Abstract
Phosphatidylinositol (PI) metabolism plays a central role in signaling pathways in both animals and higher plants. Stomatal guard cells have been reported to contain PI 3-phosphate (PI3P) and PI 4-phosphate (PI4P), the products of PI 3-kinase (PI3K) and PI 4-kinase (PI4K) activities. In this study, we tested the roles of PI3P and PI4P in stomatal movements. Both wortmannin (WM) and LY294002 inhibited PI3K and PI4K activities in guard cells and promoted stomatal opening induced by white light or the circadian clock. WM and LY294002 also inhibited stomatal closing induced by abscisic acid (ABA). Furthermore, overexpression in guard cells of GFP:EBD (green fluorescent protein:endosome binding domain of human EEA1) or GFP:FAPP1PH (PI-four-P adaptor protein-1 pleckstrin homology domain), which bind to PI3P and PI4P, respectively, increased stomatal apertures under darkness and white light and partially inhibited stomatal closing induced by ABA. The reduction in ABA-induced stomatal closing with reduced levels of PI monophosphate seemed to be attributable, at least in part, to impaired Ca2+ signaling, because WM and LY294002 inhibited ABA-induced cytosolic Ca2+ increases in guard cells. These results suggest that PI3P and PI4P play an important role in the modulation of stomatal closing and that reductions in the levels of functional PI3P and PI4P enhance stomatal opening.
INTRODUCTION
Guard cells surrounding stomata control both the influx of CO2 required for photosynthesis and water loss from plants through transpiration to the atmosphere. The size of the stomata is regulated through volume changes of guard cells under the concerted influence of light, temperature, CO2, and phytohormones. Previous experiments have shown that guard cell signaling is mediated by numerous factors, including Ca2+, pH, reactive oxygen species (ROS), protein kinases and phosphatases, the cytoskeleton, ion channels, and phosphoinositides (Assmann and Shimazaki, 1999; Hwang et al., 2000; Schroeder et al., 2001).
Phosphoinositides are a family of inositol-containing phospholipids found in all eukaryotic cells. It has been established that these lipids play many important roles throughout plant life (Drøbak et al., 1999; Stevenson et al., 2000). Phosphatidylinositol (PI) kinases catalyze the addition of phosphates to specific positions on the inositol ring of PI. The PI kinases include PI 3-kinase (PI3K) and PI 4-kinase (PI4K), which synthesize PI 3-phosphate (PI3P) and PI 4-phosphate (PI4P). PI 5-kinase has not yet been found, and PI 5-phosphate is likely to be produced from the degradation of PI 4,5-bisphosphate (PI45P2) (Hinchliffe et al., 1998). In animals, several distinct PI3K isoforms are involved in the regulation of diverse cellular processes, including vesicle trafficking, proliferation, cytoskeletal organization, Glc transport, and cell volume recovery (Rameh and Cantley, 1999). However, in plants, only one PI3K type, which is a PI-specific PI3K related to yeast Vps34p, has been found. The plant PI3K has been suggested to be involved in root nodule development, plant growth and development, vesicle trafficking from Golgi to vacuoles, and regulation of the transcriptional process (Hong and Verma, 1994; Welters et al., 1994; Bunney et al., 2000; Kim et al., 2001).
PI4K catalyzes the production of PI4P, the only known precursor of PI45P2, which can be cleaved into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol by phospholipase C; therefore, it represents a critical point of regulation of PI-dependent pathways. In mammalian and yeast cells, PI4Ks also are important for membrane biogenesis and vesicle trafficking from the ER to the Golgi and the plasma membrane (Roth, 1999). In plant cells, two PI4K genes have been cloned (Stevenson et al., 2000). Although previous studies have succeeded in localizing the enzyme activities of these PI4Ks to the plasma membrane, nucleus, cytosol, and cytoskeleton (Drøbak et al., 1999), their functions remain poorly understood.
PI3P and PI4P exist in guard cells of Commelina communis, in which they are suggested to be involved in guard cell signaling (Parmar and Brearley, 1993, 1995). Phosphoinositide metabolism has been shown to play important roles in abscisic acid (ABA)–induced cytosolic calcium concentration ([Ca2+]cyt) changes and stomatal closing (Gilroy et al., 1990; Staxén et al., 1999). In addition, endogenous PI monophosphate (PIP) levels in guard cells change rapidly in response to treatment with ABA (Lee et al., 1996). However, the specific role of PIP in guard cells has not been studied previously. In this study, we present evidence that supports the involvement of PI3P and PI4P of guard cells in stomatal movements and ABA-induced Ca2+ signaling.
RESULTS
Wortmannin and LY294002 Promote Stomatal Opening Induced by White Light or by the Circadian Clock in the Dark and Inhibit Stomatal Closing Induced by ABA
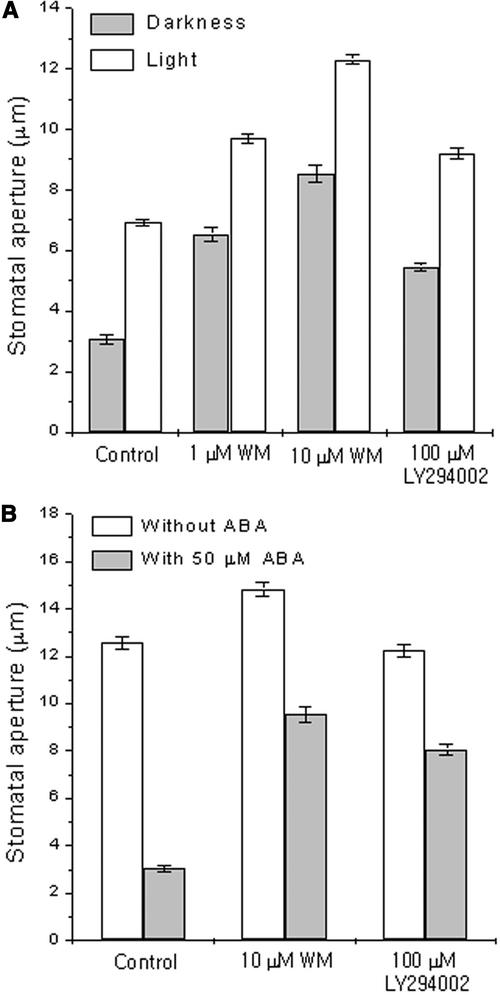
To investigate whether PI3P and PI4P in guard cells are involved in stomatal movements, we treated guard cells of Vicia faba leaves with wortmannin (WM) or LY294002, inhibitors of PI3K and PI4K. These inhibitors were used in the micromolar range because micromolar concentrations of these inhibitors have been reported previously to inhibit the activity of PI kinases in plant cells (Matsuoka et al., 1995; Xue et al., 1999; Kim et al., 2001). In the presence of 1 or 10 μM WM, stomatal opening movements induced by the circadian clock under darkness or white light were enhanced greatly (P < 0.001; Figure 1A). WM at 1 μM increased stomatal opening induced by the circadian clock in the dark to 214% compared with the control and to 140% in the light compared with the control. WM at 10 μM enhanced stomatal opening further (an increase of 279 and 178% compared with the control under darkness and white light, respectively). LY294002, a PI kinase inhibitor structurally distinct from WM, also enhanced stomatal opening under darkness and white light at 100 μM (P < 0.001).
Figure 1.
Effects of WM and LY294002 on Stomatal Movements in Vicia.
(A) Effects on stomatal opening. Epidermal fragments were incubated with WM, LY294002, or 0.2% DMSO (as a solvent control) for 3 h under darkness or white light beginning at h 3 to 4 of the usual photoperiod.
(B) Effects on stomatal closing. Fully opened stomata were pretreated with WM, LY294002, or 0.2% DMSO (as a solvent control) for 30 min and treated with 50 μM ABA for 1 h. Final apertures (average ± se) from 120 measurements from three independent experiments are presented.
We also tested the effects of these inhibitors on ABA-induced stomatal closing. In the absence of the inhibitors, stomatal apertures after 1 h of treatment with 50 μM ABA were 24% of control without ABA (Figure 1B). In the presence of the inhibitor WM at 10 μM or LY294002 at 100 μM, stomatal apertures remained at 64 and 66% of controls without ABA, respectively (P < 0.001; Figure 1B). Similar results were obtained from another plant species; in C. communis guard cells, stomatal opening induced by white light or the circadian clock was enhanced, and stomatal closing induced by ABA was inhibited (data not shown). These findings suggest an important role for PI3P and PI4P in guard cells for stomatal movements.
WM and LY294002 Commonly Inhibit Both PI3K and PI4K Activities in Guard Cells
To determine whether these inhibitors reduce the enzyme activities of PI3K and PI4K in guard cells, we prepared protein extracts from guard cell–enriched epidermis pretreated with the inhibitors and then assayed the kinase activities in these extracts. PI3K and PI4K activities were detected in the membrane fraction but not in the cytosolic fraction of proteins extracted from guard cell–enriched samples in an in vitro assay with γ-32P-ATP (Figure 2). This is in contrast to findings in other plant cells, in which PI kinase activity has been detected in both soluble and membrane fractions as well as in the cytoskeleton and nucleus (Hong and Verma, 1994; Munnik et al., 1998). The level of radiolabeling of PI4P was approximately 9- to 10-fold higher than that of PI3P, indicating that PI4K activity dominates PI3K activity. Treatment with WM or LY294002 inhibited both PI3K and PI4K activities. Compared with the control, WM reduced PI4K activity to 41% ± 15% at 1 μM, to 16% ± 3% at 10 μM, and almost abolished all activity at 30 μM (to 8% ± 3%). WM also inhibited PI3K activity almost completely at 1 μM (to 9% ± 7%). LY294002 (100 μM) inhibited the activities of PI3K (to 35% ± 14%) and PI4K (to 64% ± 13%) compared with the controls.
Figure 2.
Effects of WM and LY294002 on the Activities of PI3K and PI4K in Guard Cells of Vicia.
Epidermal fragments were illuminated for 3 h in 10 mM KCl and 10 mM Mes-KOH buffer, pH 6.1, with or without inhibitors. Proteins were extracted from guard cell–enriched epidermal tissues, separated into membrane and soluble fractions as described in Methods, and incubated with γ-32P-ATP and PI for 15 min. The lipid products were extracted and separated on a trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid–treated thin layer chromatography plate with a borate-based separation system. Proteins prepared from wild-type (WT) and mutant ΔVPS34 (defective in PI3K) yeast also were assayed for kinase activities to help identify the PI3P and PI4P bands. A representative from two independent experiments with similar results is shown. DGPP, diacylglycerolpyrophosphate; PA, phosphatidic acid.
WM has been reported to inhibit not only PI kinases but also other related lipid kinases (Vanhaesebroeck et al., 2001). If PI kinase inhibitors inhibit PI4P 5-kinase (PI4P5K) as well, then they may act by reducing the level of PI45P2, which could decrease the ABA-induced release of IP3, an important second messenger in guard cells (Gilroy et al., 1990; Lee et al., 1996; Staxén et al., 1999). Thus, the possibility that PI kinase inhibitors also might inhibit PI4P5K was tested using guard cell–enriched samples. The PI bisphosphate band appeared only when exogenous PI4P was present in the in vitro kinase assay (Figure 3). Because PI4P 3-kinase has never been reported in plant cells, we suggest that the band corresponds to the PI4P5K product, PI45P2. WM at 1, 10, and 30 μM reduced PI4P5K activity in guard cells to 58, 38, and 20%, respectively. However, LY294002 at 100 μM did not inhibit PI4P5K activity significantly (to 92% ± 5% of control; P > 0.05, n = 3). Interestingly, under experimental conditions in which only endogenous but no exogenous PI was present in the assay, LY294002 specifically inhibited PI3K activity (to 65% ± 4% of control; P < 0.05, n = 3) but not PI4K activity (to 92% ± 11% of control; P > 0.05, n = 3). The specificity of LY294002 for PI kinases supports the notion that the inhibitory effects of this inhibitor are attributable mainly to reduced PIP levels rather than to reduced PI45P2 levels.
Figure 3.
Effects of WM and LY294002 on PI4P5K Activity in Guard Cells of Vicia.
All experimental procedures were as described in Figure 2 except that the membrane fraction of the guard cell preparation was incubated for 30 min with γ-32P-ATP in the presence or absence of PI4P. In control samples without any exogenous lipid substrate, no PI45P2 band was detected. In all samples, some endogenous lipids from the membrane fraction were phosphorylated. C, control; DGPP, diacylglycerolpyrophosphate; PA, phosphatidic acid.
Overexpression of the PI3P Binding Endosome Binding Domain Construct GFP:EBD Increases Stomatal Apertures under Darkness and White Light and Inhibits Stomatal Closing Induced by ABA
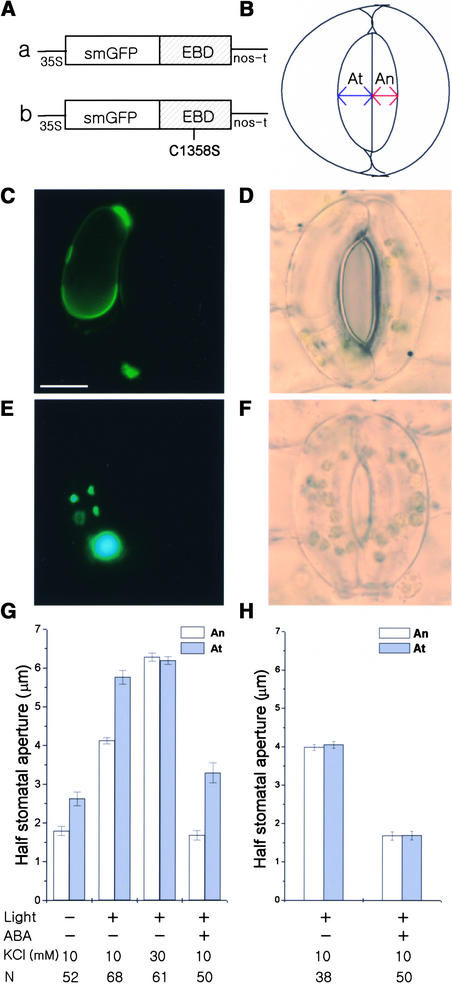
WM and LY294002 interfered with the activity of both PI3K and PI4K; therefore, it was difficult to determine which PIP contributed to stomatal movements. To answer this question, we overexpressed in guard cells GFP:EBD (green fluorescent protein:endosome binding domain of human EEA1) (Figure 4A, construct a), which binds specifically to PI3P (Kim et al., 2001). Figure 4C shows a fluorescence image of a guard cell expressing GFP:EBD. GFP:EBD, which has been reported to localize to membranous structures (Gillooly et al., 2000; Kim et al., 2001), appeared to localize at, or close to, vesicles and tonoplast membranes in guard cells (Figure 4C). We compared the apertures of half-stoma bordered by transformed guard cells (At) expressing GFP:EBD with those of nontransformed neighbor guard cells (An) (Figure 4B).
Figure 4.
Effects of Overexpressed GFP:EBD on Stomatal Movements in Vicia.
(A) Diagram of the constructs GFP:EBD (a) and GFP:EBDC1358S (b).
(B) Cartoon of the method of measurement of half-stomatal apertures bordered by transformed (At) or nontransformed (An) guard cells.
(C) and (D) A guard cell expressing GFP:EBD treated with 50 μM ABA for 1 h.
(C) Fluorescence image. Bar = 10 μm.
(D) Corresponding bright-field image.
(E) and (F) A guard cell expressing GFP:EBDC1358S treated with 50 μM ABA for 1 h.
(E) Fluorescence image.
(F) Corresponding bright-field image.
The guard cells at right in (D) and (F) were not transformed. The fluorescence images shown in (C) and (E) were taken at the same exposure time. The intense fluorescence of accumulated GFP:EBDC1358S looks bluish in (E).
(G) and (H) Half-stomatal apertures of GFP:EBD-transformed (G) or GFP:EBDC1358S-transformed (H) guard cells and nontransformed guard cells under darkness after white light–induced stomatal opening for 3 h and 50 μM ABA-induced stomatal closing for 1 h. Results shown are from three independent experiments (average ± se). N, number of cells observed.
Overexpressed GFP:EBD increased stomatal apertures under darkness and white light (Figure 4G; P < 0.001) and inhibited stomatal closing in response to 50 μM ABA (Figures 4D and 4G; P < 0.001). In the darkness, At was 2.6 ± 0.18 μm, whereas An was 1.8 ± 0.12 μm. After 3 h of illumination with white light, At was 5.8 ± 0.18 μm, whereas An was 4.1 ± 0.08 μm. To determine the effects of the overexpressed GFP:EBD on stomatal closing, At and An stomata were induced to open to the same extent, using 30 mM KCl and increasing temperature to 29°C. Subsequent treatment with 50 μM ABA for 1 h reduced An to 1.7 ± 0.12 μm, whereas At remained open at 3.3 ± 0.26 μm. As a con-trol for the expression of GFP:EBD, we expressed GFP:EBDC1358S (Figure 4A, construct b), which does not bind to PI3P (Kim et al., 2001). GFP:EBDC1358S showed a spotty pattern of expression in the cytosol of guard cells (Figure 4E), which was similar to that found in Arabidopsis cells (Kim et al., 2001), and it did not alter stomatal movements (Figures 4F and 4H). These results provide evidence of an important role for PI3P in stomatal movements.
Overexpression of the PI4P Binding Construct GFP:FAPP1PH Increases Stomatal Apertures under Darkness and White Light and Inhibits Stomatal Closing Induced by ABA
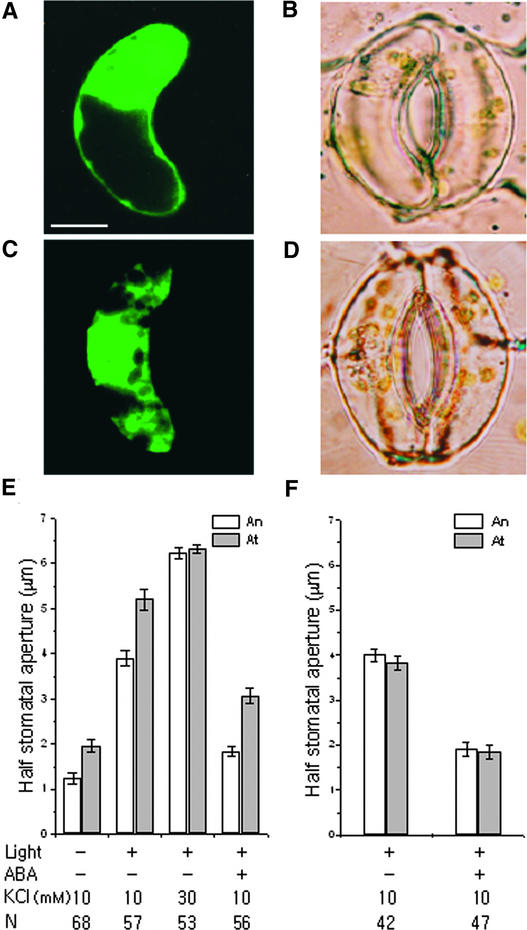
To investigate a possible role for PI4P in stomatal movements, we overexpressed in guard cells GFP:FAPP1PH (PI-four-phosphate adaptor protein-1 pleckstrin homology domain). Dowler et al. (2000) reported previously that FAPP1PH fused to glutathione S-transferase binds specifically to PI4P in vitro. The fusion protein GFP:FAPP1PH (Figure 5A) used in our study specifically bound to PI4P in vitro (Figure 5C) and was localized mainly to membrane regions in Arabidopsis mesophyll cell protoplasts (Figures 5E and 5G). However, a mutant form, GFP:FAPP1PH[K7E, R18A] (Figure 5B), did not bind to PI4P in vitro (Figure 5D) and was distributed mainly in the cytosol of Arabidopsis mesophyll cell protoplasts (Figures 5F and 5G).
Figure 5.
PI4P-Specific Binding of GFP:FAPP1PH and Its Localization at the Plasma Membrane in Arabidopsis Mesophyll Cell Protoplasts.
(A) and (B) Diagram of the constructs GFP:FAPP1PH (A) and GFP:FAPP1PH[K7E, R18A] (B).
(C) and (D) Phosphoinositide binding properties of GFP:FAPP1PH (C) and GFP:FAPP1PH[K7E, R18A] (D). The phosphoinositide binding affinity of His-tagged GFP:FAPP1PH and GFP:FAPP1PH[K7E, R18A] was assessed by fat-protein gel blot analysis. The binding of GFP:FAPP1PH and GFP:FAPP1PH[K7E, R18A] was detected using a monoclonal anti-His antibody.
(E) and (F) In vivo targeting of GFP:FAPP1PH (E) and GFP: FAPP1PH[K7E, R18A] (F). Protoplasts were transformed with GFP:FAPP1PH and GFP:FAPP1PH[K7E, R18A] and examined at 12 to 36 h after transformation. The images show representative protoplasts at 17 h after transformation. The region where red chloroplasts and cytosolic GFP:FAPP1PH[K7E, R18A] overlap appears yellow in (F). CH, chloroplasts; PM, plasma membrane; V, central vacuole. Bar = 20 μm.
(G) Protein gel blot analysis of the localization of GFP:FAPP1PH and GFP:FAPP1PH[K7E, R18A]. Whole cell extracts (T) obtained from the transformed protoplasts were separated into soluble (S) and membrane (M) fractions by ultracentrifugation at 100,000g for 1 h. The membrane fraction was resuspended in the original volume. Proteins in equal volume (30 μL) of each fraction were separated by SDS-PAGE and probed by protein gel blot analysis using monoclonal anti-GFP antibody.
Figure 6A shows a fluorescence image of a guard cell expressing GFP:FAPP1PH. GFP:FAPP1PH appeared to localize to or near the plasma membrane and nucleus in guard cells (Figure 6A), as has been found for other eukaryotic cells (Payrastre et al., 1992; Balla, 1998; Bunney et al., 2000). We compared apertures of half-stoma bordered by transformed guard cells (At) expressing GFP:FAPP1PH with those of their nontransformed neighbor guard cells (An). As observed for PI3P binding of GFP:EBD, overexpressed GFP:FAPP1PH increased stomatal apertures under darkness and white light (Figure 6E; P < 0.001) and inhibited stomatal closing in response to 50 μM ABA (Figures 6B and 6E; P < 0.001). In the darkness, At was 2.0 ± 0.15 μm, whereas An was 1.2 ± 0.12 μm. After 3 h of illumination with white light, At was 5.2 ± 0.22 μm, whereas An was 3.9 ± 0.16 μm. To determine the effects of the overexpressed GFP:FAPP1PH on stomatal closing, At and An stomata were induced to open to the same extent, using 30 mM KCl and increasing temperature to 29°C. Subsequent treatment with 50 μM ABA for 1 h reduced An to 1.8 ± 0.11 μm, whereas At remained open at 3.1 ± 0.18 μm. As a control for the expression of GFP:FAPP1PH, we expressed GFP:FAPP1PH[K7E, R18A], which does not bind to PI4P. GFP:FAPP1PH[K7E, R18A] appeared to localize mainly in the cytosol of guard cells (Figure 6C), as found in Arabidopsis mesophyll cell protoplasts (Figure 5F), and some also appeared as spots and did not alter stomatal movements (Figures 6D and 6F). These results suggest that PI4P plays an important role in stomatal movements.
Figure 6.
Effects of Overexpressed GFP:FAPP1PH on Stomatal Movements in Vicia.
(A) and (B) A guard cell expressing GFP:FAPP1PH treated with 50 μM ABA for 1 h.
(A) Fluorescence image. Bar = 10 μm.
(B) Corresponding bright-field image.
(C) and (D) A guard cell expressing GFP:FAPP1PH[K7E, R18A] treated with 50 μM ABA for 1 h.
(C) Fluorescence image.
(D) Corresponding bright-field image.
The guard cells at right in (B) and (D) were not transformed.
(E) and (F) Half-stomatal apertures of GFP:FAPP1PH-transformed (E) or GFP:FAPP1PH[K7E, R18A]-transformed (F) (At) and nontransformed (An) guard cells under darkness after white light–induced stomatal opening for 3 h and 50 μM ABA-induced stomatal closing for 1 h. Results shown are from three independent experiments (average ± se). N, number of cells observed.
The Effect of the PI4P Binding Construct on Stomatal Opening Is Not Mediated through Reductions in the Level of PI45P2 or IP3
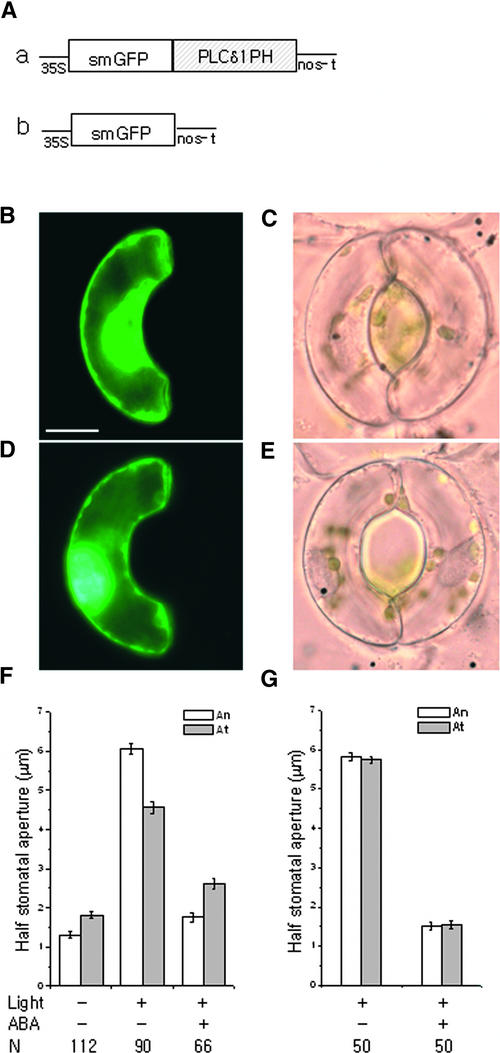
Because PI4P is a precursor of PI45P2, it is possible that the PI4P binding construct inhibits PI45P2 synthesis and subsequent stimulus-dependent IP3 release in guard cells. If this is the case, then the PI45P2 binding construct should show the same effect as the PI4P binding construct. However, the expression of GFP:PLCδ1PH (phospholipase Cδ1 pleckstrin homology domain), a PI45P2 and IP3 binding construct (Stauffer et al., 1998; Hirose et al., 1999; Kost et al., 1999), in guard cells inhibited white light–induced stomatal opening (Figures 7C and 7F), an effect that was opposite to that of the PI4P binding construct GFP:FAPP1PH (Figure 6). The effect of GFP:PLCδ1PH on stomatal opening also was opposite to those of WM and LY294002 (Figure 1A). ABA-induced closing of stomata bordered by guard cells expressing GFP:PLCδ1PH was similar to that of guard cells expressing GFP:EBD or GFP:FAPP1PH (Figures 4, 6, and 7). Therefore, the PI4P binding construct may reduce ABA-induced stomatal closing, at least in part, by reducing the level of PI45P2. The expression of GFP alone did not have any effect on stomatal movement.
Figure 7.
Effects of Overexpressed GFP:PLCδ1PH on Stomatal Movements in Vicia.
(A) Diagram of the constructs GFP:PLCδ1PH (a) and GFP (b).
(B) and (C) A guard cell expressing GFP:PLCδ1PH irradiated with white light for 3 h.
(B) Fluorescence image. Bar = 10 μm.
(C) Corresponding bright-field image.
(D) and (E) A guard cell expressing GFP irradiated with white light for 3 h.
(D) Fluorescence image.
(E) Corresponding bright-field image.
The guard cells at right in (C) and (E) were not transformed.
(F) and (G) Half-stomatal apertures of GFP:PLCδ1PH-transformed (F) or GFP-transformed (G) guard cells and nontransformed guard cells under darkness after white light–induced stomatal opening for 3 h and 50 μM ABA-induced stomatal closing for 1 h. The guard cells were incubated in 10 mM KCl and 10 mM Mes-KOH buffer, pH 6.1, at 29°C throughout the experiment. Results shown are from three independent experiments (average ± se). N, number of cells observed.
WM and LY294002 Inhibit ABA-Induced Cytosolic Ca2+ Increases in Guard Cells
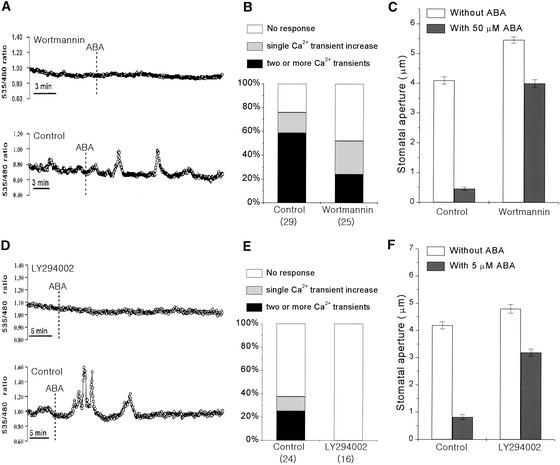
To elucidate the possible mechanisms by which PI3P and PI4P participate in stomatal movements, we determined if these lipids are involved in ABA-induced Ca2+ signaling, an important mediator in stomatal movements (Assmann and Shimazaki, 1999; Schroeder et al., 2001). We measured changes in [Ca2+]cyt induced by ABA in the presence or absence of inhibitors (WM or LY294002) in Arabidopsis guard cells expressing yellow cameleon 2.1 as a Ca2+ indicator (Allen et al., 1999). WM (10 μM) reduced the probability of ABA-induced [Ca2+]cyt increases (Figures 8A and 8B; χ2 = 3.34, P < 0.05) as well as the stomatal closing induced by ABA (Figure 8C; P < 0.001, n = 120). In the control group, ABA (50 μM) induced two or more Ca2+ transient increases in 59% of guard cells (n = 17 of 29), a single transient [Ca2+]cyt increase in 17% of guard cells (n = 5 of 29), and no response in [Ca2+]cyt in 24% of guard cells (n = 7 of 29). However, in WM-treated guard cells, the percentage of cells showing two or more ABA-induced Ca2+ transient increases declined to 24% (n = 6 of 25). Guard cells showing a single transient increase or no change of [Ca2+]cyt were 28 and 48%, respectively (n = 7 of 25 and n = 12 of 25; Figure 8B). In addition, WM decreased the average peak of [Ca2+]cyt changes even in those guard cells showing Ca2+ increases (P < 0.005). The average peak [Ca2+]cyt change was 211 nM in WM-treated cells and 488 nM in control cells. LY294002 (100 μM) had the same effects on ABA-induced [Ca2+]cyt increases (Figures 8D and 8E) and stomatal closing (Figure 8F; P < 0.001, n = 120).
Figure 8.
Effects of WM and LY294002 on the ABA-Induced Ca2+ Increases in Guard Cells and Stomatal Closing in Arabidopsis.
(A) and (D) ABA-induced cytosolic Ca2+ changes in the presence or absence of the inhibitors 10 μM WM (A) and 100 μM LY294002 (D). Vertical lines indicate the time when cells were treated with 50 μM ABA (for the WM assay) or 5 μM ABA (for the LY294002 assay).
(B) and (E) Percentage of guard cells showing different types of ABA-induced Ca2+ responses in the presence of WM and 50 μM ABA (B) or LY294002 and 5 μM ABA (E). Numbers in parentheses indicate the total numbers of cells analyzed.
(C) and (F) Effects of 10 μM WM (C) and 100 μM LY294002 (F) on ABA-induced closing in Arabidopsis stomata. Results shown are from 120 measurements from three independent experiments (average ± se).
Calcium imaging experiments were performed at 5 μM ABA in LY294002 to analyze inhibitor effects at a lower ABA concentration. In control cells, ABA (5 μM) induced two or more Ca2+ transient increases in 25% of guard cells (n = 6 of 24), a single transient [Ca2+]cyt increase in 12.5% of guard cells (n = 3 of 24), and no response in [Ca2+]cyt in 62.5% of guard cells (n = 15 of 24). By contrast, none of the LY294002-treated guard cells displayed changes in [Ca2+]cyt in response to ABA (χ2 = 7.74, P < 0.005, n = 16). These results suggest that PI3P and/or PI4P are important for ABA-induced Ca2+ signaling.
DISCUSSION
To determine whether PI3P and PI4P are important for stomatal movements, we used several different methods, including kinase inhibitor tests, biochemical assays, biolistic transformation of guard cells with chimeric genes encoding GFP-tagged phosphoinositide-specific binding proteins, and calcium imaging. The results consistently showed that PI3P and PI4P in guard cells are involved in normal stomatal movements. Furthermore, the results suggested that these lipids are essential for normal Ca2+ signaling induced by ABA.
In the kinase inhibitor test, WM and LY294002 enhanced circadian clock– or white light–induced stomatal opening and inhibited ABA-induced stomatal closing (Figures 1A and 1B). Biochemical assays showed that WM inhibited PI3K, PI4K and PI4P5K, whereas LY294002 inhibited PI3K and PI4K but not PI4P5K, in protein extracts from guard cell–enriched epidermal samples (Figures 2 and 3). The specificity of LY294002 for PI kinases is consistent with the notion that alterations in stomatal movement by these inhibitors are caused by the depletion of PI3P and PI4P. We further tested this hypothesis using GFP:EBD and GFP:FAPP1PH, which bind specifically to PI3P and PI4P, respectively. These constructs, when introduced into guard cells by the biolistic bombardment technique, had effects similar to those of WM and LY294002: they increased stomatal apertures under darkness and white light and partially inhibited ABA-induced stomatal closing (Figures 4G and 6E). These results support the notion that both PI3P and PI4P are necessary for normal stomatal movements. Overexpression of the lipid binding proteins in guard cells might have blocked the function of target lipids by competing with endogenous lipid binding proteins.
The fusion protein GFP:EBD has been used previously to study trafficking of PI3P in Arabidopsis protoplasts (Kim et al., 2001), and the results showed trafficking of PI3P from the trans-Golgi network to the lumen of the central vacuole. The EBD protein consists of a FYVE domain (PI3P binding domain) and a Rab5 binding domain; therefore, it is possible that other mechanisms, such as depletion of a Rab5 homolog by EBD, contributed to the altered responses of stomata bordered by EBD-expressing guard cells. To eliminate this possibility, we used an EBD that has a point mutation at the zinc binding motif of the FYVE domain. The overexpressed EBDC1358S, unlike normal EBD, failed to alter stomatal responses (Figure 4H), suggesting that the effects of EBD on stomatal movements result from the specific binding of PI3P to the FYVE domain of EBD.
GFP:FAPP1PH bound specifically to PI4P (Figure 5C), as shown previously for glutathione S-transferase:FAPP1PH (Dowler et al., 2000), was localized at or near the plasma membrane and nucleus of guard cells (Figure 6A), and increased stomatal apertures (Figure 6E). In contrast, GFP: FAPP1PH mutated at two amino acids in a region of high interspecies homology (Figure 5B), showed decreased affinity to PI4P (Figure 5D), was localized mainly to the cytosol of guard cells (Figure 6C), and had no effects on stomatal apertures (Figure 6F). These results suggest that the binding of PI4P by GFP:FAPP1PH has an effect on stomatal movements.
GFP:PLCδ1PH, which binds specifically to PI45P2 and its metabolite IP3, has been used widely to visualize PI45P2 (Stauffer et al., 1998). Introduction of GFP:PLCδ1PH into guard cells inhibited white light–induced stomatal opening (Figures 7C and 7F), an effect that was opposite to that of the PI4P binding construct GFP:FAPP1PH (Figures 6 and 7). This result suggests that the effect of the PI4P binding construct on stomatal opening is not attributable to a reduction in the PI45P2 or IP3 level but to interference with the normal function of PI4P. Therefore, we suggest that PI4P plays a role in stomatal opening movement as part of a PI45P2- and IP3-independent pathway in guard cells.
The effect of GFP:PLCδ1PH on stomatal opening also was opposite to that of WM (Figures 1A and 7). Therefore, the effects of WM on stomatal opening are not likely to be attributable to an inhibition of PI4P5K activity but to its inhibitory effect on PI kinase activity. This conclusion is supported further by the fact that the effect of WM was similar to the effects of the two PIP binding constructs, GFP:EBD and GFP:FAPP1PH (Figures 1, 4, and 6). The effect of GFP:PLCδ1PH on ABA-induced stomatal closing was similar to the effects of PIP binding proteins (Figures 4, 6, and 7). Therefore, the effect of PI4P binding protein may be mediated at least partly by reductions in the PI45P2 level, which could reduce ABA-induced IP3 release and consequently inhibit ABA signaling in guard cells.
The level of a signal mediator for stomatal closing may increase in response to physiological stimuli that induce stomatal closure. For example, IP3 levels in guard cells increase transiently in response to ABA (Lee et al., 1996), and activities of PI kinases in plant cells have been suggested to increase in response to hyperosmotic stress (Monks et al., 2001). Previously, we showed that the PIP level in guard cell protoplasts fluctuates considerably during ABA treatment (Lee et al., 1996), reaching 140% of the control at 3 and 10 min after ABA treatment, whereas the levels of structural lipids, such as phosphatidylcholine, phosphatidylethanolamine, and phosphatidylglycerol, remain stable. This observation supports a role for PIP in ABA signaling. The possibility that PI kinase activity in guard cells changes in response to ABA was tested by the in vitro PI kinase assay using protein extracts from guard cell–enriched epidermal samples. Although our assay of the enzyme activities seemed reliable, as indicated by the measurable decreases in enzyme activities in the presence of WM and LY294002 (Figure 2), we did not detect any consistent changes in PI kinase activity after ABA treatment. The changes in the enzyme activities were variable and remained within ±40% of control at 3, 5, and 30 min after ABA treatment (data not shown).
It is possible that the activity of PI3K and PI4K may not be changed by closing signals such as ABA, and the roles of their lipid products may be to prime guard cells for subsequent stimulation—for example, by controlling important signal transducers (Tsukazaki et al., 1998). Alternatively, changes in the kinase activities may depend on particular conditions in the membrane, which we were unable to simulate in our in vitro experiments. Regardless of any signal-dependent changes in PI kinase activity, basal levels of kinase activity probably always are present in these cells, and the lipid products of these kinases probably always are undergoing rapid turnover, because inhibitors of PI kinases increased stomatal apertures under all conditions tested, including darkness, light, and ABA treatment (Figure 1 and our unpublished results).
Therefore, how do PI3P and PI4P participate in stomatal movements? Oscillations in [Ca2+]cyt have critical roles in stomatal movements (Allen et al., 1999, 2001), and phosphoinositide metabolism plays important roles in ABA-induced [Ca2+]cyt changes and stomatal closing (Gilroy et al., 1990; Staxén et al., 1999). Therefore, we studied the effects of WM or LY294002 on [Ca2+]cyt changes induced by ABA using Arabidopsis guard cells expressing yellow cameleon 2.1 as a Ca2+ indicator (Allen et al., 1999). We were able to confirm the previously reported effects of ABA on [Ca2+]cyt in control guard cells (Figure 8). The patterns of ABA-induced [Ca2+]cyt responses included two or more transient increases, single transient increases, or no perceivable changes. In guard cells pretreated with WM or LY294002, [Ca2+]cyt increases were reduced substantially compared with those in the control cells, suggesting that ABA-induced [Ca2+]cyt signaling is disrupted, although not abolished entirely. These results suggest important functions for PI3P and/or PI4P in Ca2+ signaling in guard cells.
Because PI3K has been reported to activate ROS formation in platelets (Bae et al., 2000), PI3K in guard cells also may activate ROS formation, which in turn may activate plasma membrane Ca2+ channels (Pei et al., 2000). Recent studies have shown that fungal elicitors and ABA cause ROS production in guard cells and that ROS induce stomatal closing (Lee et al., 1999; Pei et al., 2000), suggesting a critical role for ROS in the mediation of stomatal closing.
WM or LY294002 increased stomatal apertures during opening as well as closing movements. Ca2+ acts as a negative regulator of stomatal opening (Schwartz, 1985); thus, if WM or LY294002 decreases [Ca2+]cyt, they can reduce the negative effect of Ca2+ on stomatal opening and increase stomatal apertures. Guard cells commonly oscillate between two ranges of membrane voltages, one close to the K+ equilibrium voltage and the other far negative of the K+ equilibrium voltage, and hyperpolarized membrane potential induces Ca2+ influx (Hamilton et al., 2000). The inhibitors may have blocked Ca2+ influx during the hyperpolarization phase of the normal oscillations in membrane voltage, thereby increasing stomatal apertures under all conditions.
The evidence for a role of PI3P or PI4P in Ca2+ signaling is compelling. However, it does not exclude additional modes of action of these lipids in guard cells. PIP has been reported to be involved in endocytosis in yeast and mammalian cells (Li et al., 1995; Wurmser and Emr, 1998; Audhya et al., 2000). Vesicle trafficking, including endocytosis, has been suggested to be important for stomatal movements (Blatt, 2000; Kubitscheck et al., 2000). Therefore, the reduction of functional PIP by GFP-tagged proteins may have inhibited endocytosis during stomatal movements. Clearly, further investigation is required to fully reveal the mechanisms by which PI3P and PI4P function in stomatal movements.
In conclusion, we have presented evidence that both PI3P and PI4P in guard cells are involved in normal stomatal movements and that their mechanism of action involves the mediation of [Ca2+]cyt increases in response to a closing stimulus. These phosphoinositides also may regulate diverse cellular processes, including signal transduction and vesicle trafficking in guard cells. The results presented here demonstrate roles for PIP in guard cells and suggest that these plant lipids may be as versatile in their functions as those found in nonplant systems.
METHODS
Plant Materials
Seeds of Vicia faba and Arabidopsis thaliana (ecotype Landsberg erecta) were planted in a vermiculite-humus soil mixture and fertilized with HYPONeX solution (1 g/L; Hyponex Corp., Marysville, OH). Vicia plants were grown in a greenhouse at 22 ± 2°C with light/dark cycles of 16/8 h. Arabidopsis plants (wild type for measurements of stomatal aperture and transgenic plants expressing yellow cameleon 2.1 for calcium imaging) were grown in a growth chamber at 20 ± 2°C with light/dark cycles of 16/8 h. Fully differentiated young leaves from 3- to 4-week-old plants were used.
Chemicals
Wortmannin (WM; 10 mM) and LY294002 (50 or 100 mM) stock solutions were prepared in 100% DMSO. Both chemicals were purchased from Sigma (St. Louis, MO).
Fluorescent Gene Constructs of Lipid Binding Proteins
Four constructs were used in this study. To generate the chimeric fusion construct GFP:EBD, the endosome binding domain (EBD) encoding the C-terminal fragment of human EEA1 (amino acid residues 1257 to 1411) was ligated in frame to the C terminus of the soluble modified green fluorescent protein (smGFP) coding region lacking the terminator codon. The FYVEC1358S mutant also was fused to smGFP, resulting in construct GFP:EBDC1358S (Kim et al., 2001).
Mouse FAPP1 cDNA (Dowler et al., 2000) corresponding to the pleckstrin homology (PH) domain was amplified by PCR from a cDNA library using two specific primers (5′-CTCGAGATGGAG-GGGGTTCTGTACAAG-3′ and 5′-TCACGCTTTGGAGCTCCCAAG-GGC-3′). The PCR product was subcloned into pBluescript KS+ (Stratagene) and then ligated to the C terminus of the GFP coding region lacking the termination codon. The two mutations, K7E and R18A, were introduced into FAPP1PH using two sequential PCR amplifications and two sets of primers (R18A, 5′-CAGAACAAACCA-TGCAGGCTGCCAACC-3′ and 5′-GGTTGGCAGCCTGCATGGTTT-GTTCTG-3′; K7E, 5′-CTCGAGATGGAGGGGGTTCTGTACGAGTGG-ACCAAC-3′ and 5′-TCACGCTTTGGAGCTCCCAAGGGC-3′). The resulting FAPP1PH[K7E, R18A] construct was ligated to the C terminus of the GFP coding region lacking the termination codon. GFP:PLCδ1PH was constructed according to the method described previously (Kost et al., 1999). All chimeric GFP fusion constructs were placed under the control of the 35S promoter in a pUC vector for overexpression in guard cells.
Biochemical Assay of Phosphatidylinositol and Phosphatidylinositol Monophosphate Kinases from Guard Cells
The abaxial epidermis of Vicia leaves was peeled from 15 fully expanded leaves for each sample. The epidermal peels were collected in ice-cold 10 mM KCl and 10 mM Mes-KOH, pH 6.1, and blended in a Waring blender (7010) for 30 s at 18,000 rpm to remove epidermal and mesophyll cells. After this step, only guard cells remained viable (data not shown) (Fukuda et al., 1998). They were washed and collected on a 220-μm nylon mesh. The prepared epidermal samples were homogenized in liquid N2 using a mortar and pestle and extracted with buffer (50 mM Hepes-KOH, pH 7.4, 5 mM MgCl2, 1 mM EDTA, 10 mM DTT, 1 mM 2-mercaptoethanol, 0.7 μg/mL pepstatin A, 5 μg/mL aprotinin, 20 μg/mL leupeptin, and 0.5 mM phenylmethylsulfonyl fluoride). In several experiments, the samples were ground at 4°C for 5 min in the buffer. The homogenate then was centrifuged at 10,000g for 5 min at 4°C. The resulting supernatant was further centrifuged at 100,000g for 1 h at 4°C for separation of the membrane and soluble fractions. Protein was quantified using the Bio-Rad protein assay kit with BSA as a standard, and the same amount of protein was used for all samples in the same experiment.
The phosphatidylinositol (PI) and PI monophosphate (PIP) kinase assay mixture contained 50 mM Hepes-KOH, pH 7.4, 5 mM MgCl2, 10 mM DTT, 4 to 10 μg of protein extracts from guard cell–enriched epidermal samples, and 20 to 100 μM ATP in a total volume of 50 μL. The reaction was started by adding 10 μg of PI or PI 4-phosphate (Sigma) and 10 μCi of γ-32P-ATP (Amersham), left at room temperature for 10 to 30 min, and then stopped by the addition of 100 μL of 1 M HCl. For extraction of lipids, 200 μL of chloroform:methanol (1:1, v/v) was added to the sample and vortexed for 20 s. Phase separation was facilitated by centrifugation at 5000 rpm for 2 min in a tabletop centrifuge. The upper phase was removed, and the lower chloroform phase was washed once more with clear upper phase. The washed chloroform phase was dried under a stream of nitrogen gas and redissolved in 30 μL of chloroform. The labeled PI phosphates then were spotted onto trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid–treated silica gel 60 thin layer chromatography (TLC) plates (Merck) and separated with a borate buffer system (Walsh et al., 1991). The plates were autoradiographed or quantified using a phosphorimager (FLA-2000R). Yeast wild-type SEY6210 and the VPS34 deletion mutant PHY102 (SEY6210 Vps34Δ1::TRP1) (Stack et al., 1993), kindly provided by S.D. Emr (University of California, San Diego), were used as references for the identification of PI 3-phosphate and PI 4-phosphate bands on TLC plates. To help in the identification of phosphatidic acid, PI 4-phosphate, and PI 4,5-bisphosphate bands, cold phospholipid standards were run in different lanes of the same TLC plate and were visualized by spraying the plate with 1.3% molybdenum oxide in 4.2 M sulfuric acid (Sigma).
Measurements of the Stomatal Aperture
Fully expanded leaves from 3- to 4-week-old Vicia were excised, and epidermal pieces were peeled from the abaxial surface. The epidermal peels were blended in a Waring blender (see above), washed, and collected on a 220-μm nylon mesh. To test the effects of WM and LY294002 on stomatal openings, epidermal fragments were floated on 10 mM KCl and 10 mM Mes-KOH buffer, pH 6.1, with or without added chemicals under darkness or white light (0.2 mmol·m−2·s−1) at 25°C for 3 h beginning at h 3 or 4 of the usual photoperiod. Apertures were measured. For the assay of stomatal closing induced by abscisic acid (ABA), epidermal fragments were exposed to white light (0.2 mmol·m−2·s−1) for 3 h in 30 mM KCl and 10 mM Mes-KOH, pH 6.1. To close the stomata, the epidermis was transferred to 10 mM KCl and 10 mM Mes-KOH, pH 6.1, preincubated for 30 min with or without added chemicals, and treated with 50 μM [±]-cis,trans-ABA for 1 h. Stomatal apertures then were measured using an eyepiece micrometer.
For measurements of stomatal apertures in biolistically transformed leaves, bombarded leaf fragments were floated on the same buffer used above. After stomatal opening or closing, abaxial epidermal pieces were peeled, and each half of the stomatal apertures, bordered by transformed guard cells, and their untransformed neighbors were measured with a fluorescence microscope.
For stomatal closing experiments in Arabidopsis, fully expanded leaves were floated on 10 mM KCl and 10 mM Mes-KOH buffer, pH 6.1, under white light (0.2 mmol·m−2·s−1) for 3 h. The inhibitors were added to the medium at 30 min before ABA treatment in closing buffers. The closing buffer for WM experiments contained 10 mM Mes-Tris, pH 6.15, 2 mM KCl, and 200 μM CaCl2; for LY294002 experiments, the closing buffer contained 10 mM Mes-Tris, pH 6.15, 5 mM KCl, and 50 μM CaCl2. One hour after the onset of ABA treatment, epidermal pieces were peeled from the abaxial surface, and stomatal apertures were measured using an eyepiece micrometer.
Biolistic Gene Bombardment into Vicia Guard Cells
Biolistic bombardment of guard cells with fluorescent gene constructs was performed as described previously (Marc et al., 1999) with minor modifications to facilitate gene expression in guard cells. In brief, 2.5 μg of plasmid DNA was mixed with 0.5 mg of 1.0-μm gold particles (Bio-Rad) in a 50-μL aqueous solution. The gold-DNA suspension was dispersed with moderate vortexing and sonication in the presence of 1.25 M CaCl2 and 17 mM spermidine and kept at room temperature for 5 min. The DNA-coated gold particles then were collected by brief centrifugation, washed, resuspended in ethanol, and spread onto plastic carrier discs for biolistic transformation (Particle Delivery System-1000/He; Bio-Rad). Young leaves (4.5 to 6 cm long) of Vicia were excised from 3- to 4-week-old plants and placed with their lower side up onto moist filter paper in 60-mm plastic Petri dishes. The dishes were inserted into the firing chamber, the vacuum was pumped to 27 inches of Hg, and the DNA-coated gold particles were fired into the leaves from a distance of 60 mm at a helium pressure of 1350 p.s.i. Bombarded leaves then were placed onto a humid Petri dish and kept in the dark at room temperature. Within 24 to 84 h, epidermal peels were taken from the bombarded leaves, and transformed guard cells were observed with an Axioskop 2 fluorescence microscope (Carl Zeiss, Jena, Germany).
Protoplast Transformation and Protein Gel Blot Analysis
For transient expression of GFP:FAPP1PH and GFP:FAPP1PH[K7E, R18A], Arabidopsis mesophyll cell protoplasts were isolated from seedlings grown in a greenhouse. Plasmids were introduced into 300 μL of protoplast suspension (5 × 106/mL) by polyethylene glycol–mediated transformation (Jin et al., 2001). Expression of constructs was monitored using a Zeiss Axioplan fluorescence microscope. To obtain whole cell extracts, protoplasts were harvested 17 h after transformation by centrifugation at 50g for 5 min and resuspended in 200 μL of 50 mM Hepes buffer, pH 7.5, containing 0.1 M NaCl, 2 mM EDTA, 2 mM 2-mercaptoethanol, and 1 mM phenylmethylsulfonyl fluoride. Protoplasts were lysed by sonication and clarified by a brief centrifugation at 5000g. The whole cell extracts were fractionated into soluble and membrane fractions by ultracentrifugation at 100,000g for 1 h. The pellet was resuspended in an original volume of Hepes buffer. These fractions were probed for the presence of GFP:FAPP1PH and GFP:FAPP1PH[K7E, R18A] by protein gel blot analysis using a monoclonal anti-GFP antibody (Clontech, Palo Alto, CA).
Phospholipid Binding Assay
To prepare recombinant GFP:FAPP1PH and GFP:FAPP1PH[K7E, R18A] proteins, DNA fragments encoding GFP:FAPP1PH and GFP:FAPP1PH[K7E, R18A] were subcloned into a pRSET-A expression vector (Invitrogen, Carlsbad, CA). The constructs were introduced into BL21 (DE3)LysS, and expression was induced by 0.1 mM isopropylthio-β-galactoside for 2 h. His-tagged GFP:FAPP1PH and GFP:FAPP1PH[K7E, R18A] proteins were purified using a nickel–nitrilotriacetic acid agarose affinity column according to the manufacturer's instructions (Invitrogen). To assess the phosphoinositide binding properties of GFP:FAPP1PH and GFP:FAPP1PH[K7E, R18A], a lipid binding assay was performed by fat-protein gel blot analysis (Dowler et al., 2000). Briefly, 0.5 and 1.5 μg of phospholipids dissolved in chloroform were spotted onto a nitrocellulose membrane (NitroBind; Micron Separations, Westborough, MA) and allowed to dry at room temperature. The membrane was blocked with 1.5% (w/v) fatty acid–free BSA in TBST (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.1% [v/v] Tween 20) for 1 h. The membrane was incubated for 12 h at 4°C with gentle stirring in the same solution containing 0.5 μg/mL affinity-purified GFP:FAPP1PH and GFP:FAPP1PH[K7E, R18A]. After washing three times with TBST, the blot was incubated with a monoclonal anti-His antibody (Qiagen, Valencia, CA) for 1 h and then washed again with TBST three times. An anti-mouse IgG antibody conjugated with horseradish peroxidase was used as the secondary antibody. Binding of proteins to phospholipids was detected with the enhanced chemiluminescence detection system (Amersham).
Imaging of Cytosolic Calcium Concentration in Intact Guard Cells of Arabidopsis
Experimental procedures for epidermal strip preparation and fluorescence ratio measurements were performed essentially as described by Allen et al. (1999)(2001) with a minor modification. In brief, rosette leaves of Arabidopsis (ecotype Landsberg erecta) expressing p35S–yellow cameleon 2.1–BAR were mounted onto a glass cover slip that had been coated with medical adhesive (Hollister Inc., Libertyville, IL). Then the adaxial surface of leaves was removed with a razor blade, and the remaining abaxial epidermis was incubated immediately in a small Petri dish containing a bathing medium (for WM experiments, 10 mM Mes-Tris, pH 6.15, 2 mM KCl, and 200 μM CaCl2; for LY294002 experiments, 10 mM Mes-Tris, pH 6.15, 5 mM KCl, and 50 μM CaCl2). To open the stomates, the Petri dish was illuminated for 2 h (120 μmol·m−2·s−1) before fluorescence ratio measurements. DMSO, WM, or LY294002 was added to the solution at 30 min before measurements were taken. [±]-cis,trans-ABA (5 or 50 μM) was added by perfusion at 5 to 10 min after fluorescence measurements began. Ca2+ transient increases were counted when cytosolic calcium concentration ratio change was ≥0.1 unit above the baseline and distinguishable from the background.
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes. No restrictions or conditions will be placed on the use of any materials described in this article that would limit their use for noncommercial research purposes.
Acknowledgments
We thank S.D. Emr for yeast strains and B. Drøbak and S. Lee for providing protocols for the PI kinase assay. We also thank Myungki Min for performing the fat-protein gel blot experiment. This research was supported by the Crop Functional Genomics Center of Korea (Grant CG1-1-15) and the Pohang University of Science and Technology Basic Science Research Institute research fund awarded to Y.L. and in part by National Science Foundation Grant MCB 0077791 (to J.I.S.). J.M.K. was supported by a fellowship from the Human Frontier Science Program Organization. This work also was supported in part by a grant from the National Creative Research Initiatives program of the Ministry of Science and Technology (Korea) to I.H.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.004143.
References
- Allen, G.J., Chu, S.P., Harrington, C.L., Schumacher, K., Hoffmann, T., Tang, Y.Y., Grill, E., and Schroeder, J.I. (2001). A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411, 1053–1057. [DOI] [PubMed] [Google Scholar]
- Allen, G.J., Kwak, J.M., Chu, S.P., Llopis, J., Tsien, R.Y., Harper, J.F., and Schroeder, J.I. (1999). Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J. 19, 735–747. [DOI] [PubMed] [Google Scholar]
- Assmann, S.M., and Shimazaki, K. (1999). The multisensory guard cell: Stomatal responses to blue light and abscisic acid. Plant Physiol. 119, 809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya, A., Foti, M., and Emr, S.D. (2000). Distinct roles for the yeast phosphatidylinositol 4-kinases, Stt4p and Pik1p, in secretion, cell growth, and organelle membrane dynamics. Mol. Biol. Cell 11, 2673–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae, Y.S., Sung, J.Y., Kim, O.S., Kim, Y.J., Hur, K.C., Kazlauskas, A., and Rhee, S.G. (2000). Platelet-derived growth factor-induced H2O2 production requires the activation of phosphatidylinositol 3-kinase. J. Biol. Chem. 275, 10527–10531. [DOI] [PubMed] [Google Scholar]
- Balla, T. (1998). Phosphatidylinositol 4-kinases. Biochim. Biophys. Acta 1436, 69–85. [DOI] [PubMed] [Google Scholar]
- Blatt, M.R. (2000). Ca2+ signalling and control of guard-cell volume in stomatal movements. Curr. Opin. Plant Biol. 3, 196–204. [PubMed] [Google Scholar]
- Bunney, T.D., Watkins, P.A., Beven, A.F., Shaw, P.J., Hernandez, L.E., Lomonossoff, G.P., Shanks, M., Peart, J., and Drøbak, B.K. (2000). Association of phosphatidylinositol 3-kinase with nuclear transcription sites in higher plants. Plant Cell 12, 1679–1688.11006340 [Google Scholar]
- Dowler, S., Currie, R.A., Campbell, D.G., Deak, M., Kular, G., Downes, C.P., and Alessi, D.R. (2000). Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem. J. 351, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drøbak, B.K., Dewey, R.E., and Boss, W.F. (1999). Phosphoinositide kinases and the synthesis of polyphosphoinositides in higher plant cells. Int. Rev. Cytol. 189, 95–130. [DOI] [PubMed] [Google Scholar]
- Fukuda, M., Hasezawa, S., Asai, N., Nakajima, N., and Kondo, N. (1998). Dynamic organization of microtubules in guard cells of Vicia faba L. with diurnal cycle. Plant Cell Physiol. 39, 80–86. [DOI] [PubMed] [Google Scholar]
- Gillooly, D.J., Morrow, I.C., Lindsay, M., Gould, R., Bryant, N.J., Gaullier, J.M., Parton, R.G., and Stenmark, H. (2000). Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 19, 4577–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy, S., Read, N.D., and Trewavas, A.J. (1990). Elevation of cytoplasmic calcium by caged calcium or caged inositol trisphosphate initiates stomatal closure. Nature 346, 769–771. [DOI] [PubMed] [Google Scholar]
- Hamilton, D.W., Hills, A., Kohler, B., and Blatt, M.R. (2000). Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc. Natl. Acad. Sci. USA 97, 4967–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchliffe, K.A., Ciruela, A., and Irvine, R.F. (1998). PIPkins1, their substrates and their products: New functions for old enzymes. Biochim. Biophys. Acta 1436, 87–104. [DOI] [PubMed] [Google Scholar]
- Hirose, K., Kadowaki, S., Tanabe, M., Takeshima, H., and Iino, M. (1999). Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science 284, 1527–1530. [DOI] [PubMed] [Google Scholar]
- Hong, Z., and Verma, D.P.S. (1994). A phosphatidylinositol 3-kinase is induced during soybean nodule organogenesis and is associated with membrane proliferation. Proc. Natl. Acad. Sci. USA 91, 9617–9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, J.U., Eun, S.O., and Lee, Y. (2000). Structure and function of actin filaments in mature guard cells. In Actin: A Dynamic Framework for Multiple Plant Cell Functions, C.J. Staiger, F. Baluska, D. Volkmann, and P.W. Barlow, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 427–436.
- Jin, J.B., Kim, Y.A., Kim, S.J., Lee, S.H., Kim, D.H., Cheong, G.W., and Hwang, I.H. (2001). A new dynamin-like protein, ADL6, is involved in trafficking from the trans-Golgi network to the central vacuole in Arabidopsis. Plant Cell 13, 1511–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D.H., Eu, Y.J., Yoo, C.M., Kim, Y.W., Pih, K.T., Jin, J.B., Kim, S.J., Stenmark, H., and Hwang, I.H. (2001). Trafficking of phosphatidylinositol 3-phosphate from the trans-Golgi network to the lumen of the central vacuole in plant cells. Plant Cell 13, 287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost, B., Lemichez, E., Spielhofer, P., Hong, Y., Tolias, K., Carpenter, C., and Chua, N.H. (1999). Rac homologues and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J. Cell Biol. 145, 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitscheck, U., Homann, U., and Thiel, G. (2000). Osmotically-evoked shrinking of guard cell protoplasts causes retrieval of plasma membrane into the cytoplasm. Planta 210, 423–431. [DOI] [PubMed] [Google Scholar]
- Lee, S., Choi, H., Suh, S., Doo, I.S., Oh, K.Y., Choi, E.J., Taylor, A.T.S., Low, P.S., and Lee, Y. (1999). Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol. 121, 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y.S., Choi, Y.B., Suh, S., Lee, J., Assmann, S.M., Joe, C.O., Kelleher, J.F., and Crain, R.C. (1996). Abscisic acid-induced phosphoinositide turnover in guard-cell protoplasts of Vicia faba. Plant Physiol. 110, 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G., D'Souza-Schorey, C., Barbieri, M.A., Roberts, R.L., Klippel, A., Williams, L.T., and Stahl, P.D. (1995). Evidence for phosphatidylinositol 3-kinase as a regulator of endocytosis via activation of Rab5. Proc. Natl. Acad. Sci. USA 92, 10207–10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc, J., Granger, C.L., Brincat, J., Fisher, D.D., Kao, T., McCubbin, A.G., and Cyr, R.J. (1999). A GFP-MAP4 reporter gene for visualizing cortical microtubule rearrangements in living epidermal cells. Plant Cell 10, 1927–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka, K., Bassham, D.C., Raikhel, N.V., and Nakamura, K. (1995). Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J. Cell Biol. 130, 1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks, D.E., Aghoram, K., Courtney, P.D., DeWald, D.B., and Dewey, R.E. (2001). Hyperosmotic stress induces the rapid phosphorylation of a soybean phosphatidylinositol transfer protein homolog through activation of the protein kinases SPK1 and SPK2. Plant Cell 13, 1205–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik, T., Irvine, R.F., and Musgrave, A. (1998). Phospholipid signalling in plants. Biochim. Biophys. Acta 1389, 222–272. [DOI] [PubMed] [Google Scholar]
- Parmar, P.N., and Brearley, C.A. (1993). Identification of 3-phosphorylated and 4-phosphorylated phosphoinositides and inositol phosphates in stomatal guard cells. Plant J. 4, 255–263. [Google Scholar]
- Parmar, P.N., and Brearley, C.A. (1995). Metabolism of 3-phosphorylated and 4-phosphorylated phosphatidylinositols in stomatal guard cells of Commelina communis L. Plant J. 8, 425–433. [Google Scholar]
- Payrastre, B., Nievers, M., Boonstra, J., Breton, M., Verkleij, A.J., and Van Bergen en Henegouwen, P.M. (1992). A differential location of phosphoinositide kinases, diacylglycerol kinase, and phospholipase C in the nuclear matrix. J. Biol. Chem. 267, 5078–5084. [PubMed] [Google Scholar]
- Pei, Z.M., Murata, Y., Benning, G., Thomine, S., Klusener, B., Allen, G.J., Grill, E., and Schroeder, J.I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734. [DOI] [PubMed] [Google Scholar]
- Rameh, L.E., and Cantley, L.C. (1999). The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem. 274, 8347–8350. [DOI] [PubMed] [Google Scholar]
- Roth, M.G. (1999). Lipid regulators of membrane traffic through the Golgi complex. Trends Cell Biol. 9, 174–179. [DOI] [PubMed] [Google Scholar]
- Schroeder, J.I., Kwak, J.M., and Allen, G.J. (2001). Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 410, 327–330. [DOI] [PubMed] [Google Scholar]
- Schwartz, A. (1985). Role of Ca2+ and EGTA on stomatal movements in epidermal peels of Commelina communis L. Plant Physiol. 79, 1003–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack, J.H., Herman, P.K., Schu, P.V., and Emr, S.D. (1993). A membrane-associated complex containing the Vps15 protein kinase and the Vps34 PI 3-kinase is essential for protein sorting to the yeast lysosome-like vacuole. EMBO J. 12, 2195–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer, T.P., Ahn, S., and Meyer, T. (1998). Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr. Biol. 8, 343–346. [DOI] [PubMed] [Google Scholar]
- Staxén, I., Pical, C., Montgomery, L.T., Gray, J.E., Hetherington, A.M., and McAinsh, M.R. (1999). Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc. Natl. Acad. Sci. USA 96, 1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson, J.M., Perera, I.Y., Heilmann, I., Persson, S., and Boss, W.F. (2000). Inositol signaling and plant growth. Trends Plant Sci. 5, 252–258. [DOI] [PubMed] [Google Scholar]
- Tsukazaki, T., Chiang, T.A., Davison, A.F., Attisano, L., and Wrana, J.L. (1998). SARA, a FYVE domain protein that recruits Smad2 to the TGFβ receptor. Cell 95, 779–791. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck, B., Leevers, S.J., Ahmadi, K., Timms, J., Katso, R., Driscoll, P.C., Woscholski, R., Parker, P.J., and Waterfield, M.D. (2001). Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 70, 535–602. [DOI] [PubMed] [Google Scholar]
- Walsh, J.P., Caldwell, K.K., and Majerus, P.W. (1991). Formation of phosphatidylinositol 3-phosphate by isomerization from phosphatidylinositol 4-phosphate. Proc. Natl. Acad. Sci. USA 88, 9184–9187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welters, P., Takegawa, K., Emr, S.D., and Chrispeels, M.J. (1994). AtVPS34, a phosphatidylinositol 3-kinase of Arabidopsis thal-iana, is an essential protein with homology to a calcium-dependent lipid binding domain. Proc. Natl. Acad. Sci. USA 91, 11398–11402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurmser, A.E., and Emr, S.D. (1998). Phosphoinositide signaling and turnover: PtdIns(3)P, a regulator of membrane traffic, is transported to the vacuole and degraded by a process that requires lumenal vacuolar hydrolase activities. EMBO J. 17, 4930–4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, H.W., Pical, C., Brearley, C., Elge, S., and Muller-Rober, B. (1999). A plant 126-kDa phosphatidylinositol 4-kinase with a novel repeat structure: Cloning and functional expression in baculovirus-infected insect cells. J. Biol. Chem. 274, 5738–5745. [DOI] [PubMed] [Google Scholar]