar]
- Melnick C. R., Michals K. K., Matalon R. Linguistic development of children with phenylketonuria and normal intelligence. J Pediatr. 1981 Feb;98(2):269–272. doi: 10.1016/s0022-3476(81)80658-0. [DOI] [PubMed] [Google Scholar]
- Pennington B. F., van Doorninck W. J., McCabe L. L., McCabe E. R. Neuropsychological deficits in early treated phenylketonuric children. Am J Ment Defic. 1985 Mar;89(5):467–474. [PubMed] [Google Scholar]
- Smith I., Beasley M. G., Ades A. E. Intelligence and quality of dietary treatment in phenylketonuria. Arch Dis Child. 1990 May;65(5):472–478. doi: 10.1136/adc.65.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I., Beasley M. G., Wolff O. H., Ades A. E. Behavior disturbance in 8-year-old children with early treated phenylketonuria. Report from the MRC/DHSS Phenylketonuria Register. J Pediatr. 1988 Mar;112(3):403–408. doi: 10.1016/s0022-3476(88)80320-2. [DOI] [PubMed] [Google Scholar]
- Thompson A. J., Smith I., Brenton D., Youl B. D., Rylance G., Davidson D. C., Kendall B., Lees A. J. Neurological deterioration in young adults with phenylketonuria. Lancet. 1990 Sep 8;336(8715):602–605. doi: 10.1016/0140-6736(90)93401-a. [DOI] [PubMed] [Google Scholar]
- Thompson A. J., Tillotson S., Smith I., Kendall B., Moore S. G., Brenton D. P. Brain MRI changes in phenylketonuria. Associations with dietary status. Brain. 1993 Aug;116(Pt 4):811–821. doi: 10.1093/brain/116.4.811. [DOI] [PubMed] [Google Scholar]
- Welsh M. C., Pennington B. F., Ozonoff S., Rouse B., McCabe E. R. Neuropsychology of early-treated phenylketonuria: specific executive function deficits. Child Dev. 1990 Dec;61(6):1697–1713. [PubMed] [Google Scholar]
- de Sonneville L. M., Schmidt E., Michel U., Batzler U. Preliminary neuropsychological test results. Eur J Pediatr. 1990;149 (Suppl 1):S39–S44. doi: 10.1007/BF02126298. [DOI] [PubMed] [Google Scholar]
- van Spronsen F. J., van Rijn M., van Dijk T., Smit G. P., Reijngoud D. J., Berger R., Heymans H. S. Plasma phenylalanine and tyrosine responses to different nutritional conditions (fasting/postprandial) in patients with phenylketonuria: effect of sample timing. Pediatrics. 1993 Oct;92(4):570–573. [PubMed] [Google Scholar]


