INTRODUCTION
Calcium Signaling and Calcium Sensors: A General Paradigm
Many extracellular signals, including light, biotic, and abiotic stress factors, elicit changes in cellular Ca2+ concentration in plants (Trewavas and Knight, 1994; Bush, 1995; Braam et al., 1997; McAinsh et al., 1997; Sanders et al., 1999; Rudd and Franklin-Tong, 2001). In addition, many intrinsic growth and developmental processes, such as elongation of root hairs and pollen tube formation, are controlled by Ca2+ transients (Felle and Hepler, 1997; Holdaway-Clarke et al., 1997; Wymer et al., 1997). Because different signals often elicit distinct and specific cellular responses, it is important to determine how cells can distinguish the Ca2+ signals produced by different stimuli. Recent studies in both animal and plant cells suggest that a Ca2+ signal is presented not only by the concentration of Ca2+ but also by its spatial and temporal information (Franklin-Tong et al., 1996; Holdaway-Clarke et al., 1997; Dolmetsch et al., 1998; Li et al., 1998; Trewavas, 1999). A combination of changes in all Ca2+ parameters produced by a particular signal is referred to as a “Ca2+ signature.” Although such Ca2+ signatures may partially explain the specificity of cellular responses triggered by a particular stimulus, the molecules that “sense” and “interpret” the Ca2+ signals provide additional specificity to the coupling of Ca2+ parameters to cellular responses.
If Ca2+ signaling pathways are composed of “molecular relays,” the first “runner” after Ca2+ would be a Ca2+ “sensor,” which monitors temporal and spatial changes in Ca2+ concentrations. Such sensors often are proteins that bind Ca2+ and change their conformation in a Ca2+-dependent manner. Several families of Ca2+ sensors have been identified in higher plants. Perhaps the best known is calmodulin (CaM) and CaM-related proteins, which typically contain four elongation factor (EF)–hand domains for Ca2+ binding (Zielinski, 1998; Snedden and Fromm, 2001). A new family of Ca2+ sensors from Arabidopsis consists of proteins similar to both the regulatory B-subunit of calcineurin and the neuronal Ca2+ sensor in animals (Liu and Zhu, 1998; Kudla et al., 1999). We refer to these plant Ca2+ sensors as calcineurin B–like (CBL) proteins (Kudla et al., 1999). The third major class is exemplified by the Ca2+-dependent protein kinases (CDPKs), which contain CaM-like Ca2+ binding domains and a kinase domain in a single protein (Roberts and Harmon, 1992; Harmon et al., 2000). The CDPK proteins are unique because they function both as Ca2+ sensors and as effectors of their Ca2+-sensing activity.
CaM and CBL are small proteins that contain multiple Ca2+ binding domains but lack other effector domains, such as the kinase domain in CDPKs. To transmit the Ca2+ signal, CaMs and CBLs interact with target proteins and regulate their activity. CaM target proteins have been identified in higher plants and include protein kinases, metabolic enzymes, cytoskeleton-associated proteins, and others (Reddy et al., 1996, 2002; Snedden et al., 1996; Zielinski, 1998; Snedden and Fromm, 2001). A family of SNF1-like protein kinases has been identified as targets for CBL proteins (Shi et al., 1999; Halfter et al., 2000). The target proteins of Ca2+ sensors then regulate activities that constitute cellular responses triggered by an external signal. Ca2+ sensors, therefore, are part of a complex signaling network of interconnected pathways. It will be interesting to determine how this network is established and how it functions to link discrete signals to specific responses. In this review, we focus on the two families of small Ca2+ sensors (CaM and CBL) and their targets to explore how specific signals may be transmitted through the combined action of these proteins. The CDPK-type of calcium sensors are reviewed separately in this issue.
GENES AND PROTEINS
CaMs and CBLs: Diversity and Structural Basis for Function
CaM is one of the most conserved eukaryotic proteins, although the number and organization of CaM genes can vary greatly among different organisms. As shown in Figure 1, the extended superfamily of CaMs consists of proteins with a diverse number of Ca2+ binding EF hands and additional domains (Snedden and Fromm, 1998, 2001; Zielinski, 1998). Table 1 lists the known and putative CaM genes in Arabidopsis. Common criteria for the definition of CaM versus CaM-like and CaM-related proteins, however, are currently not established in the field. Here, we define three groups: typical CaMs, CaM-like proteins, and CaM-related proteins. In Arabidopsis, typical CaM members include CaM1 to CaM7, which are highly similar to animal CaM and to each other (>95% identical in amino acid sequence). Other proteins listed in Table 1 (CaM8 to CaM14) share 50 to 75% amino acid identity to the typical CaM2, and some of these have been shown to have CaM activity. They are referred to as CaM-like proteins (CaM8, CaM9, CaM13, and CaM14) or, when they have additional non-CaM domains, CaM-related proteins (CaM10 to CaM12).
Figure 1.

Scheme of CaMs and CaM-Related Proteins in Plants.
Typical CaMs (such as Arabidopsis [At] CaM2 and petunia [Ph] CaM81) contain four EF hands (red ovals). CaM-related proteins show additional domains (indicated by boxes) and/or either removal or addition of EF hands. For instance, Arabidopsis CaM11 contains an insertion of 22 Gln residues (22xQ) that follow the first four amino acids of the protein (Table 1). Petunia CaM53 and rice (Os) CaM61 contain a C-terminal extension that comprises a polybasic domain (+) and a CaaL box that specifies prenylation by GGTase-I (Rodriguez-Concepcion et al., 1999; Dong et al., 2002). A conserved C-terminal extension of unknown function is present in both Arabidopsis CaM10 and CaM12, but the latter contains two additional EF hands (Table 1). Extra EF hands and unrelated sequences also are found in the protein encoded by Arabidopsis TCH3, a touch-induced gene (Sistrunk et al., 1994). Substitution of a EF hand for a nonconserved sequence is observed in wheat (Ta) CaM2-1 (Yang et al., 1996) and tobacco (Nt) rgsCaM, which was found to be a suppressor of post-transcriptional gene silencing (Anandalakshmi et al., 2000).
Table 1.
Conserved (Boldface) and Divergent CaM Genes in Arabidopsis
| Name | Accession Number |
CaM Function? |
Expressed Sequence Tags |
Amino Acids |
Extension | % Identity (% Similarity) to CaM2 |
Closest CaM Homolog (% Similarity) |
|---|---|---|---|---|---|---|---|
| CaM1 | At5g37780 | Yes | 7 | 149 | No | 96.6 (100) | CaM4 (100) |
| CaM2 | At2g41110 | Yes | 12 | 149 | No | 100 (100) | CaM3, CaM5 (100) |
| CaM3 | At3g56800 | Yes | 4 | 149 | No | 100 (100) | CaM2, CaM5 (100) |
| CaM4 | At1g66410 | Yes | 18 | 149 | No | 96.6 (100) | CaM1 (100) |
| CaM5 | At2g27030 | Yes | 6 | 149 | No | 100 (100) | CaM2, CaM3 (100) |
| CaM6 | (Q03509) | Yes | 4 | 149 | No | 98.6 (99.3) | CaM7 (99.3) |
| CaM7 | At3g43810 | Yes | 9 | 149 | No | 99.3 (100) | CaM2, CaM3, CaM5 (100) |
| CaM8 | At4g14640 | Yes | 3 | 151 | No | 72.5 (79.8) | CaM11 (89.3) |
| CaM9 | At3g51920 | (Yes)a | 5 | 151 | No | 49.6 (60.4) | CaM1, CaM4 (60.4) |
| CaM10 (CaBP-22) |
At2g41090 | (Yes)b | 4 | 191 | C terminus (no homology found with any other database sequences) |
66.4 (73.3) | CaM1, CaM4 (74.6) |
| CaM11 | At3g22930 | —c | 2 | 173 | N terminus (22 Gln residues) | 74.5 (83.2) | CaM8 (90.0) |
| CaM12 | At2g41100 | — | 6 | 324 | N terminus (2 EF hands); C terminus (similar to that of CaM10) |
63.7 (69.8) | CaM1, CaM4 (70.5) |
| CaM13 | At1g12310 | — | 11 | 148 | No | 50.3 (62.6) | CaM14 (95.9) |
| CaM14 | At1g62820 | — | 1 | 148 | No | 49.6 (61.2) | CaM13 (95.9) |
Does not display Ca2+-induced electrophoretic mobility shifting but partially complements a CaM-defective yeast mutant (Zielinski, 2002).
Displays Ca2+-induced electrophoretic mobility shifting (Ling and Zielinski, 1993).
—, unknown.
For example, Arabidopsis CaM8 is a CaM-like protein because of its more divergent sequence. This protein can function as a CaM in Ca2+ binding and yeast complementation experiments, but it appears to interact with a more limited set of target proteins compared with typical CaM isoforms (Zielinski, 2002). A good example of a CaM-related protein is petunia CaM53, which has been demonstrated to have CaM activity but which contains a polybasic C-terminal domain that is not found in typical CaMs (Figure 1). As discussed below, this extra domain in CaM53 regulates its cellular localization (Rodriguez-Concepcion et al., 1999). It is also interesting that the genes encoding CaM10, CaM12, and CaM2 are organized in a tandem array in this order in chromosome 2. This could result from gene duplication and the incorporation of additional domains in a sequence of events from CaM2 to CaM10 to CaM12 (Figure 1, Table 1).
CBLs also are encoded by a multigene family of at least 10 members in Arabidopsis (Table 2) that have similar structural domains with small variations in the length of the coding regions (Kudla et al., 1999; Kim et al., 2000; Albrecht et al., 2001; Guo et al., 2001b). Their amino acid sequence identity, which ranges from 20 to 90%, would be sufficient for functional redundancy among the closely related members while allowing for functional specificity among more diverged members. Unlike CaM genes, CBLs have been identified previously only in higher plants, suggesting that CBLs may function in plant-specific signaling processes.
Table 2.
CBL Genes and Proteins in Arabidopsis
| Name | Protein Accession Number | Nucleotide Accession Number (Verified cDNAs) |
MIPS/TAIR Accession Number |
Synonyms | Amino Acids | Introns |
|---|---|---|---|---|---|---|
| AtCBL1 | AAC26008 | AF076251 | At4g17615 | 214 | Yes | |
| AtCBL2 | AAC26009 | AF076252 | At5g55990 | 227 | Yes | |
| AtCBL3 | AAC26010 | AF076253 | At4g26570 | 226 | Yes | |
| AtCBL4 | AAG28402 | AF192886 | At5g24270 | SOS3 | 223 | Yes |
| AtCBL5 | AAG28401 | AF192885 | At4g01420 | 214 | Yes | |
| AtCBL6 | AAG28400 | AF192884 | At4g16350 | 227 | Yes | |
| AtCBL7 | AAG10059 | AF290434 | At4g26560 | 214 | Yes | |
| AtCBL8 | AAL10300 | AF411957 | At1g64480 | 214 | Yes | |
| AtCBL9 | AAL10301 | AF411958 | At5g47100 | 213 | Yes | |
| AtCBL10 | In progress | AF490607 | At4g33000 | 256 | Yes |
The CaM- and CBL-type Ca2+ binding proteins are characterized by a common helix-loop-helix structural motif (the EF hands) that acts as the Ca2+ binding site (Figure 2). The EF-hand consensus sequence consists of a 12–amino acid loop that uses amino acids at positions 1, 3, 5, 7, 9, and 12 for interaction with Ca2+. The Asp at position 1, Gly at position 6, and Glu at position 12 are the most highly conserved amino acids in the loop. The Gly at position 6 is required to maintain the structure of the loop, which cannot accommodate any other amino acid at this site. Comparing CaM with CBL proteins, the two families do not show significant similarity in their primary amino acid sequences except for the conserved positions in the EF-hand motifs. In addition to a general sequence difference, CaMs and CBLs also differ in the number of EF-hand motifs in their basic structures. Typically, CaMs contain four EF hands and CBLs contain three. Because the structure of no CBL has yet been characterized, we focus on the structural analysis of CaM to illustrate the molecular basis for calcium binding and target interaction.
Figure 2.
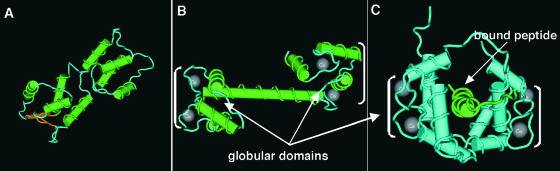
Structural Analysis of Apo-CaM, Ca2+-CaM, and the Ca2+-CaM–Target Complex.
Strand-rod presentation of Apo-CaM (A) and Ca2+-CaM (B) showing substantial changes upon Ca2+ binding. (C) shows a solution structure solved by NMR of peptide-bound Ca2+-CaM. Peptide binding causes disruption of the flexible tether, bringing the globular domains closer to form a channel around the peptide. The majority of contacts between Ca2+-CaM and target peptide are nonspecific van der Waals bonds made by residues in the hydrophobic surfaces. Brackets indicate globular domains.
The EF hands in CaM are organized into two distinct globular domains, each of which contains one pair of EF hands. Each pair of EF hands is considered the basic functional unit. Pairing of EF hands is thought to stabilize the protein and increase its affinity toward Ca2+ (Seamon and Kreetsinger, 1983). Although each globular domain binds Ca2+ and undergoes conformational changes independently, the two domains act in concert to bind target proteins (Nelson and Chazin, 1998). Upon increase of Ca2+ to submicromolar or low micromolar levels, all CaM molecules are activated. Cooperative binding is required for this “on/off” mechanism to function efficiently. The cooperativity of Ca2+ binding ensures that full activation of the CaM occurs in a narrow region of calcium concentration during a signaling event.
The selectivity of CaM toward Ca2+ also is an important factor in effective transduction of the Ca2+ signal. CaMs bind Ca2+ selectively in the presence of high concentrations of Mg2+ and monovalent cations in the cell. The cation selectivity is achieved by optimizations in the structure folds of the binding loop (Figure 2). For example, discrimination between Ca2+ and Mg2+ is accomplished through reduction in the size of the binding loop. Binding of Mg2+ ions would collapse the EF-hand loop, thereby reducing the distance between negatively charged side chains and destabilizing the CaM-Mg2+ complex (Falke et al., 1994). Even small changes in the chemical properties of the Ca2+ binding loop (e.g., Glu-12→Gln) can drastically reduce the binding affinity to Ca2+ (Beckingham, 1991; Haiech et al., 1991). The Glu-12→Gln mutation changes the carboxylate side chain into carboxylamide, which removes the oxygen ligand for Ca2+ (Nelson and Chazin, 1998). Together, structural analyses in combination with site-directed mutagenesis established that CaMs (and other EF hand–containing proteins, including CBLs) have evolved as highly specific Ca2+ sensors.
Structural analysis of the Ca2+-free and Ca2+-bound states of CaM proteins reveals the conformational changes induced by Ca2+ binding (Figure 2). In the Ca2+-free state, CaM adopts a closed conformation. Ca2+ binding triggers a conformational change, and the protein adopts an open conformation with nearly perpendicular interhelical angles between the globular domains. This open conformation exposes a hydrophobic surface within each globular domain and permits the binding of protein targets (Babu et al., 1988; Kuboniwa et al., 1995; Zhang et al., 1995).
Ca2+-CaM binds and regulates the activity of a wide range of proteins that are not necessarily related in structure. How can Ca2+-CaMs bind to so many different proteins? More specifically, the plasticity of the Ca2+-CaM structure must accommodate the variation in both the molecular size and the composition of the target proteins. This issue has been addressed by structural analyses of Ca2+-CaM and target-bound Ca2+-CaM. Figure 2C shows that the two globular domains of Ca2+-CaM are connected by a flexible tether that can accommodate peptides of varying sizes (Nelson and Chazin, 1998). Upon binding a peptide, the two globular domains fold toward each other to form a hydrophobic channel rich in Met residues that have flexible hydrophobic side chains. In this channel, Ca2+-CaM interacts with peptides mostly through nonspecific van der Waals interactions that form between the exposed hydrophobic domains of Ca2+-CaM and the target peptides, which explains why Ca2+-CaM can bind many target proteins (O'Neil and DeGrado, 1990; Osawa et al., 1998; Zhang and Yuan, 1998). Together, the structures of CaM illustrate how this class of proteins can function as extremely efficient Ca2+ sensors and on/off switches, allowing them to transduce Ca2+ signals with high efficiency and accuracy. Different affinities for Ca2+-CaM interactions with specific target proteins may be sufficient for the differential transduction of the Ca2+ signal.
Expression Patterns of CaMs and CBLs: Clues for Function
The temporal and spatial expression of a gene and the subcellular localization of the protein often provide important clues to their functions. As expected for CaMs and CBLs that participate in numerous signaling pathways, their expression and subcellular localization are regulated by multiple signals, including light, mechanical stress, heat/cold shock, wounding, osmotic stress, pathogens, and plant hormones. Certain CaM/CBL genes also are regulated developmentally and show tissue- and cell-specific expression patterns. As discussed below, the diversity of gene expression and protein localization patterns is important for generating functional diversity and specificity.
Touch-induced genes (TCH) encode CaM-related proteins (Figure 1), which are induced rapidly by mechanical manipulation, cold and heat shock, phytohormones, and Ca2+ itself (Braam et al., 1997). The magnitude and kinetics of mRNA induction differ between the different TCH genes (Braam and Davis, 1990; Braam, 1992a, 1992b; Antosiewicz et al., 1995; Polisensky and Braam, 1996; Braam et al., 1997). Extensive work with TCH3 established that it is expressed in the shoot apical meristem, vascular tissue, and root pericycle cells during vegetative growth in Arabidopsis (Sistrunk et al., 1994; Antosiewicz et al., 1995). After wind stimuli, TCH3 becomes abundant in branch points of leaf primordia and stipule, pith parenchyma, and vascular tissues, although the functional consequences of this induction are not understood.
CaM proteins have an important function in pathogenesis and wounding (Bergey and Ryan, 1999; Heo et al., 1999; Yamakawa et al., 2001) and in the hypersensitive response (Levine et al., 1996; Harding et al., 1997; Harding and Roberts, 1998; Heo et al., 1999; Blume et al., 2000). Constitutive ectopic expression of CaM genes alone sometimes can trigger a pathogen response in the absence of any elicitor, resulting in acquired resistance to a wide range of pathogens and suggesting that CaM proteins are perhaps rate-limiting factors in the pathogen response (Heo et al., 1999). Other genes for CaM proteins are induced by salt (Jang et al., 1998) or expressed in a developmentally regulated and tissue-specific manner (Yang et al., 1996, 1998). For example, a novel prenylated CaM protein from petunia (Rodriguez-Concepcion et al., 1999; Caldelari et al., 2001) accumulates to high levels in meristematic tissues (Figure 3A). As more genome-wide gene expression profiling experiments are completed, we expect a broader view of CaM gene expression during plant development and under various growth conditions.
Figure 3.

Subcellular and Tissue-Specific Localization of CaM53.
(A) A CaM53-specific antibody (Rodriguez-Concepcion et al., 1999) was used for immunolocalization of the protein on a paraffin-embedded petunia longitudinal section showing a floral meristem. Secondary antibodies coupled to alkaline phosphatase were used to visualize the localization of cross-reacting polypeptides with bright-field illumination.
(B) to (D) Subcellular localization was studied in petunia protoplasts transformed with the plasmid to express the full-length CaM53 protein fused to green fluorescent protein as described previously (Rodriguez-Concepcion et al., 1999). After electroporation, protoplasts were incubated for 16 hr in the presence of 5 μM mevinolin (B), 1 mM CaCl2 (C), or 1 mM EGTA (D). Localization of the green fluorescent protein–CaM53 fusion protein was detected by green fluorescence. Red fluorescence corresponds to chlorophyll. Arrow in (B) indicates the nucleus.
(E) Ectopic overexpression of the wild-type CaM53 (CaM53wt) or a nonprenylated mutant version (CaM53mS) in Nicotiana benthamiana plants using the potato virus x viral vector induced distinct developmental abnormalities. An empty potato virus x vector–infected plant is shown as a control (Rodriguez-Concepcion et al., 1999). The photograph was taken 35 days after inoculation.
Different members of the Arabidopsis CBL gene family also show specific expression patterns. For example, CBL1 expression is induced strongly by wounding, drought, high salt, cold, and abscisic acid (Kudla et al., 1999; Piao et al., 2001; S. Luan, unpublished results). Both CBL1 and CBL2 respond to light, but CBL2 lacks the other responses of CBL1 (Nozawa et al., 2001). Such expression patterns suggest that CBL1 and CBL2 have both overlapping and specific functions in certain signal transduction pathways.
Subcellular Localization Patterns of CaMs and CBLs: A New Paradigm for Ca2+ Signaling?
It is becoming increasingly clear that plants can establish specific cellular Ca2+ signatures by restricting Ca2+ to a specific compartment of the cell (for review, see Rudd and Franklin-Tong, 2001). Certain CaMs and CBLs also are found in different subcellular locations that can change upon the perception of extracellular signals. A good example of this type of regulation is found in petunia CaM53 (Rodriguez-Concepcion et al., 1999). Like rice OsCaM61 (Figure 1), CaM53 contains a polybasic 34-residue C-terminal extension ending with a CaaX-box motif for prenylation. CaM53 is efficiently prenylated (Caldelari et al., 2001) and processed (Rodriguez-Concepcion et al., 2000) to be targeted to the plasma membrane. When prenylation is prevented by either mutation of the Cys residue of the CaaX box (Rodriguez-Concepcion et al., 1999) or inhibition of the mevalonate pathway (which provides the prenyl groups) with mevinolin (Figure 3B), the polybasic domain targets the protein to the nucleus. A similar prenylation-dependent membrane versus nuclear localization has been reported for OsCaM61 (Dong et al., 2002).
Several factors can regulate CaM53 prenylation and subcellular localization, including the availability of the prenyl groups (Figure 3B), which can depend in turn on the metabolic status of the cell (Yalovsky et al., 1999). Prenylation and plasma membrane targeting of CaM53, however, do not depend on calcium binding (Figures 3C and 3D). The prenylation status of CaM53 is likely an important aspect of its function, because the set of proteins with which CaM53 could interact upon calcium binding is expected to be very different depending on the subcellular localization of the protein. Consistently, the phenotype of Nicotiana benthamiana plants ectopically expressing plasma membrane–targeted CaM53 is very different from that of plants expressing the nucleus-localized CaaX-box mutant protein (Figure 3E). CaM53 may be a good example of a plant-specific CaM that incorporates a novel mechanism (prenylation-dependent differential localization) to coordinate the metabolic activity of plant cells with calcium-activated responses in the plasma membrane and the nucleus.
Certain structural features of CBLs also suggest that these Ca2+ sensors can change their cellular localization. Several CBLs have a conserved myristoylation site in their N-terminal regions (Liu and Zhu, 1998; Kudla et al., 1999; Kim et al., 2000, Albrecht et al., 2001). It would be expected that these CBLs are localized to cell membranes, which could serve as a regulatory mechanism for establishing a local signal cascade similar to the model discussed for CaM53 above. For example, a significant amount of SOS3 (CBL4 in Table 2) is always found associated with the membrane fraction, and the myristoylation site is required for the function of the protein (Ishitani et al., 2000). CBL1 also is associated with the membrane and could be subject to regulation by a Ca2+-myristoyl switch (J. Kudla and S. Luan, unpublished results), similar to mammalian recoverin (Resh, 1999).
In the Ca2+-free state, the myristoyl moiety in recoverin is inaccessible to membranes. The Ca2+-induced conformational change exposes the myristoyl group and facilitates the association of recoverin with the membrane (Resh, 1999). In the case of CBLs, the association of a particular CBL protein would target the CBL–CBL-interacting protein kinase (CIPK) complex to the membrane, thereby enabling CIPK phosphorylation of membrane-associated protein substrate(s). The view that is emerging is that in plants, certain CaM/CBL proteins have acquired protein domains that restrict their localization, perhaps as a mechanism to establish local signal transduction pathways that initiate specific cellular responses.
TARGETS AND FUNCTIONS
CaM Targets a Diverse Group of Proteins
Small Ca2+ sensors such as CaMs and CBLs contain the Ca2+ binding domains but lack other effector modules (e.g., the kinase domain in the CDPK proteins). They transmit Ca2+ signals by interacting with a number of target proteins. The interaction between CaM and CaM-dependent protein kinases in animal cells provides a good model that illustrates how Ca2+-CaM regulates the activity of the target. For example, CaMKII contains an autoinhibitory domain that occludes the active site in the resting state. Ca2+-CaM binds to a site near or overlapping the autoinhibitory domain, thereby releasing it from the active site and activating the enzyme (for review, see Hook and Means, 2001). This model appears to be applicable to interactions between CaMs/CBLs and their target proteins in plant cells. CaM targets in plants have been reviewed extensively (Snedden and Fromm, 1998, 2001; Zielinski, 1998; Reddy et al., 2002); therefore, we introduce the conceptual framework using only a few examples to explain how CaMs regulate protein target activity in plants.
CaM target proteins can be identified using labeled CaMs to screen expression cDNA libraries (Fromm and Chua, 1992). A large number of CaM binding proteins have been identified from plants. Glu decarboxylase (GAD) is one of the best studied (Baum et al., 1993, 1996; Snedden et al., 1996; Zik et al., 1998). The enzyme catalyzes the conversion of l-Glu to γ-aminobutyric acid (GABA) and is activated rapidly during several stress responses (Snedden and Fromm, 1998, 2001). GAD is activated by binding either to CaM or to a monoclonal antibody that recognizes the CaM binding domain of GAD. In analogy to the Ca2+-CaM–CaMK interaction, the binding of Ca2+-CaM to GAD probably relieves the autoinhibitory effect of the CaM binding domain, because mutant GAD lacking the CaM binding domain (GADΔC) is constitutively active. Overexpression of GADΔC in transgenic tobacco induced developmental abnormalities associated with increased GABA levels concomitant with reduced levels of Glu (Baum et al., 1996). The activation of GAD by environmental stimuli via the Ca2+-CaM signaling system is very rapid, exemplifying the highly cooperative on/off switch of the CaM response (Snedden and Fromm, 1998).
Ca2+-ATPases are localized in the endomembranes or plasma membrane and play a key role in removing Ca2+ from the cytoplasm to terminate a signaling event, which is critical for Ca2+ homeostasis in all eukaryotic cells (for review, see Sze et al., 2000). Among the Ca2+-ATPases in higher plants, type IIB Ca2+-ATPases are major targets of Ca2+-CaM regulation. Unlike homologs in animal cells, plant type IIB ATPases are located in both endomembranes (endoplasmic reticulum and tonoplast) and the plasma membrane (Sze et al., 2000). Ca2+-CaM interacts with type IIB ATPases to activate the pump by releasing an autoinhibitory domain from the active site, similar to the Ca2+-CaM–CaMKII interaction in animals. It is noteworthy that plant Ca2+-ATPases are subject to regulation by CDPKs, another type of Ca2+ sensor. Interestingly, although Ca2+-CaM activates the pump, CDPK phosphorylation inhibits the pump, demonstrating the complexity of the regulation of Ca2+ signal termination by feedback from two different types of Ca2+ sensors (Hwang et al., 2000). Several plant nucleotide-gated ion channels also may be regulated by Ca2+-CaM (Schuurink et al., 1998; Arazi et al., 1999, 2000; Kohler et al., 1999; Leng et al., 1999). These channel proteins contain six transmembrane domains and a high-affinity CaM binding site overlapping a cyclic nucleotide binding domain (Arazi et al., 2000).
Although typical Ca2+-CaM–dependent protein kinases similar to CaMKII in animals have not been identified in plants, certain plant protein kinases are regulated by CaMs. These are exemplified by CCaMK, a chimeric plant Ca2+-CaM–dependent protein kinase with a visinin-like Ca2+ binding domain and a CaM binding domain in one molecule (Patil et al., 1995). Although Ca2+ can regulate the kinase via a visinin-like domain, Ca2+-CaM enhances the kinase activity toward a substrate and inhibits its autophosphorylation activity, suggesting that Ca2+-CaM may regulate substrate specificity in vivo (Takezawa et al., 1996). Several genes that encode CaMKII-like protein kinases have been identified by homology-based cloning from apple (Watillon et al., 1995) and through CaM interaction cloning from maize (Lu and Feldman, 1997). The structural domains of these kinases resemble those in animal CaMKII, with high-affinity CaM binding domains and a kinase domain. However, the activity of these plant CaMKII-like kinases and their regulation by Ca2+-CaM have not been characterized.
Calcium Targets in the Nucleus
Ca2+ signaling and the role of CaM in the nucleus are drawing increased interest (Rudd and Franklin-Tong, 2001; Snedden and Fromm, 2001). CaMs participate in transcriptional regulation either directly, by binding to transcription factors (Szymanski et al., 1996), or indirectly, by activating kinases or phosphatases that control transcription factor activity (Marechal et al., 1999). Studies in animal cells have demonstrated that CaM localization to the nucleus could be facilitated by differential Ca2+ oscillations (Craske et al., 1999; Teruel et al., 2000; Teruel and Meyer, 2000), suggesting additional and complex levels of transcriptional regulation. As discussed above, changing the metabolic status of plant cells induced the translocation of CaM53 to the nucleus, where it appears to activate specific signaling (Rodriguez-Concepcion et al., 1999).
Selective Ca2+ signals were measured in the cytoplasm and nucleus of transgenic plants expressing either cytoplasmic or nuclear forms of the Ca2+ reporter protein aequorin (van Der Luit et al., 1999; Pauly et al., 2000). Such Ca2+ signals may be required for the expression of specific genes. For example, expression of tobacco NpCaM1 (but not NpCaM2, which encodes an identical CaM protein) in response to wind was stimulated by a nuclear Ca2+ transient, whereas cold-responsive expression was induced primarily by a cytoplasmic Ca2+ transient (van Der Luit et al., 1999). Thus, spatially separated Ca2+ signals also can control the function of closely related CaM proteins through the regulation of their genes.
Several more recent studies implicate nuclear Ca2+ signals in important cellular processes. A Ca2+ binding protein required for light response has been localized in the nucleus (Guo et al., 2001a). This protein (called SUB1) also contains a putative domain for DNA binding, suggesting that it may combine the function of Ca2+ sensing and transcriptional regulation in one protein. Another study identified a CaM-related protein as a critical component in the suppression of post-transcriptional gene silencing in plants (Anandalakshmi et al., 2000). It remains to be established how these Ca2+ binding proteins mediate Ca2+ signals and regulate corresponding nuclear processes.
The CBL-CIPK Paradigm
Unlike CaMs, which interact with a large variety of target proteins, CBLs appear to interact with a single family of protein kinases (Shi et al., 1999). These kinases, referred to as CIPKs, are most similar to Suc nonfermenting (SNF) protein kinase from yeast. CBL1 interacts with CIPKs through the C-terminal nonkinase domain that contains a conserved region among different CIPK members (Shi et al., 1999). Interestingly, interaction between CBL1 and CIPK1 requires micromolar levels of Ca2+. This Ca2+-dependent interaction is consistent with the general paradigm established for Ca2+ sensor interactions with target proteins in animals (e.g., the Ca2+-CaM–CaMKII interaction).
Another study (Halfter et al., 2000) using SOS3 (equivalent to CBL4 in Table 2) as “bait” also identified several interacting protein kinases that belong to the CIPK family. In particular, SOS3 interaction with SOS2 (equivalent to CIPK24 in Table 3) stimulates kinase activity against a peptide substrate, suggesting that SOS3 serves as a regulatory subunit of SOS2. SOS2 and SOS3 were identified initially by a genetic screen for Arabidopsis mutants that are salt overly sensitive (SOS; not to be confused with earlier terms such as SOS [Son-Of-Sevenless in mitogen-activated protein kinase pathways in animals] or the SOS response in Escherichia coli) (Liu and Zhu, 1998; Liu et al., 2000; for review, see Hasegawa et al., 2000). Mutations in SOS genes render Arabidopsis plants hypersensitive to NaCl in the growth medium, implicating SOS genes in salt tolerance functions.
Table 3.
CIPK Genes and Proteins in Arabidopsis
| Name | Protein Accession Number |
Nucleotide Accession Number (Verified cDNAs) | MIPS/TAIR Accession Number | Synonyms | Amino Acids | Introns |
|---|---|---|---|---|---|---|
| AtCIPK1 | AAG28776 | AF302112 | At3g17510 | 444 | Yes | |
| AtCIPK2 | AAF86506 | AF286050 | At5g07070 | 456 | No | |
| AtCIPK3 | AAF86507 | AF286051 | At2g26980 | 375 | Yes | |
| AtCIPK4 | AAG01367 | AY007221 | At4g14580 | 426 | No | |
| AtCIPK5 | AAF86504 | AF285105 | At5g10930 | 431 | No | |
| AtCIPK6 | AAF86505 | AF285106 | At4g30960 | 441 | No | |
| AtCIPK7 | AAK16682 | AF290192 | At3g23000 | AtSR1 | 429 | No |
| AtCIPK8 | AAK16683 | AF290193 | At4g24400 | 445 | Yes | |
| AtCIPK9 | AAK16684 | AF295664 | At1g01140 | 449 | Yes | |
| AtCIPK10 | AAK16685 | AF295665 | At5g58380 | 479 | No | |
| AtCIPK11 | AAK16686 | AF295666 | At2g30360 | 435 | No | |
| AtCIPK12 | AAK16687 | AF295667 | At4g18700 | 489 | No | |
| AtCIPK13 | AAK16688 | AF295668 | At2g34180 | 502 | No | |
| AtCIPK14 | AAK16689 | AF295669 | At5g01820 | AtSR2 | 442 | No |
| AtCIPK15 | AAK16692 | AF302111 | At5g01810 | AtPK10 | 421 | No |
| AtCIPK16 | AAK50348 | AY030304 | At2g25090 | 469 | Yes | |
| AtCIPK17 | AAK64513 | AY036958 | At1g48260 | 421 | Yes | |
| AtCIPK18 | AAK59695 | AY034099 | At1g29230 | 520 | No | |
| AtCIPK19 | AAK50347 | AY030303 | At5g45810 | 483 | No | |
| AtCIPK20 | AAK61493 | AY035225 | At5g45820 | 439 | No | |
| AtCIPK21 | AAK59696 | AY034100 | At5g57630 | 417 | Yes | |
| AtCIPK22 | AAL47845 | AF450478 | At2g38490 | 445 | Yes | |
| AtCIPK23 | AAK61494 | AY035226 | At1g30270 | 482 | Yes | |
| AtCIPK24 | AAK72257 | AF395081 | At5g35410 | SOS2 | 446 | Yes |
| AtCIPK25 | AAL41008 | AF448226 | At5g25110 | 487 | No |
The Arabidopsis genome contains a large number of genes for putative CIPK proteins. Table 3 lists 25 CIPK genes that have been confirmed by cDNA cloning and sequencing. Further experiments have extended the analysis of CBL–CIPK interactions to the entire family of CBLs and a large fraction of the CIPK family in an effort to determine the functional pairs of CBLs and CIPKs. These studies revealed interaction specificity among various members of the CBL and CIPK families. Considering the number of genes in the two families (∼10 CBLs versus 25 CIPKs), one would expect that some CBLs interact with more than one CIPK. Indeed, certain CBLs interact with multiple CIPKs; likewise, some CIPKs interact with multiple CBLs (Kim et al., 2000; Albrecht et al., 2001; Guo et al., 2001b). It must be noted, however, that these interaction studies were performed primarily using the yeast two-hybrid system; therefore, they may not necessarily represent the physiological situations in plants. In addition to matching the CBLs with their target kinases, the interaction studies further defined the functional domains of CBLs and CIPKs. For example, the CBL-interacting domain in the C-terminal region of CIPKs was localized to a small region of ∼20 amino acids (Kim et al., 2000; Albrecht et al., 2001; Guo et al., 2001b). This domain may function in kinase regulation by releasing the autoinhibitory domain (Guo et al., 2001b).
The diversity in the protein sequence/structure and expression patterns of CBLs and CIPKs suggests that these proteins perform many different functions. To date, a physiological function has been established for only a few CBL and CIPK proteins. As discussed above, SOS2 and SOS3 have been identified by a genetic screen and appear to play a role in salt tolerance in Arabidopsis (Liu and Zhu, 1998; Hasegawa et al., 2000; Liu et al., 2000). Because high salt induces an increase in Ca2+ levels in the cytoplasm (Pauly et al., 2000), salt tolerance could involve Ca2+ signaling and the signal could be transmitted via SOS3/SOS2 to components required for salt detoxification (Hasegawa et al., 2000). In this context, it is interesting that one of the three identified SOS genes, SOS1, encodes a putative Na+/H+ antiporter (Shi et al., 2000), reinforcing the view that Ca2+ signaling has a role in salt detoxification.
In addition to the SOS3/SOS2 pair, biochemical studies have shown that CBL1 expression is highly responsive to a variety of abiotic stress conditions, including wounding, cold, drought, and high salt, implicating CBL1 in signaling these stress signals (Kudla et al., 1999). This hypothesis is supported by further genetic analysis of the CBL1 gene. Disruption of CBL1 gene function renders mutant plants hypersensitive to drought, high salt, and hyperosmotic stress (V. Albrecht and J. Kudla, unpublished results; Y.H. Cheong, K.N. Kim, and S. Luan, unpublished results). These phenotypes suggest that the CBL1 gene functions not only in salt tolerance but in other abiotic stress responses as well, which is distinct from SOS3/CBL4, which functions specifically in ionic homeostasis under high-salt conditions.
A CIPK member, AtSR2 (CIPK14 in Table 3), is regulated by Suc (Chikano et al., 2001). Mutant plants disrupted in AtSR2 gene function are more sensitive to Glc but not to the general osmotic stress conferred by mannitol, indicating that AtSR2 is involved specifically in a sugar-sensing mechanism (N. Koizumi and H. Sano, personal communication). With at least 10 CBLs and 25 CIPKs encoded in the Arabidopsis genome, many functional CBL-CIPK pairs can be formed that potentially function in a large array of signaling processes that involve calcium signal. Therefore, establishing the function of CBLs and CIPKs presents a unique opportunity to elucidate the decoding process of Ca2+ signals in plant cells.
PERSPECTIVES
Ca2+ and the “Combination Code” for Specific Signal-Response Coupling
Most, if not all, signaling pathways in plants involve Ca2+ signals (Rudd and Franklin-Tong, 2001; for review, see Trewavas, 1999). This leads to the question, how can a cell distinguish different extracellular signals by specific responses if they all use Ca2+ as a messenger? If one examines the nature of Ca2+ signals produced by various stimuli in both animals and plants, they appear to vary in their spatial and temporal information, which is referred to as the Ca2+ signature (for review, see Rudd and Franklin-Tong, 2001). Subtle differences in these signatures may be responsible in part for the “specificity” in cellular responses. There is little doubt that Ca2+ sensors and their targets are involved in further defining such signaling specificity.
First, as shown here, in plants there exist a number of distinct CaMs and CBLs, their target proteins, and additional downstream components in the signaling pathways. What pathway to take to decode a particular Ca2+ signature may depend largely on the presence of these signaling components in a specific cell. This is consistent with the distinct temporal and spatial expression patterns of CaMs and CBLs and their target proteins, which determine the abundance of the signaling proteins in a particular cell under particular conditions. In addition, targeting of certain proteins, such as CaM53 and CBL1, to specific subcellular locations will further define their functions in the decoding of Ca2+ signatures.
Second, the specificity of CaMs (or CBLs) in interactions with their targets certainly plays an important role in the diversity of cellular responses. For example, different CBLs interact with different subgroups of CIPKs or different CBLs may interact with the same subgroup of CIPKs with different affinities. The combinatorial potential of these proteins would contribute to the mechanism of a discrete response.
Third, the substrate specificity and differential cofactor dependence of CIPKs present an additional level of regulation in the CBL–CIPK system. For example, CIPK1 and CIPK7 depend on Mn2+ as a cofactor, but SOS2 (CIPK24) activity depends solely on Mg2+. The availability of a particular substrate for CIPKs in a cell also contributes to specificity.
In conclusion, specificity in the Ca2+ signaling system results from a multifactorial decision process, ranging from a specific Ca2+ signature to the availability of a specific set of calcium sensors and their target proteins that are coupled to downstream components. Each step in this process constrains the Ca2+ signal, ultimately leading to specificity in cellular responses, yet provides opportunities at every step for potential cross-talk to parallel or competing pathways. To fully understand the Ca2+ signaling pathways, we must not only decode the Ca2+ signatures but also dissect the “combination code” that consists of calcium sensors and downstream target proteins.
Accession Numbers
The Protein Data Bank accession numbers for the proteins shown in Figure 2 are 1CFC (Apo-CaM), 1CLL (Ca2+-CaM), and 1CFF (Ca2+-CaM).
Acknowledgments
We thank colleagues in our laboratories for helpful discussions and Drs. H. Sano and N. Koizumi for providing unpublished results. Our apologies to colleagues in the field whose work was not discussed because of space limitations. Our research discussed in this review is supported by the National Institutes of Health and the National Science Foundation (S.L.), the Deutsche Forschungsgemeinschaft (J.K.), the Spanish Ministry of Science and Technology (M.R.-C.), the Israel Science Foundation and Binational Science Foundation (S.Y.), and the Swiss Federal Institute of Technology (W.G.).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.001115.
References
- Albrecht, V., Ritz, O., Linder, S., Harter, K., and Kudla, J. (2001). The NAF domain defines a novel protein-protein interaction module conserved in Ca2+-regulated kinases. EMBO J. 20 1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandalakshmi, R., Marathe, R., Ge, X., Herr, J.M., Jr., Mau, C., Mallory, A., Pruss, G., Bowman, L., and Vance, V.B. (2000). A calmodulin-related protein that suppresses posttranscriptional gene silencing in plants. Science 290 142–144. [DOI] [PubMed] [Google Scholar]
- Antosiewicz, D.M., Polisensky, D.H., and Braam, J. (1995). Cellular localization of the Ca2+ binding TCH3 protein of Arabidopsis. Plant J. 8 623–636. [DOI] [PubMed] [Google Scholar]
- Arazi, T., Kaplan, B., and Fromm, H. (2000). A high-affinity calmodulin-binding site in a tobacco plasma-membrane channel protein coincides with a characteristic element of cyclic nucleotide-binding domains. Plant Mol. Biol. 42 591–601. [DOI] [PubMed] [Google Scholar]
- Arazi, T., Sunkar, R., Kaplan, B., and Fromm, H. (1999). A tobacco plasma membrane calmodulin-binding transporter confers Ni2+ tolerance and Pb2+ hypersensitivity in transgenic plants. Plant J. 20 171–182. [DOI] [PubMed] [Google Scholar]
- Babu, Y.S., Bugg, C.E., and Cook, W.J. (1988). Structure of calmodulin refined at 2.2 A resolution. J. Mol. Biol. 204 191–204. [DOI] [PubMed] [Google Scholar]
- Baum, G., Chen, Y., Arazi, T., Takatsuji, H., and Fromm, H. (1993). A plant glutamate decarboxylase containing a calmodulin binding domain: Cloning, sequence, and functional analysis. J. Biol. Chem. 268 19610–19617. [PubMed] [Google Scholar]
- Baum, G., Lev-Yadun, S., Fridmann, Y., Arazi, T., Katsnelson, H., Zik, M., and Fromm, H. (1996). Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J. 15 2988–2996. [PMC free article] [PubMed] [Google Scholar]
- Beckingham, K. (1991). Use of site-directed mutations in the individual Ca2(+)-binding sites of calmodulin to examine Ca2(+)-induced conformational changes. J. Biol. Chem. 266 6027–6030. [PubMed] [Google Scholar]
- Bergey, D.R., and Ryan, C.A. (1999). Wound- and systemin-inducible calmodulin gene expression in tomato leaves. Plant Mol. Biol. 40 815–823. [DOI] [PubMed] [Google Scholar]
- Blume, B., Nürnberger, T., Nass, N., and Scheel, D. (2000). Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell 12 1425–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam, J. (1992. a). Regulated expression of the calmodulin-related TCH genes in cultured Arabidopsis cells: Induction by calcium and heat shock. Proc. Natl. Acad. Sci. USA 89 3213–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam, J. (1992. b). Regulation of expression of calmodulin and calmodulin-related genes by environmental stimuli in plants. Cell Calcium 13 457–463. [DOI] [PubMed] [Google Scholar]
- Braam, J., and Davis, R.W. (1990). Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell 60 357–364. [DOI] [PubMed] [Google Scholar]
- Braam, J., Sistrunk, M.L., Polisensky, D.H., Xu, W., Purugganan, M.M., Antosiewicz, D.M., Campbell, P., and Johnson, K.A. (1997). Plant responses to environmental stress: Regulation and functions of the Arabidopsis TCH genes. Planta 203 (suppl.), S35–S41. [DOI] [PubMed] [Google Scholar]
- Bush, D.S. (1995). Calcium regulation in plant cells and its role in signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46 95–122. [Google Scholar]
- Caldelari, D., Sternberg, H., Rodriguez-Concepcion, M., Gruissem, W., and Yalovsky, S. (2001). Efficient prenylation by a plant geranylgeranyltransferase-I requires a functional CaaL box motif and a proximal polybasic domain. Plant Physiol. 126 1416–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikano, H., Ogawa, M., Ikeda, Y., Koizumi, N., Kusano, T., and Sano, H. (2001). Two novel genes encoding SNF-1 related protein kinases from Arabidopsis thaliana: Differential accumulation of AtSR1 and AtSR2 transcripts in response to cytokinins and sugars, and phosphorylation of sucrose synthase by AtSR2. Mol. Gen. Genet. 264 674–681. [DOI] [PubMed] [Google Scholar]
- Craske, M., Takeo, T., Gerasimenko, O., Vaillant, C., Torok, K., Petersen, O.H., and Tepikin, A.V. (1999). Hormone-induced secretory and nuclear translocation of calmodulin: Oscillations of calmodulin concentration with the nucleus as an integrator. Proc. Natl. Acad. Sci. USA 96 4426–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch, R.E., Xu, K., and Lewis, R.S. (1998). Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392 933–936. [DOI] [PubMed] [Google Scholar]
- Dong, A., Xin, H., Yu, Y., Sun, C., Cao, K., and Shen, W.H. (2002). The subcellular localization of an unusual rice calmodulin isoform, OsCaM61, depends on its prenylation status. Plant Mol. Biol. 48 203–210. [DOI] [PubMed] [Google Scholar]
- Falke, J.J., Drake, S.K., Hazard, A.L., and Peersen, O.B. (1994). Molecular tuning of ion binding to calcium signaling proteins. Q. Rev. Biophys. 27 219–290. [DOI] [PubMed] [Google Scholar]
- Felle, H.H., and Hepler, P.K. (1997). The cytosolic Ca2+ concentration gradient of Sinapis alba root hairs as revealed by Ca2+-selective microelectrode tests and fura-dextran ratio imaging. Plant Physiol. 114 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin-Tong, V.E., Trobak, B.K., Watkins, P.A.C., and Trewavas, A.J. (1996). Growth of pollen tubes of Papaver rhoeas is regulated by a slow moving calcium wave propagated by inositol 1,4,5-triphosphate. Plant Cell 8 1305–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm, H., and Chua, N.H. (1992). Cloning of plant cDNAs encoding calmodulin-binding proteins using 35S labeled recombinant calmodulin as a probe. Plant Mol. Biol. 10 199–206. [Google Scholar]
- Guo, H., Mockler, T., Duong, H., and Lin, C. (2001. a). SUB1, an Arabidopsis Ca2+-binding protein involved in cryptochrome and phytochrome coaction. Science 291 487–490. [DOI] [PubMed] [Google Scholar]
- Guo, Y., Halfter, U., Ishitani, M., and Zhu, J.K. (2001. b). Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell 13 1383–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiech, J., Kilhoffer, M.C., Lukas, T.J., Craig, T.A., Roberts, D.M., and Watterson, D.M. (1991). Restoration of the calcium binding activity of mutant calmodulins toward normal by the presence of a calmodulin binding structure. J. Biol. Chem. 266 3427–3431. [PubMed] [Google Scholar]
- Halfter, U., Ishitani, M., and Zhu, J.K. (2000). The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc. Natl. Acad. Sci. USA 97 3735–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S.A., Oh, S.H., and Roberts, D.M. (1997). Transgenic tobacco expressing a foreign calmodulin gene shows an enhanced production of active oxygen species. EMBO J. 16 1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S.A., and Roberts, D.M. (1998). Incompatible pathogen infection results in enhanced reactive oxygen and cell death responses in transgenic tobacco expressing a hyperactive mutant calmodulin. Planta 206 253–258. [Google Scholar]
- Harmon, A.C., Gribskov, M., and Harper, J.F. (2000). CDPKs: A kinase for every Ca2+ signal? Trends Plant Sci. 5 154–159. [DOI] [PubMed] [Google Scholar]
- Hasegawa, M., Bressan, R., and Pardo, J.M. (2000). The dawn of plant salt tolerance genetics. Trends Plant Sci. 5 317–319. [DOI] [PubMed] [Google Scholar]
- Heo, W.D., Lee, S.H., Kim, M.C., Kim, J.C., Chung, W.S., Chun, H.J., Lee, K.J., Park, C.Y., Park, H.C., Choi, J.Y., and Cho, M.J. (1999). Involvement of specific calmodulin isoforms in salicylic acid-independent activation of plant disease resistance responses. Proc. Natl. Acad. Sci. USA 96 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke, T.L., Feijo, J.A., Hackett, G.R., Kunkel, J.G., and Hepler, P.K. (1997). Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell 9 1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook, S.S., and Means, A.R. (2001). Ca2+/CaM-dependent kinases: From activation to function. Annu. Rev. Pharmacol. Toxicol. 41 471–505. [DOI] [PubMed] [Google Scholar]
- Hwang, I., Sze, H., and Harper, J.F. (2000). A calcium-dependent protein kinase can inhibit a calmodulin-stimulated Ca2+ pump (ACA2) located in the endoplasmic reticulum of Arabidopsis. Proc. Natl. Acad. Sci. USA 97 6224–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani, M., Liu, J., Halfter, U., Kim, C.S., Shi, W., and Zhu, J.K. (2000). SOS3 function in plant salt tolerance requires N-myristoylation and calcium binding. Plant Cell 12 1667–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, H.J., Pih, K.T., Kang, S.G., Lim, J.H., Jin, J.B., Piao, H.L., and Hwang, I. (1998). Molecular cloning of a novel Ca2+-binding protein that is induced by NaCl stress. Plant Mol. Biol. 37 839–847. [DOI] [PubMed] [Google Scholar]
- Kim, K.N., Cheong, Y.H., Gupta, R., and Luan, S. (2000). Interaction specificity of Arabidopsis calcineurin B-like calcium sensors and their target kinases. Plant Physiol. 124 1844–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler, C., Merkle, T., and Neuhaus, G. (1999). Characterisation of a novel gene family of putative cyclic nucleotide- and calmodulin-regulated ion channels in Arabidopsis thaliana. Plant J. 18 97–104. [DOI] [PubMed] [Google Scholar]
- Kuboniwa, H., Tjandra, N., Grzesiek, S., Ren, H., Klee, C.B., and Bax, A. (1995). Solution structure of calcium-free calmodulin. Nat. Struct. Biol. 2 768–776. [DOI] [PubMed] [Google Scholar]
- Kudla, J., Xu, Q., Harter, K., Gruissem, W., and Luan, S. (1999). Genes for calcineurin B-like proteins in Arabidopsis are differentially regulated by stress signals. Proc. Natl. Acad. Sci. USA 96 4718–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng, Q., Mercier, R.W., Yao, W., and Berkowitz, G.A. (1999). Cloning and first functional characterization of a plant cyclic nucleotide-gated cation channel. Plant Physiol. 121 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, A., Pennell, R.I., Alvarez, M.E., Palmer, R., and Lamb, C. (1996). Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr. Biol. 6 427–437. [DOI] [PubMed] [Google Scholar]
- Li, W., Llopis, J., Whitney, M., Zlokarnik, G., and Tsien, R.Y. (1998). Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature 392 936–941. [DOI] [PubMed] [Google Scholar]
- Ling, V., and Zielinski, R.E. (1993). Isolation of an Arabidopsis cDNA sequence encoding a 22-kD calcium-binding protein (CaBP-22) related to calmodulin. Plant Mol. Biol. 22 207–214. [DOI] [PubMed] [Google Scholar]
- Liu, J., Ishitani, M., Halfter, U., Kim, C.S., and Zhu, J.K. (2000). The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA 97 3730–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., and Zhu, J.K. (1998). A calcium sensor homolog required for plant salt tolerance. Science 280 1943–1945. [DOI] [PubMed] [Google Scholar]
- Lu, Y.T., and Feldman, L.J. (1997). Light-regulated root gravitropism: A role for, and characterization of, a calcium/calmodulin-dependent protein kinase homolog. Planta 203 (suppl.), S91–S97. [DOI] [PubMed] [Google Scholar]
- Marechal, E., Hiratsuka, K., Delgado, J., Nairn, A., Qin, J., Chait, B.T., and Chua, N.H. (1999). Modulation of GT-1 DNA-binding activity by calcium-dependent phosphorylation. Plant Mol. Biol. 40 373–386. [DOI] [PubMed] [Google Scholar]
- McAinsh, M.R., Brownlee, C., and Hetherington, A.M. (1997). Calcium ions as second messengers in guard cell signal transduction. Physiol. Plant. 100 16–29. [Google Scholar]
- Nelson, M.R., and Chazin, W. (1998). Calmodulin as a calcium sensor. In Calmodulin and Signal Transduction, L.J. Van Eldik and D.M. Watterman, eds (San Diego, CA: Academic Press), pp. 17–64.
- Nozawa, A., Koizumi, N., and Sano, H. (2001). An Arabidopsis snf1-related protein kinase, atsr1, interacts with a calcium-binding protein, AtCBL2, of which transcripts respond to light. Plant Cell Physiol. 42 976–981. [DOI] [PubMed] [Google Scholar]
- O'Neil, K.T., and DeGrado, W.F. (1990). How calmodulin binds its targets: Sequence independent recognition of amphiphilic alpha-helices. Trends Biochem. Sci. 15 59–64. [DOI] [PubMed] [Google Scholar]
- Osawa, M., Swindells, M.B., Tanikawa, J., Tanaka, T., Mase, T., Furuya, T., and Ikura, M. (1998). Solution structure of calmodulin-W-7 complex: The basis of diversity in molecular recognition. J. Mol. Biol. 276 165–176. [DOI] [PubMed] [Google Scholar]
- Patil, S., Takezawa, D., and Poovaiah, B.W. (1995). Chimeric plant calcium/calmodulin-dependent protein kinase gene with a neural visinin-like calcium-binding domain. Proc. Natl. Acad. Sci. USA 92 4897–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly, N., Knight, M.R., Thuleau, P., van Der Luit, A.H., Moreau, M., Trewavas, A.J., Ranjeva, R., and Mazars, C. (2000). Control of free calcium in plant cell nuclei. Nature 405 754–755. [DOI] [PubMed] [Google Scholar]
- Piao, H.L., Lim, J.H., Kim, S.J., Cheong, G.W., and Hwang, I. (2001). Constitutive over-expression of AtGSK1 induces NaCl stress responses in the absence of NaCl stress and results in enhanced NaCl tolerance in Arabidopsis. Plant J. 27 305–314. [DOI] [PubMed] [Google Scholar]
- Polisensky, D.H., and Braam, J. (1996). Cold-shock regulation of the Arabidopsis TCH genes and the effects of modulating intracellular calcium levels. Plant Physiol. 111 1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, A.S., Narasimhulu, S.B., Safadi, F., and Golovkin, M. (1996). A plant kinesin heavy chain-like protein is a calmodulin-binding protein. Plant J. 10 9–21. [DOI] [PubMed] [Google Scholar]
- Reddy, V.S., Ali, G.S., and Reddy, A.S. (2002). Genes encoding calmodulin-binding proteins in the Arabidopsis genome. J. Biol. Chem. 277 9840–9852. [DOI] [PubMed] [Google Scholar]
- Resh, M.D. (1999). Fatty acylation of proteins: New insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta 1451 1–16. [DOI] [PubMed] [Google Scholar]
- Roberts, D.M., and Harmon, A.C. (1992). Calcium modulated protein targets of intracellular calcium signals in higher plants. Annu. Rev. Plant. Physiol. Plant Mol. Biol. 43 375–414. [Google Scholar]
- Rodriguez-Concepcion, M., Toledo-Ortiz, G., Yalovsky, S., Caldelari, D., and Gruissem, W. (2000). Carboxyl-methylation of prenylated calmodulin CaM53 is required for efficient plasma membrane targeting of the protein. Plant J. 24 775–784. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Concepcion, M., Yalovsky, S., Zik, M., Fromm, H., and Gruissem, W. (1999). The prenylation status of a novel plant calmodulin directs plasma membrane or nuclear localization of the protein. EMBO J. 18 1996–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd, J.J., and Franklin-Tong, V.E. (2001). Unravelling response-specificity in Ca2+ signaling pathways in plant cells. New Phytol. 151 7–33. [DOI] [PubMed] [Google Scholar]
- Sanders, D., Brownlee, C., and Harper, J.F. (1999). Communicating with calcium. Plant Cell 11 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurink, R.C., Shartzer, S.F., Fath, A., and Jones, R.L. (1998). Characterization of a calmodulin-binding transporter from the plasma membrane of barley aleurone. Proc. Natl. Acad. Sci. USA 95 1944–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon, K.B., and Kreetsinger, R.H. (1983). Calcium-modulated proteins. Met. Ions Biol. 6 1–51. [Google Scholar]
- Shi, H., Ishitani, M., Kim, C., and Zhu, J.K. (2000). The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA 97 6896–6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J., Kim, K.N., Ritz, O., Albrecht, V., Gupta, R., Harter, K., Luan, S., and Kudla, J. (1999). Novel protein kinases associated with calcineurin B–like calcium sensors in Arabidopsis. Plant Cell 11 2393–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistrunk, M.L., Antosiewicz, D.M., Purugganan, M.M., and Braam, J. (1994). Arabidopsis TCH3 encodes a novel Ca2+ binding protein and shows environmentally induced and tissue-specific regulation. Plant Cell 6 1553–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedden, W.A., and Fromm, H. (1998). Calmodulin, calmodulin-regulated proteins and plant responses to the environment. Trends Plant Sci. 3 299–304. [Google Scholar]
- Snedden, W.A., and Fromm, H. (2001). Calmodulin as a versatile calcium signal transducer in plants. New Phytol. 151 35–66. [DOI] [PubMed] [Google Scholar]
- Snedden, W.A., Koutsia, N., Baum, G., and Fromm, H. (1996). Activation of a recombinant petunia glutamate decarboxylase by calcium/calmodulin or by a monoclonal antibody which recognizes the calmodulin binding domain. J. Biol. Chem. 271 4148–4153. [DOI] [PubMed] [Google Scholar]
- Sze, H., Liang, F., Hwang, I., Curran, A.C., and Harper, J.F. (2000). Diversity and regulation of plant Ca2+ pumps: Insights from expression in yeast. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51 433–462. [DOI] [PubMed] [Google Scholar]
- Szymanski, D.B., Liao, B., and Zielinski, R.E. (1996). Calmodulin isoforms differentially enhance the binding of cauliflower nuclear proteins and recombinant TGA3 to a region derived from the Arabidopsis Cam-3 promoter. Plant Cell 8 1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takezawa, D., Ramachandiran, S., Paranjape, V., and Poovaiah, B.W. (1996). Dual regulation of a chimeric plant serine/threonine kinase by calcium and calcium/calmodulin. J. Biol. Chem. 271 8126–8132. [DOI] [PubMed] [Google Scholar]
- Teruel, M.N., Chen, W., Persechini, A., and Meyer, T. (2000). Differential codes for free Ca(2+)-calmodulin signals in nucleus and cytosol. Curr. Biol. 10 86–94. [DOI] [PubMed] [Google Scholar]
- Teruel, M.N., and Meyer, T. (2000). Translocation and reversible localization of signaling proteins: A dynamic future for signal transduction. Cell 103 181–184. [DOI] [PubMed] [Google Scholar]
- Trewavas, A.J. (1999). How plants learn. Proc. Natl. Acad. Sci. USA 96 4216–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas, A.J., and Knight, M.R. (1994). Mechanical signalling, calcium and plant form. Plant Mol. Biol. 26 1329–1341. [DOI] [PubMed] [Google Scholar]
- van Der Luit, A.H., Olivari, C., Haley, A., Knight, M.R., and Trewavas, A.J. (1999). Distinct calcium signaling pathways regulate calmodulin gene expression in tobacco. Plant Physiol. 121 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watillon, B., Kettmann, R., Boxus, P., and Burny, A. (1995). Structure of a calmodulin-binding protein kinase gene from apple. Plant Physiol. 108 847–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymer, C.L., Bibikova, T.N., and Gilroy, S. (1997). Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J. 12 427–439. [DOI] [PubMed] [Google Scholar]
- Yalovsky, S., Rodriguez-Concepcion, M., and Gruissem, W. (1999). Lipid modifications of proteins: Slipping in and out of membranes. Trends Plant Sci. 4 439–445. [DOI] [PubMed] [Google Scholar]
- Yamakawa, H., Mitsuhara, I., Ito, N., Seo, S., Kamada, H., and Ohashi, Y. (2001). Transcriptionally and post-transcriptionally regulated response of 13 calmodulin genes to tobacco mosaic virus-induced cell death and wounding in tobacco plant. Eur. J. Biochem. 268 3916–3929. [DOI] [PubMed] [Google Scholar]
- Yang, T., Lev-Yadun, S., Feldman, M., and Fromm, H. (1998). Developmentally regulated organ-, tissue-, and cell-specific expression of calmodulin genes in common wheat. Plant Mol. Biol. 37 109–120. [DOI] [PubMed] [Google Scholar]
- Yang, T., Segal, G., Abbo, S., Feldman, M., and Fromm, H. (1996). Characterization of the calmodulin gene family in wheat: Structure, chromosomal location, and evolutionary aspects. Mol. Gen. Genet. 252 684–694. [DOI] [PubMed] [Google Scholar]
- Zhang, M., Tanaka, T., and Ikura, M. (1995). Calcium-induced conformational transition revealed by the solution structure of apo calmodulin. Nat. Struct. Biol. 2 758–767. [DOI] [PubMed] [Google Scholar]
- Zhang, M., and Yuan, T. (1998). Molecular mechanisms of calmodulin's functional versatility. Biochem. Cell Biol. 76 313–323. [DOI] [PubMed] [Google Scholar]
- Zielinski, R.E. (1998). Calmodulin and calmodulin binding proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49 697–725. [DOI] [PubMed] [Google Scholar]
- Zielinski, R.E. (2002). Characterization of three new members of the Arabidopsis thaliana calmodulin gene family: Conserved and highly diverged members of the gene family functionally complement a yeast calmodulin null. Planta 214 446–455. [DOI] [PubMed] [Google Scholar]
- Zik, M., Arazi, T., Snedden, W.A., and Fromm, H. (1998). Two isoforms of glutamate decarboxylase in Arabidopsis are regulated by calcium/calmodulin and differ in organ distribution. Plant Mol. Biol. 37 967–975. [DOI] [PubMed] [Google Scholar]


