Abstract
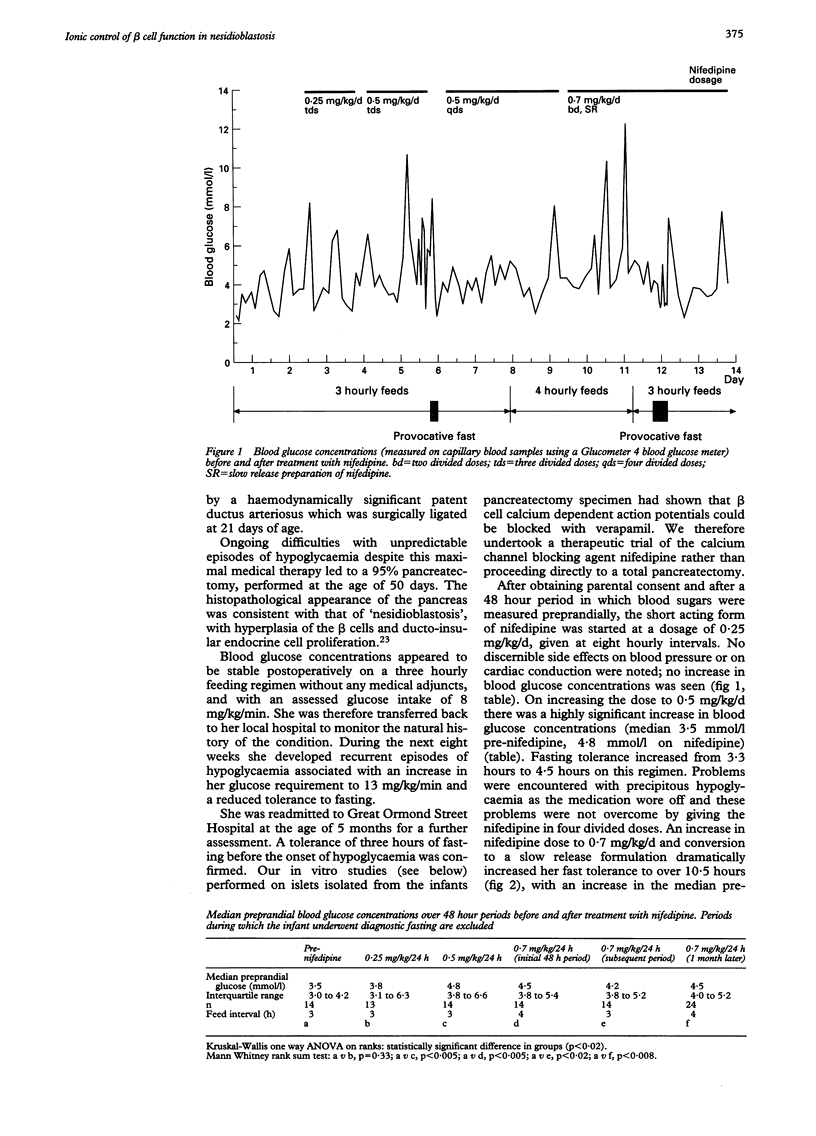
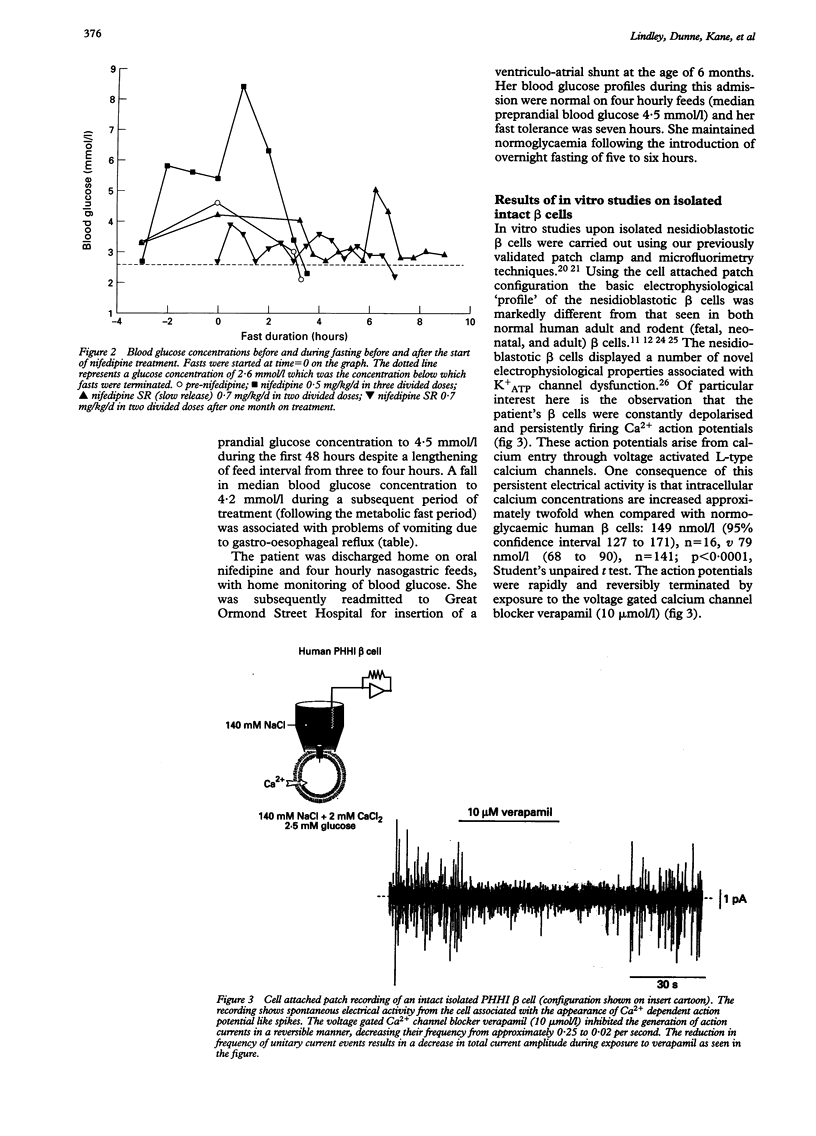
A preterm female infant presented with intractable hypoglycaemia within 10 minutes of delivery. Normoglycaemia could be maintained only by the intravenous infusion of glucose at a rate of 20-22 mg/kg/min. Persistent hyperinsulinaemic hypoglycaemia of infancy was diagnosed from an inappropriately raised plasma insulin concentration (33 mU/l) at the time of hypoglycaemia (blood glucose < 0.5 mmol/l). Medical treatment with glucagon, somatostatin, and diazoxide led to only a modest reduction in the intravenous glucose requirement; a 95% pancreatectomy was performed and histological 'nesidioblastosis' confirmed. In vitro electrophysiological studies using patch clamp techniques on isolated pancreatic beta cells characterised the ionic basis for insulin secretion in nesidioblastosis. The beta cells were depolarised in low ambient glucose concentrations with persistently firing action potentials; these were blocked reversibly by the calcium channel blocking agent verapamil. Persistent postoperative hyperinsulinaemic hypoglycaemia was treated with oral nifedipine. This increased median blood glucose concentrations from 3.5 to 4.8 mmol/l and increased in duration the child's tolerance to fasting from 3 to 10.5 hours. These data allude to an abnormality in the ionic control of insulin release in nesidioblastosis and offer a new logical approach to treatment which requires further evaluation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar-Bryan L., Nichols C. G., Wechsler S. W., Clement J. P., 4th, Boyd A. E., 3rd, González G., Herrera-Sosa H., Nguy K., Bryan J., Nelson D. A. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995 Apr 21;268(5209):423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- Aguilar-Bryan L., Nichols C. G., Wechsler S. W., Clement J. P., 4th, Boyd A. E., 3rd, González G., Herrera-Sosa H., Nguy K., Bryan J., Nelson D. A. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995 Apr 21;268(5209):423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Harrison D. E., Ashcroft S. J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. 1984 Nov 29-Dec 5Nature. 312(5993):446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Proks P., Smith P. A., Ammälä C., Bokvist K., Rorsman P. Stimulus-secretion coupling in pancreatic beta cells. J Cell Biochem. 1994;55 (Suppl):54–65. doi: 10.1002/jcb.240550007. [DOI] [PubMed] [Google Scholar]
- Aynsley-Green A., Polak J. M., Bloom S. R., Gough M. H., Keeling J., Ashcroft S. J., Turner R. C., Baum J. D. Nesidioblastosis of the pancreas: definition of the syndrome and the management of the severe neonatal hyperinsulinaemic hypoglycaemia. Arch Dis Child. 1981 Jul;56(7):496–508. doi: 10.1136/adc.56.7.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning G. M., Anderson M. P., Amara J. F., Marshall J., Smith A. E., Welsh M. J. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992 Aug 27;358(6389):761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Petersen O. H. Potassium selective ion channels in insulin-secreting cells: physiology, pharmacology and their role in stimulus-secretion coupling. Biochim Biophys Acta. 1991 Mar 7;1071(1):67–82. doi: 10.1016/0304-4157(91)90012-l. [DOI] [PubMed] [Google Scholar]
- Dunne M. J. Phorbol myristate acetate and ATP-sensitive potassium channels in insulin-secreting cells. Am J Physiol. 1994 Aug;267(2 Pt 1):C501–C506. doi: 10.1152/ajpcell.1994.267.2.C501. [DOI] [PubMed] [Google Scholar]
- Glaser B., Chiu K. C., Anker R., Nestorowicz A., Landau H., Ben-Bassat H., Shlomai Z., Kaiser N., Thornton P. S., Stanley C. A. Familial hyperinsulinism maps to chromosome 11p14-15.1, 30 cM centromeric to the insulin gene. Nat Genet. 1994 Jun;7(2):185–188. doi: 10.1038/ng0694-185. [DOI] [PubMed] [Google Scholar]
- Glaser B., Hirsch H. J., Landau H. Persistent hyperinsulinemic hypoglycemia of infancy: long-term octreotide treatment without pancreatectomy. J Pediatr. 1993 Oct;123(4):644–650. doi: 10.1016/s0022-3476(05)80970-9. [DOI] [PubMed] [Google Scholar]
- Goossens A., Gepts W., Saudubray J. M., Bonnefont J. P., Nihoul-Fekete, Heitz P. U., Klöppel G. Diffuse and focal nesidioblastosis. A clinicopathological study of 24 patients with persistent neonatal hyperinsulinemic hypoglycemia. Am J Surg Pathol. 1989 Sep;13(9):766–775. [PubMed] [Google Scholar]
- Gould V. E., Memoli V. A., Dardi L. E., Gould N. S. Nesidiodysplasia and nesidioblastosis of infancy: ultrastructural and immunohistochemical analysis of islet cell alterations with and without associated hyperinsulinaemic hypoglycaemia. Scand J Gastroenterol Suppl. 1981;70:129–142. [PubMed] [Google Scholar]
- Kaiser N., Corcos A. P., Tur-Sinai A., Ariav Y., Glaser B., Landau H., Cerasi E. Regulation of insulin release in persistent hyperinsulinaemic hypoglycaemia of infancy studied in long-term culture of pancreatic tissue. Diabetologia. 1990 Aug;33(8):482–488. doi: 10.1007/BF00405110. [DOI] [PubMed] [Google Scholar]
- Milner R. D. Nesidioblastosis unravelled. Arch Dis Child. 1996 May;74(5):369–372. doi: 10.1136/adc.74.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P., Arkhammar P., Bokvist K., Hellerström C., Nilsson T., Welsh M., Welsh N., Berggren P. O. Failure of glucose to elicit a normal secretory response in fetal pancreatic beta cells results from glucose insensitivity of the ATP-regulated K+ channels. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4505–4509. doi: 10.1073/pnas.86.12.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. L., Mealing G. A., Whitfield J. F., Braaten J. T. Long-term culture of neonatal rat pancreatic endocrine cells as model for insulin-secretion and ion-channel studies. Diabetes. 1990 Nov;39(11):1353–1360. doi: 10.2337/diab.39.11.1353. [DOI] [PubMed] [Google Scholar]
- Sheppard D. N., Rich D. P., Ostedgaard L. S., Gregory R. J., Smith A. E., Welsh M. J. Mutations in CFTR associated with mild-disease-form Cl- channels with altered pore properties. Nature. 1993 Mar 11;362(6416):160–164. doi: 10.1038/362160a0. [DOI] [PubMed] [Google Scholar]
- Spitz L., Bhargava R. K., Grant D. B., Leonard J. V. Surgical treatment of hyperinsulinaemic hypoglycaemia in infancy and childhood. Arch Dis Child. 1992 Feb;67(2):201–205. doi: 10.1136/adc.67.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires P. E., James R. F., London N. J., Dunne M. J. ATP-induced intracellular Ca2+ signals in isolated human insulin-secreting cells. Pflugers Arch. 1994 May;427(1-2):181–183. doi: 10.1007/BF00585959. [DOI] [PubMed] [Google Scholar]
- Thomas P. M., Cote G. J., Hallman D. M., Mathew P. M. Homozygosity mapping, to chromosome 11p, of the gene for familial persistent hyperinsulinemic hypoglycemia of infancy. Am J Hum Genet. 1995 Feb;56(2):416–421. [PMC free article] [PubMed] [Google Scholar]
- Thomas P. M., Cote G. J., Wohllk N., Haddad B., Mathew P. M., Rabl W., Aguilar-Bryan L., Gagel R. F., Bryan J. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995 Apr 21;268(5209):426–429. doi: 10.1126/science.7716548. [DOI] [PubMed] [Google Scholar]
- Thornton P. S., Alter C. A., Katz L. E., Baker L., Stanley C. A. Short- and long-term use of octreotide in the treatment of congenital hyperinsulinism. J Pediatr. 1993 Oct;123(4):637–643. doi: 10.1016/s0022-3476(05)80969-2. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Sharp G. W. Regulation of insulin release by calcium. Physiol Rev. 1981 Oct;61(4):914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]


