Abstract
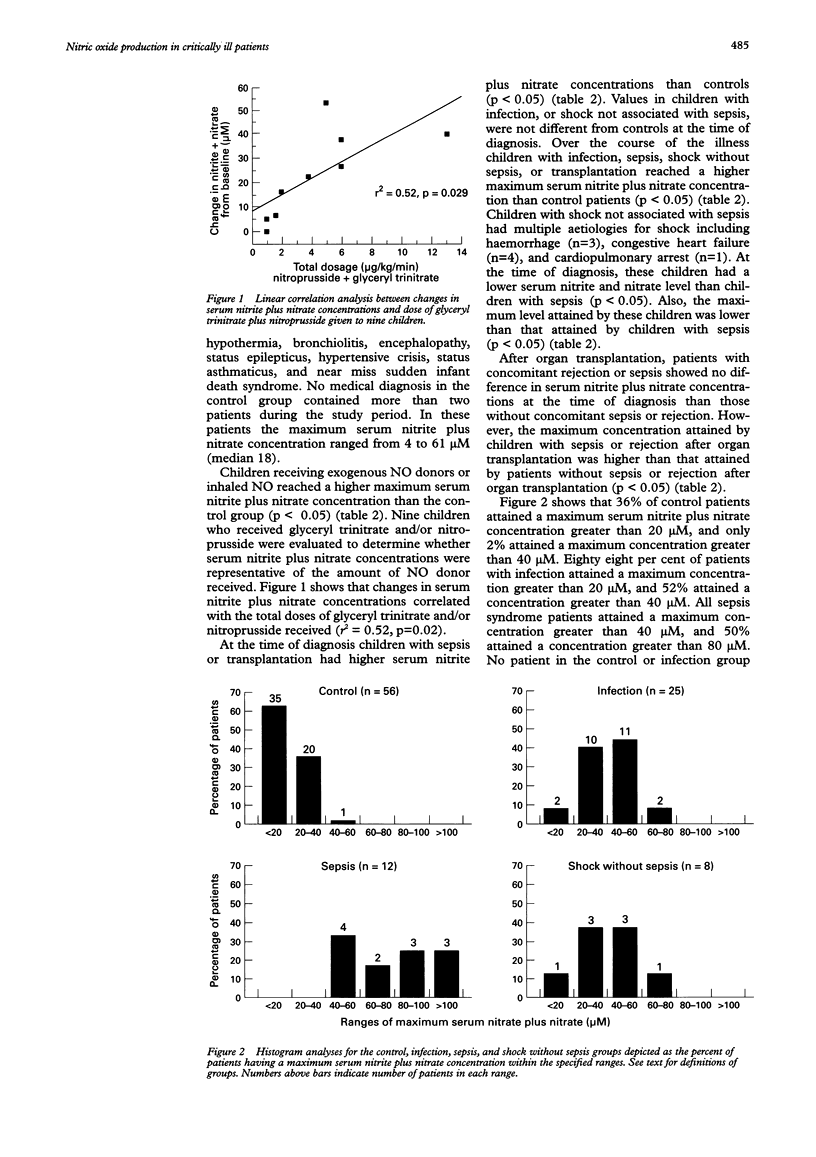
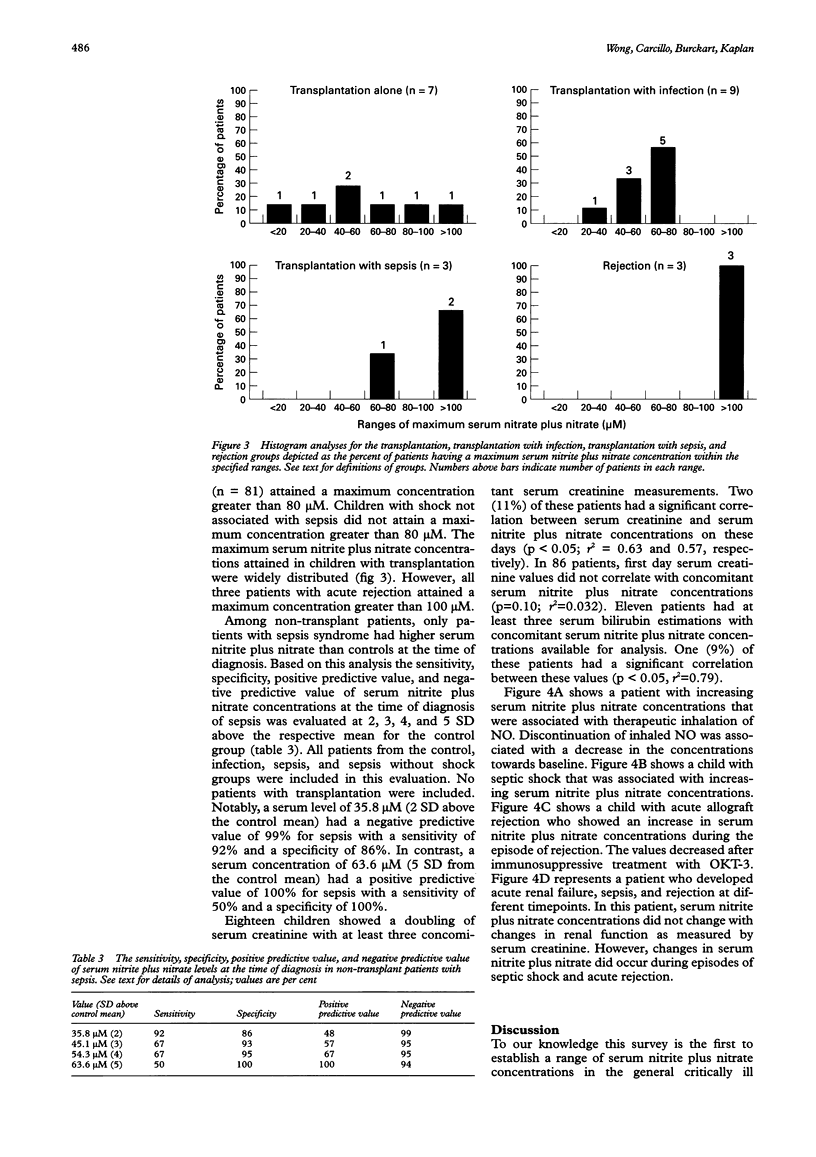
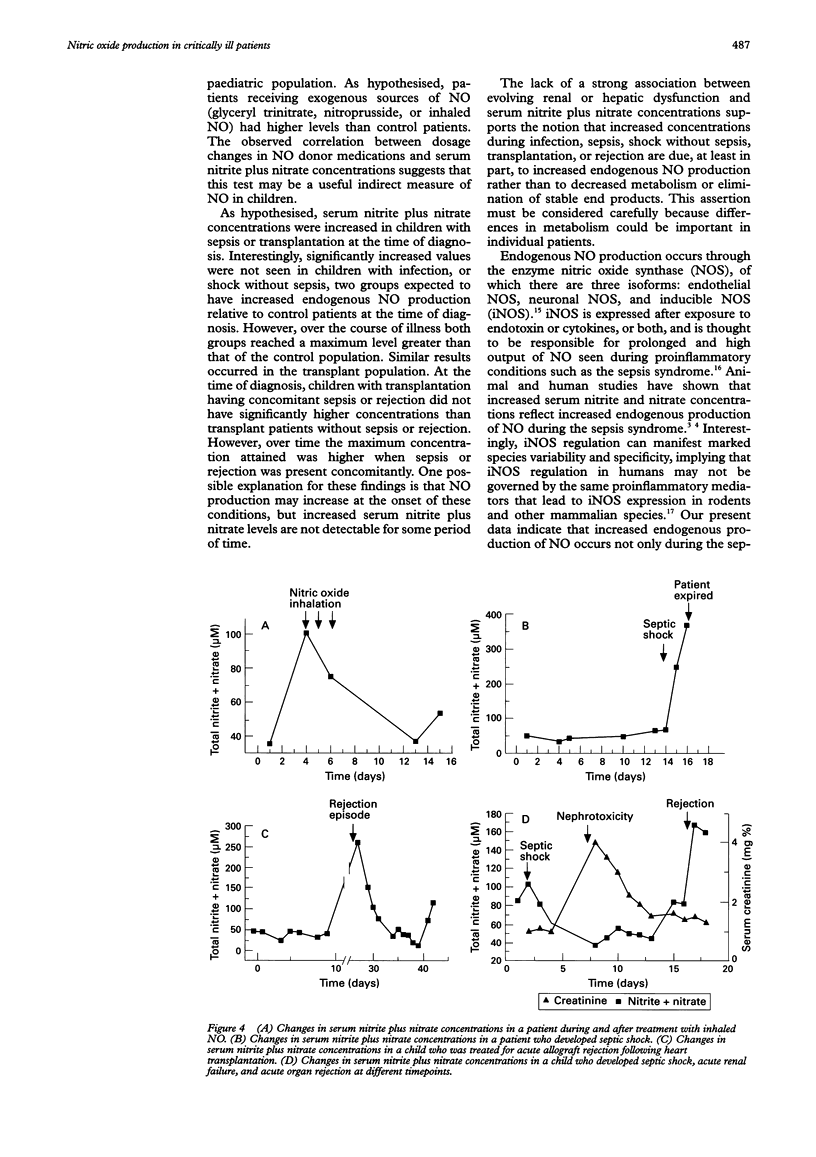
OBJECTIVE: To measure serum nitrite and nitrate levels in critically ill children as indicators of endogenous nitric oxide (NO) production. HYPOTHESIS: Endogenous NO production is increased in children with conditions characterised by immune stimulation. DESIGN: Prospective descriptive study in a multidisciplinary paediatric intensive care unit. PATIENTS: 137 consecutive critically ill children with a variety of clinical conditions. INTERVENTIONS: Using a rapid microtitre plate technique, daily serum nitrite and nitrate levels were measured from serum samples that remained in the clinical laboratory after daily routine phlebotomy. Clinical and laboratory information was also gathered daily for each patient. RESULTS: The maximum serum nitrite plus nitrate levels (microM) reached by children with infection (41.8 (SD 18.1)), sepsis syndrome (85.1 (39.9)), shock without sepsis (36.4 (19.1)), transplantation alone (61.0 (43.4)), transplantation with sepsis (200.7 (150.5)), or rejection (161.7 (70.4)), were higher than in controls (18.1 (9.3)). In the absence of exogenous NO donors, levels greater than 80 microM were reached only in children with the sepsis syndrome, organ transplantation, or acute rejection. CONCLUSIONS: Increased endogenous NO production occurs in children with clinical conditions associated with immune stimulation. Further investigation is warranted to determine the value of this simple and rapid test as a clinically useful diagnostic tool and therapeutic monitor in the evaluation of children at risk for the sepsis syndrome or acute allograft rejection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bone R. C., Balk R. A., Cerra F. B., Dellinger R. P., Fein A. M., Knaus W. A., Schein R. M., Sibbald W. J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992 Jun;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Bone R. C., Fisher C. J., Jr, Clemmer T. P., Slotman G. J., Metz C. A., Balk R. A. Sepsis syndrome: a valid clinical entity. Methylprednisolone Severe Sepsis Study Group. Crit Care Med. 1989 May;17(5):389–393. [PubMed] [Google Scholar]
- Felley-Bosco E. Species specificity at the molecular level: the case of nitric oxide synthases. Arch Toxicol Suppl. 1995;17:357–366. doi: 10.1007/978-3-642-79451-3_31. [DOI] [PubMed] [Google Scholar]
- Geller D. A., Nussler A. K., Di Silvio M., Lowenstein C. J., Shapiro R. A., Wang S. C., Simmons R. L., Billiar T. R. Cytokines, endotoxin, and glucocorticoids regulate the expression of inducible nitric oxide synthase in hepatocytes. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):522–526. doi: 10.1073/pnas.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Habib F., Dutka D., Crossman D., Oakley C. M., Cleland J. G. Enhanced basal nitric oxide production in heart failure: another failed counter-regulatory vasodilator mechanism? Lancet. 1994 Aug 6;344(8919):371–373. doi: 10.1016/s0140-6736(94)91402-8. [DOI] [PubMed] [Google Scholar]
- Jacob T. D., Ochoa J. B., Udekwu A. O., Wilkinson J., Murray T., Billiar T. R., Simmons R. L., Marion D. W., Peitzman A. B. Nitric oxide production is inhibited in trauma patients. J Trauma. 1993 Oct;35(4):590–597. doi: 10.1097/00005373-199310000-00015. [DOI] [PubMed] [Google Scholar]
- Langrehr J. M., Hoffman R. A., Lancaster J. R., Jr, Simmons R. L. Nitric oxide--a new endogenous immunomodulator. Transplantation. 1993 Jun;55(6):1205–1212. doi: 10.1097/00007890-199306000-00001. [DOI] [PubMed] [Google Scholar]
- Langrehr J. M., Murase N., Markus P. M., Cai X., Neuhaus P., Schraut W., Simmons R. L., Hoffman R. A. Nitric oxide production in host-versus-graft and graft-versus-host reactions in the rat. J Clin Invest. 1992 Aug;90(2):679–683. doi: 10.1172/JCI115911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrehr J. M., Müller A. R., Lee T. K., Schraut W. H., Simmons R. L., Hoffman R. A. Serum NO2-/NO3- from oxidative L-arginine metabolism: a possible marker for small bowel allograft rejection. Transplant Proc. 1992 Jun;24(3):1148–1148. [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nathan C., Xie Q. W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994 Sep 23;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Ochoa J. B., Udekwu A. O., Billiar T. R., Curran R. D., Cerra F. B., Simmons R. L., Peitzman A. B. Nitrogen oxide levels in patients after trauma and during sepsis. Ann Surg. 1991 Nov;214(5):621–626. doi: 10.1097/00000658-199111000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Cellek S., Palmer R. M., Moncada S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun. 1990 Dec 14;173(2):541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- Shi Y., Li H. Q., Shen C. K., Wang J. H., Pan J., Qin S. W., Liu R. Association between protective efficacy of antibodies to tumor necrosis factor and suppression of nitric oxide production in neonatal rats with fatal infection. Pediatr Res. 1993 Sep;34(3):345–348. doi: 10.1203/00006450-199309000-00021. [DOI] [PubMed] [Google Scholar]
- Shi Y., Li H. Q., Shen C. K., Wang J. H., Qin S. W., Liu R., Pan J. Plasma nitric oxide levels in newborn infants with sepsis. J Pediatr. 1993 Sep;123(3):435–438. doi: 10.1016/s0022-3476(05)81753-6. [DOI] [PubMed] [Google Scholar]
- Wagner D. A., Young V. R., Tannenbaum S. R. Mammalian nitrate biosynthesis: incorporation of 15NH3 into nitrate is enhanced by endotoxin treatment. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4518–4521. doi: 10.1073/pnas.80.14.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H. R., Carcillo J. A., Burckart G., Shah N., Janosky J. E. Increased serum nitrite and nitrate concentrations in children with the sepsis syndrome. Crit Care Med. 1995 May;23(5):835–842. doi: 10.1097/00003246-199505000-00010. [DOI] [PubMed] [Google Scholar]
- Yokokawa K., Mankus R., Saklayen M. G., Kohno M., Yasunari K., Minami M., Kano H., Horio T., Takeda T., Mandel A. K. Increased nitric oxide production in patients with hypotension during hemodialysis. Ann Intern Med. 1995 Jul 1;123(1):35–37. doi: 10.7326/0003-4819-123-1-199507010-00005. [DOI] [PubMed] [Google Scholar]


