INTRODUCTION
Calcium Signals: A Central Paradigm in Stimulus–Response Coupling
Cells must respond to an array of environmental and developmental cues. The signaling networks that have evolved to generate appropriate cellular responses are varied and are normally composed of elements that include a sequence of receptors, nonprotein messengers, enzymes and transcription factors. Receptors are normally highly specific for the physiological stimulus, and therefore are disparate in their identities. Likewise enzymes and transcription factors tend toward specificity, and this fact is reflected in abundance at the genome level. The Arabidopsis genome, for example, potentially encodes in the region of 1000 protein kinases, 300 protein phosphatases, and 1500 transcription factors. By contrast, nonprotein messengers are relatively few. They include cyclic nucleotides (Newton et al., 1999), hydrogen ions (Guern et al., 1991), active oxygen species (Van Breusegem et al., 2001), lipids (Ng and Hetherington, 2001; Nurnberger and Scheel, 2001; Munne-Bosch and Alegre, 2002), and, above all, calcium.
Changes in cytosolic free calcium ([Ca2+]c) are apparent during the transduction of a very wide variety of abiotic and biotic signals. The spectrum of stimuli that evokes rapid changes in [Ca2+]c has been cataloged in a number of recent reviews (Sanders et al., 1999; Knight, 2000; Anil and Rao, 2001; Knight and Knight, 2001; Rudd and Franklin-Tong, 2001). Abiotic stimuli include light—with red, blue, and UV/B irradiation each acting via different receptors and leading to distinct developmental responses (Shacklock et al., 1992; Baum et al., 1999; Frohnmeyer et al., 1999), low and high temperature, touch, hyperosmotic stress, and oxidative stress. Biotic stimuli include the hormones abscissic acid (ABA) and gibberellin, fungal elicitors, and nodulation (Nod) factors.
The Specificity Question
An all-pervading question during the last decade of calcium signaling research has revolved around the issue of specificity (McAinsh and Hetherington, 1998). How can a simple nonprotein messenger be involved in so many signal transduction pathways and yet still convey stimulus specificity within a variety of pathways? Ostensibly there are a number reasonable nonexclusive answers to this question. First, the Ca2+ signal itself might be a necessary but insufficient trigger for the response, with effective signal transduction occurring only should another signal change in parallel. Second, specificity might be encoded by the spatial properties of the Ca2+ signal, either because the signal is compartmentally localized (for example, to the nucleus, rather than the cytosol) or because the source of the Ca2+ signal (from outside the cell or from intracellular stores) can selectively trigger response elements. Third, the dynamic properties of the Ca2+ signal might determine the efficacy with which the response is elicited. Fourth, of course, the appropriate response elements must be present in the particular cell type in which the Ca2+ signal arises. Since Ca2+ signaling was last reviewed in this journal (Sanders et al., 1999), remarkable advances have been made in addressing this central problem of specificity, in many cases thanks to the insights provided by genetic approaches. Thus, while alluding briefly to the earlier literature, the present review will focus on developments in our understanding that have occurred over the past four years.
ELEMENTS ENCODING CALCIUM SIGNALS
Calcium signals are generated through the opening of ion channels that allow the downhill flow of Ca2+ from a compartment in which the ion is present at relatively high electrochemical potential (either outside the cell, or from an intracellular store) to one in which Ca2+ is at lower potential. There has, in the past, been a tendency to refer to such channels as “Ca2+ channels,” although we prefer the term “Ca2+-permeable channels” because this reflects the likely importance of nonselective cation channels in generating plant Ca2+ signals. Maintenance of low Ca2+ electrochemical activity in the Ca2+-responsive compartment is achieved by the ATP- or proton motive force–driven removal of Ca2+ on pumps or carriers (transporters), respectively. As shown in Figure 1, the interplay between influx through channels and efflux from pumps and carriers will determine the form of a Ca2+ spike that is potentially specific to relevant decoders. Figure 2 shows the location of channels, pumps, and carriers involved in Ca2+ transport for a generalized Arabidopsis cell, as the basis for the discussion below.
Figure 1.
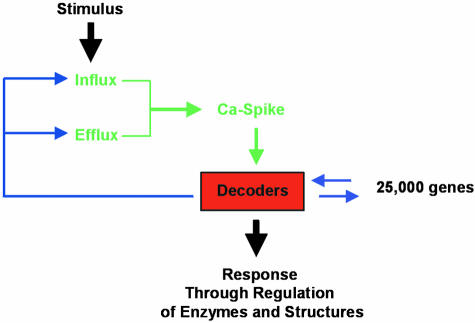
Decoding Calcium Signals Leads to a Specific Response at the Cellular Level.
Various feedback mechanisms from the calcium sensor (or “decoder”) are possible. These could include the regulation of calcium spikes via the control of calcium permeable channel gating (e.g., through EF binding hands, or via Ca2+/CaM binding) or via control of pump activity.
Figure 2.
Schematic Representation of Major Identified Ca2+ Transport Pathways in Arabidopsis Cell Membranes.
Blue circles represent energized transport systems. ACA1, ACA4, ACA8 are autoinhibited calcium ATPases identified at a molecular level. The direction of Ca2+ pumping for ACA1 is hypothetical. ECA is an ER-type calcium ATPase. ACAx in the central vacuole and in the Golgi has not been identified at a molecular level. CAX1 is a Ca2+/H+ antiporter expected to be localized at the vacuolar membrane. Red squares represent Ca2+-permeable channels. At the plasma membrane, nonselective cation (NSC) channels, depolarization activated channels (DACs) and hyperpolarization activated channels (HACs) have been characterized at an electrophysiological but not at a molecular level. A two-pore channel (TPC1) has been shown to complement a yeast mutant deficient in Ca2+ uptake, but channel location is hypothetical. Using electrophysiolocal techniques, cyclic nucleotide gated channels (CNGC1 and CNGC2) were shown to be permeable to calcium. Plasma membrane location is again hypothetical. Glutamate receptors (GLRs) might be involved in the increase of cytosolic calcium concentrations and have been identified at a molecular level. The channels identified at endomembranes have been characterized at electrophysiological and biochemical but not molecular levels. InsP3R, putative Ins3P receptor; RyR, putative ryanodine receptor activated by cADPR; NAADP-activated channels also reside in the ER as shown. SV channel, slowly activating vacuolar channel; VVCa channel, vacuolar voltage-gated Ca2+ channel.
Calcium-Permeable Ion Channels
The importance of the cellular location of ion channels in determining stimulus specificity is emphasized by a study of Ca2+-mediated stomatal closure in tobacco (Wood et al., 2000). Removal of extracellular Ca2+ with the chelator EGTA or blockage of entry with a number of ion channel blockers suggested that low temperature–induced closure involves primarily entry of Ca2+ across the plasma membrane, while intracellular mobilization appears to dominate if stomatal closure is initiated with ABA or mechanical stimulation.
Calcium-permeable channels have been investigated with electrophysiological, biochemical, and molecular approaches, and these are now beginning to yield complementary insights into the nature and control of channels that underlie the generation of Ca2+ signals.
Plasma Membrane
Electrophysiological studies during the past decade have revealed the presence in plant plasma membranes of calcium-permeable channels that are activated by membrane depolarization (reviewed by White, 2000). It has been speculated that this form of voltage gating might endow such channels with a pivotal role at an early stage in signal transduction (Ward et al., 1995). Thus, perception of a range of stimuli results in membrane depolarization, possibly as a result of the activation of anion channels, and the resultant opening of depolarization-activated Ca2+-permeable channels could lead to elevation of [Ca2+]c.
While depolarization-activation of Ca2+-permeable channels is a recurring theme in a number of biological systems, recent simultaneous and independent studies have followed pioneering work by Gelli et al. (1997) and Gelli and Blumwald (1997) on tomato cell suspensions, reporting the presence in plant plasma membranes of Ca2+-permeable channels that are activated by membrane hyperpolarization. Such channels have a high selectivity for Ca2+ over K+ and Cl− (Gelli and Blumwald, 1997; Hamilton et al., 2000; Véry and Davies, 2000). It has been known for some time that in guard cells, membrane hyperpolarization is directly associated with the elevation of [Ca2+]c that follows ABA application (Grabov and Blatt, 1998). The observation that hyperpolarization-activated Ca2+-permeable channels in the plasma membrane of guard cells are opened by ABA even in excised membrane patches implies a very close physical coupling between the channels and the sites of ABA perception (Hamilton et al., 2000). Channel opening might also be subject to negative feedback control to prevent excessive Ca2+ entry, since activity decreases around ten-fold over the range of [Ca2+]c from 0.2 to 2 μM. In root hairs, channel activity is present at the tips of growing cells, but not detectable in subapical regions or at the tips of mature cells (Véry and Davies, 2000), an observation consistent with the notion that these channels play a pivotal role in the generation of the tip-to-base Ca2+ gradient that is essential for maintaining polarization in tip-growing systems (including pollen tubes and rhizoid cells). Intriguingly, the root hair channels are, in contrast to their counterparts in guard cells, activated by elevation of [Ca2+]c, suggesting that they might play a self-sustaining role in maintaining the tip-to-base Ca2+ gradient. Hyperpolarization-activated Ca2+-permeable channels have also been reported in the growing root apex of Arabidopsis roots, but not in other more mature cells (Kiegle et al., 2000a), possibly suggesting a role for these channels in cell division and elongation.
ABA-induced stomatal closure involves the production of reactive oxygen species, notably hydrogen peroxide (Pei et al., 2000; Zhang et al., 2001), and hyperpolarization-activated Ca2+-permeable channels play a critical role in this reponse. In Arabidopsis guard cells, hydrogen peroxide stimulates hyperpolarization-activated Ca2+-permeable channels, and thereby an increase in [Ca2+]c (Pei et al., 2000). This process requires cytosolic NAD(P)H, suggesting that NAD(P)H oxidases could be part of the ABA signaling cascade (Murata et al., 2001).
It is unlikely that voltage-gated pathways comprise the sole route for Ca2+ entry across the plasma membrane. Channels that discriminate poorly between mono- and divalent cations and that exhibit at best only very weak voltage-dependence are ubiquitous in plant cells (Demidchik et al., 2002). These nonselective cation channels probably fulfill a variety of functions in addition to that of Ca2+ uptake, including the uptake of cations for general nutritional purposes, and the presence of various classes of channel is suggested by reports of diverse modes of regulation, including cyclic nucleotides (Maathuis and Sanders, 2001) and extracellular pH (Demidchik and Tester, 2002). It will therefore be important to identify these channels at a molecular level before drawing conclusions concerning their specific roles in Ca2+-based signal transduction.
The molecular basis of plasma membrane Ca2+-permeable channel activity is only just becoming apparent, and there a number of intriguing candidate genes. A unique gene in Arabidopsis, TPC1 (At4 g03560), encodes a channel with two Shaker-like domains (i.e., 2 × 6 transmembrane spans, each of which contains a putative “pore” region) connected by a hydrophilic domain that includes two EF hands (Figure 3A). The general structure resembles that of the pore-forming subunits of mammalian and yeast Ca2+ channels that contain four Shaker-like domains, and there is some sequence similarity. TPC1 expression enhances Ca2+ uptake in a yeast Ca2+-channel mutant (Furuichi et al., 2001). There are indications that TPC1, which is expressed ubiquitously, forms a depolarization-activated channel because overexpression and antisense expression appear, respectively, to enhance and suppress an increase in [Ca2+]c that occurs as a result of sugar-induced membrane depolarization. However, firm conclusions regarding voltage gating await electrophysiological characterization, and there are as yet no indications as to the physiological role(s) of TPC1.
Figure 3.
Topology Models of Putative Plasma Membrane Proteins Involved in Calcium Influx in the Cytosol in Arabidopsis.
(A) The two-pore channel (TPC1) is composed of two EF calcium binding hands, which could be involved in the feedback control of the channel activity via cytosolic calcium concentration. The pore loop (P) is localized between the 5th and 6th transmembrane domains of each repeat. The 4th transmembrane domain in each repeat is enriched in basic residues, which might suggest that the channel is voltage gated.
(B) CNGC structure also contains a P loop and, unlike counterparts in animals, overlapping of the calmodulin and cyclic nucleotide binding domains at the C terminus of the protein.
(C) GLR structure is similar to that of animal ionotropic glutamate receptors and is composed of four membrane-localized domains among which M2 is predicted not to span the membrane. Two glutamate binding domains (GlnH) are localized on the outside of the membrane.
The Arabidopsis genome also appears to encode no fewer than 20 members of a cyclic nucleotide-gated channel (CNGC) family (Maser et al., 2001; http://plantst.sdsc.edu/plantst/html/1.A.1.shtml). The general structure is shown in Figure 3B. A Shaker-like domain is supplemented at the C terminus with overlapping calmodulin and cyclic nucleotide binding domains (Arazi et al., 2000; Kohler and Neuhaus, 2000). Mammalian orthologs form tetrameric channels that are weakly selective among Group I and Group II cations. AtCNGC2 has been analyzed electrophysiologically and shown to conduct a number of cations, including Ca2+, in response to cAMP (Leng et al., 1999, 2002). A tobacco CNGC (NtCBP4) is localized at the plasma membrane and when overexpressed confers Pb2+ hypersensitivity (Arazi et al., 1999). Conversely, expression of a C-terminal truncated NtCBP4 confers Pb2+ tolerance, as does disruption of the AtCNGC1 gene (Sunkar et al., 2000). Since Pb2+ has no known physiological role in plants but is known to permeate some Ca2+-permeable channels, these observations are consistent with NtCBP4 and AtCNGC1 providing a route for Ca2+ entry across the plasma membrane in planta. Intriguingly, cngc2 mutants of Arabidopsis are defective in the hypersensitive response that follows pathogen infection, although these plants nevertheless exhibit gene-for-gene resistance (Clough et al., 2000), implying that AtCNGC2 is not an essential component in defense responses. Rather, expression analyses of AtCNGC2 support the notion that this channel might play a role in senescence or developmentally regulated cell death (Kohler et al., 2001). To date, there are no reports of cyclic nucleotide–activated channel activity in planta, although the presence of nonselective cation channels that are inhibited by cyclic nucleotides (cAMP and cGMP) has been recorded in Arabidopsis root cells (Maathuis and Sanders, 2001). It is possible that this activity relates to CNGC isoforms other than those that have been analyzed in heterologous systems. A number of reports have linked cyclic nucleotides with Ca2+ signaling (Bowler et al., 1994; Volotovski et al., 1998; Jin and Wu, 1999; Moutinho et al., 2001), and it is possible that CNGCs provide an essential link between the two messengers.
Glutamate receptors (GLRs) comprise a further class of ion channel that might provide a calcium-permeable pathway across the plasma membrane. A generalized structure is shown in Figure 3C. In animals, GLRs are neurotransmitter gated and form nonselective cation channels in the postsynaptic membrane. In Arabidopsis, the GLR family comprises 30 genes (Lacombe et al., 2001; http://plantst.sdsc.edu/plantst/html/1.A.10.shtml). Glutamate triggers in Arabidopsis roots a large transient elevation in [Ca2+]c and a membrane depolarization, both of which are sensitive to the Ca2+ antagonist La3+ (Dennison and Spalding, 2000). The response is relatively specific to glutamate. Overexpression of the AtGLR2 gene leads to Ca2+ deficiency symptoms and other ionic defects that can be alleviated by increasing external Ca2+ concentration (Kim et al., 2001). Expression analysis suggests that AtGLR2 might be involved in unloading calcium from the xylem vessels (Kim et al., 2001). It is possible that physiological activation of GLRs involves opening of nonselective anion channels at the plasma membrane, although a plasma membrane location for plant GLRs has yet to be demonstrated.
Endomembranes
The large lytic vacuole of mature plant cells is unquestionably the principal intracellular Ca2+ store, and accordingly, a number of Ca2+ release channels have been reported to reside in the vacuolar membrane (Sanders et al., 1999). Two of these channels are ligand gated respectively by inositol trisphosphate and by cyclic ADP-ribose. Two further channel types are gated by voltage, one by membrane hyperpolarization and a second by membrane depolarization. This second class of channel is known as the slowly activating vacuolar (SV) channel in reference to its voltage-activation kinetics (Hedrich and Neher, 1987), and it is activated by rises in [Ca2+]c over the physiological range. This response potentially endows the channel with the capacity to catalyze Ca2+-induced Ca2+ release (CICR) (Ward and Schroeder, 1994; Bewell et al., 1999). Although the response to Ca2+ alone would not permit CICR in vivo (Pottosin et al., 1997), the presence of Mg2+ ions at physiological concentrations potentiates the Ca2+ response, thereby suggesting a bona fide role in CICR (Pei et al., 1999; Carpaneto et al., 2001). In addition to regulation by [Ca2+]c and by phosphorylation state (Allen and Sanders, 1995), SV channels are also potently downregulated by 14-3-3 proteins (van den Wijngaard et al., 2001), implying a central role for SV channels in coordination of signaling events.
The mere presence of a large intracellular Ca2+ store does not, of course, guarantee that it is mobilized in signaling events, and recent attention has highlighted the importance of other intracellular Ca2+ stores, notably the endoplasmic reticulum (ER). Voltage-dependent Ca2+-selective channels have been identified in the ER (Klusener et al., 1995; Klusener and Weiler, 1999), and the demonstration of high-affinity InsP3 binding sites on the ER is also suggestive of the presence of InsP3-gated Ca2+ release channels (Martinec et al., 2000). Calcium release assays have also revealed the presence of cADPR-mobilizable Ca2+ in ER vesicles (Navazio et al., 2001), and have identified as well a novel and discrete Ca2+ release pathway activated by the NADP metabolite nicotinic acid adenine dinucleotide phosphate (NAADP; Navazio et al., 2000). Interestingly, NAADP does not effect Ca2+ mobilization from vacuolar membrane vesicles.
Distinct roles for some of these ligands are emerging. For InsP3, these roles include transduction of salt and hyperosmotic stress signals (Drobak and Watkins, 2000; DeWald et al., 2001) as well as involvement in gravitropism (Perera et al., 1999), while for cADPR, mediation in activation of plant defense genes seems likely (Durner et al., 1998), as well as in ABA signal transduction (Wu et al., 1997; McAinsh and Hetherington, 1998).
The full significance of this plethora of endomembrane Ca2+ release pathways has yet to be assessed rigorously. However, to date, no genes encoding endomembrane Ca2+ release channels have been identified, and until the encoded channels are characterized and localized, it is difficult to speculate on the significance of the intracellular distribution.
Calcium Efflux through H+/Ca2+ Antiporters and Calcium ATPases
The transport systems that energize efflux from the cytosol provide three critical housekeeping functions. First, following a calcium release, efflux systems restore [Ca2+]c to resting levels, thereby terminating a Ca2+ signal. Second, they load Ca2+ into compartments such as the ER and vacuole to be used as sources for a regulated Ca2+ release. Third, they supply Ca2+ to various organelles to support specific biochemical functions. For example, high levels of Ca2+ in the ER are required for proper protein processing through the secretory pathway (e.g., Durr et al., 1998).
A fundamental question is whether, in addition to their housekeeping functions, any of these efflux pathways help shape the dynamic form of a calcium spike and thereby help define the information encoded in the signal. If efflux is subject to regulation, then elucidating the signals that control these efflux systems will be equally important in identifying the signals that open various calcium channels. Pioneering work in two nonplant systems (Xenopus oocytes and Dictyostelium) has demonstrated that increasing the abundance or activity of a Ca2+ pump can indeed alter signal transduction (Camacho and Lechleiter, 1993; Lechleiter et al., 1998; Roderick et al., 2000). Thus, the potential signaling importance of efflux systems in plants must be seriously considered.
Calcium Exchangers
H+/Ca2+ antiporters can in principle drive “uphill” transport of Ca2+, in which a proton motive force is maintained, although in plants this usually requires an H+/Ca2+ stoichiometry of at least three (Blackford et al., 1990). The first plant H+/Ca2+ antiporter to be cloned and functionally expressed was CAX1 (calcium exchanger 1; Hirschi et al., 1996; Hirschi, 2001) and its projected membrane topology is shown in Figure 4A. The gene was identified by its ability to restore growth on high-Ca2+ media to a yeast mutant defective in vacuolar Ca2+ transport. CAX1 appears to transport Ca2+ with a low affinity (Km is ∼13 μM), consistent with kinetic studies on H+/Ca2+-antiport activity conducted with oat root vacuoles (Schumaker and Sze, 1986). The ionic specificity of CAX1 is partially determined by a 9-amino-acid sequence following the first predicted transmembrane domain (Shigaki et al., 2001).
Figure 4.
Topology Models of Systems Catalyzing Ca2+ Efflux from the Cytosol.
(A) Ca2+/H+ antiporters. The topology of CAX1 is based on speculation from hydropathy analyses. The number of transmembrane domains predicted varies from eight to eleven. CAX1 has recently been shown to have an N-terminal autoinhibitor. The blue highlight indicates the position of a 9-amino-acid sequence implicated in providing transport specificity for cations.
(B) Ca2+-ATPase (ACA type). The topology of calcium pumps is well-supported by homology modeling based on a crystal structure of a mammalian sacro(endo)plasmic reticulum-type Ca2+ ATPase pump. ECA-type calcium pumps are predicted to have similar topologies, but lack the distinguishing feature of an N-terminal auotoinhibitor and calmodulin binding site.
In Arabidopsis, there are 12 genes predicted to encode antiporters closely related to CAX1 (Maser et al., 2001; http://plantst.sdsc.edu/plantst/html/2.A.19.shtml). Whether all of these related antiporters can transport calcium has not been determined. CAX2 appears to transport Mn in addition to Ca (Hirschi, et al., 2000). Although CAX1 is expected to be localized to the plant vacuole, there is evidence for H+/Ca2+ antiporters in other locations, such as the plasma membrane (Kasai and Muto, 1990). The subcellular locations of all twelve Arabidopsis CAX1–related isoforms still need to be determined.
Recently, the activity of CAX1 was shown to be regulated by an N-terminal autoinhibitor (Pittman and Hirschi, 2001). The expression of a “deregulated” CAX1 in tobacco resulted in plants with increased accumulation of Ca (Hirschi, 1999), consistent with the increased activity of an antiporter that functions to sequester calcium into an endomembrane compartment. Interestingly, the plants displayed growth phenotypes that mimicked calcium deficiency symptoms. In addition, the plants displayed hypersensitivity to K and Mg and increased sensitivity to various stresses, including cold. Thus, aspects of plant development and stress tolerance are dependent on regulation of CAX1 activity. An important challenge is to understand how the activity of CAX1 and related antiporters is controlled.
Pumps
Calcium pumps belong to the superfamily of P-type ATPases that directly use ATP to drive ion translocation. Two distinct Ca2+ pump families have been proposed on the basis of protein sequence identities (Geisler et al., 2000a; Axelsen and Palmgren, 2001; http://biobase.dk/~axe/Patbase.html). Mem-bers of the type IIA and IIB families, respectively, include the ER-type calcium ATPases (ECAs) and the autoinhibited calcium ATPases (ACAs). The ACAs are distinguished from ECAs by three biochemical features: 1) The presence of an N-terminal autoinhibitor, 2) direct activation through binding Ca/calmodulin, and 3) insensitivity to inhibition by cyclopiazonic acid and thapsigargin (Figure 4B; Sze et al., 2000). Interestingly, a pump showing mixed characteristics of both ECA and ACA pumps was identified in maize (Subbaiah and Sachs, 2000). However, a corresponding “chimeric gene” has not been found in the Arabidopsis genome, suggesting that this unusual pump is not common to all land plants.
In Arabidopsis, there are four ECA- and ten ACA-type calcium pumps (Axelsen and Palmgren, 2001). Isoform ECA1 appears to be located in the ER, as determined by membrane fractionation and immunodetection (Liang et al., 1997; Hong et al., 1999). However, the potential for other isoforms targeting to non-ER locations must be considered. In tomato, there is evidence from membrane fractionation and immunodetection suggesting that related ER-type calcium pumps (LCA1-related) are present in the vacuolar and plasma membranes (Ferrol and Bennett, 1996).
The ACA subgroup of plant Ca2+ATPases is most closely related to the plasma membrane–type pumps found in animals. However, they form a distinct subgroup distinguished by two features: 1) a unique structural arrangement with the autoinhibitory domain at the N terminus instead of the C terminus, and 2) representatives that target to membranes other than the plasma membrane (i.e., endomembranes). While ACA8 has been found at the plasma membrane (Bonza et al., 2000), as expected on the basis of the animal paradigm, endomembrane locations have been identified for ACA2 (ER; Liang et al., 1997; Hong, et al., 1999) and ACA4 (small vacuoles; Geisler et al., 2000b). In addition, evidence suggests that ACA1 is located in the plastid inner envelope membrane (Huang et al., 1993). The subcellular locations have not been determined for ACA7, 9, 10, 11, 12, and 13.
Functional Overlap?
Given the probability of at least 26 calcium pumps and antiporters in Arabidopsis, it is likely that multiple efflux systems located in the same membrane system will be found. For example, in vacuoles, there is evidence for both an H+/Ca2+ antiporter (such as CAX1p) and an autoinhibited calcium pump, such as ACA4. In the ER, there is evidence for two different types of calcium pumps, ECA1 and ACA2. An important challenge is to delineate the specific and redundant functions for each of the different efflux pathways (Harper, 2001).
Regulation
It is now clear that transport activities are regulated for CAX1 and most members of the ACA-type calcium pumps, as indicated by experimental evidence and structural analogy. However, in plants, there is no experimental evidence for the regulation of ECA activity. In theory, the plant ECA pathway may provide a constitutive “house-keeping” activity that simply “cleans up” at a constant rate after any calcium release. Nevertheless, there are two rationales for expecting some kind of regulatory control. First, the most closely related ER-type calcium pumps in animal systems are highly regulated. In animals, the activity of the sacro-(endo)plasmic reticulum-type Ca2+ ATPase pump is tightly regulated by the phosphorylation status of an inhibitory subunit, phospholamban (East, 2000). In addition, there is evidence that the animal ER-type pumps can be regulated by a feedback system that maintains an appropriate Ca2+ load within the ER lumen (Bhogal and Colyer, 1998; Mogami et al., 1998). Second, the observation in plants of the regulation of both CAX1 and ACA pathways supports a speculation that all major efflux pathways in plant cells are carefully regulated. Assuming that ECAs are regulated, an important challenge is to determine for each specific efflux pathway whether the transporter's regulation is as a tuning mechanism to control the magnitude or duration of a calcium spike (i.e., a signaling function), or as feedback control to adjust the distribution and levels of calcium at the cell surface or in different endomembrane compartments (i.e., a nutritional function).
DECODING CALCIUM SIGNALS
The initial perception of a calcium signal occurs through the binding of calcium to many different calcium sensors. Sensors can be divided into two types, sensor relays and sensor responders. Sensor relays, such as calmodulin, undergo a calcium-induced conformational change (sensing) that is relayed to an interacting partner. The interacting partner then responds with some change in its enzyme activity or structure (for example, calmodulin stimulation of an ACA calcium pump activity). This type of sensor includes calmodulin and calcineurin B–like proteins that are reviewed separately in this issue by Luan et al. (2002).
In contrast, sensor responders undergo a calcium-induced conformational change (sensing) that alters the protein's own activity or structure (e.g., intramolecular activation of a Ca2+-dependent protein kinase [CDPK]). By definition, sensor relays function through bimolecular interactions, whereas sensor responders function through intramolecular interactions. These two different modes of decoding calcium signals are used extensively in plants to provide many pathways by which calcium can trigger a diverse number of responses.
Calcium-Regulated Kinases
Kinases represent an important pathway by which calcium signals are decoded and propagated downstream into changes in structure and enzyme activity (Harmon et al., 2000). In Arabidopsis, the kinases shown to be involved in decoding calcium signals all belong to the CDPK/SNF1-related kinase family (http://plantsP.sdsc.edu). Of the 84 members of this family, 59 are predicted to be regulated by calcium, based on structural similarities to biochemically characterized members, and of these 34 are CDPKs that bind calcium directly through their calmodulin-like regulatory domain (sensor-responders). The remaining 25 comprise the SNF1-related kinase 3 subgroup, members of which appear to interact with a diverse collection of calcineurin B-like sensor relays and to play a role in a number of signaling processes, including those related to salt tolerance (Halfter et al., 2000; Luan et al., 2002).
CDPKs are unique owing to the presence of a C-terminal calmodulin-like regulatory domain that functions to couple the calcium sensor (i.e., calmodulin-like domain) directly to its “responder” (i.e., kinase). This group of kinases was first discovered in plants (Harper et al., 1991; Suen and Choi, 1991). While structurally analogous kinases have been found in alveolate protists (e.g., Plasmodium; Zhao et al., 1993; Zhang and Choi, 2001), CDPKs have not been found in members of other phylogenetic branches, including fungi, insects, and humans.
In Arabidopsis, most CDPKs have four calcium binding EF hands, but there are several examples of degenerate or truncated EF hands (http://plantsP.sdsc.edu). At the extreme end of the divergence spectrum, there are eight CDPK-related kinases (CRKs) that no longer appear to be regulated by calcium (Furumoto et al., 1996) due to the high degree of divergence in their calmodulin-like domains. In addition, there are two closely related PPCKs (phosphoenolpyruvate caboxylase kinases) and PPRKs (PPCK-related kinases) that have either no C-terminal domain or one with no significant similarity to EF hand–containing proteins.
What appears to be missing from Arabidopsis are potential homologs of a CaMK (calmodulin-dependent protein kinase) and protein kinase C, which represent the two major types of calcium-regulated kinases that function in animal systems (Satterlee and Sussman, 1998). In addition, an Arabidopsis homolog has not been found for a calcium/calmodulin-dependent protein kinase, a kinase identified in lilly (Patil et al., 1995) and tobacco (Liu et al., 1998) that can be regulated by both calmodulin (analogous to a CaMK) and a visinin-like regulatory domain (analogous to a CDPK).
There is evidence for cytosolic-, nuclear-, cytoskeletal-, and membrane-associated CDPKs (e.g., Schaller et al., 1992; Satterlee and Sussman, 1998; Martin and Busconi, 2000; Patharkar and Cushman, 2000; Romeis et al., 2000; Lu and Hrabak, 2002), raising the expectation that CDPKs could participate in regulating all aspects of cell physiology. While none of the CDPKs appear to be integral membrane proteins, 24 of the 34 Arabidopsis CDPKs have potential myristoylation sites at the beginning of their highly variable N-terminal domains. Myristoylation and palmitoylation at the N terminus have been implicated in CDPK–membrane associations (Ellard-Ivey et al., 1999; Martin and Busconi, 2000; Lu and Hrabak, 2002).
The potential for different CDPKs to sense and respond to different calcium spikes is provided by the functional divergence observed in the EF hands of different calmodulin-like regulatory domains. Evidence that such differences can influence calcium activation thresholds was provided by biochemical characterization of three different soybean isoforms (Lee et al., 1998). Each of the three isoforms displayed a different calcium threshold for half-maximal activation, with the greatest difference between two isoforms being more than ten-fold (using the same syntide-2 as protein substrate). Interestingly, the protein substrate itself (e.g., histone versus syntide-2) was also observed to change the calcium threshold by more than ten-fold. Furthermore, there is evidence that the calcium stimulation of some CDPKs is potentiated by putative lipid messengers (Harper et al., 1993; Binder et al., 1994; Farmer and Choi, 1999), interactions with 14-3-3 (Camoni et al., 1998), and phosphorylation by other kinases (Romeis et al., 2000, 2001). The emerging theme is that different CDPKs can be used to sense different calcium signals, and the specific activity of a given CDPK will depend on multiple factors, including its modification by other signaling pathways and its interaction with different downstream targets.
Insights into the physiological roles of CDPKs have come from three strategies: 1) identification of potential substrates, 2) in planta expression of recombinant CDPKs, and 3) in planta suppression of CDPK activity.
There is a growing list of potential CDPK substrates identified through approaches involving in vitro phosphorylation reactions (e.g., Weaver and Roberts, 1992), protein–protein interactions (Patharkar and Cushman, 2000), and consensus site predictions (e.g., Huang et al., 2001). Potential targets include important enzymes/proteins involved in carbon and nitrogen metabolism, such as nitrate reductase and sucrose synthase (Huber et al., 1996; Douglas et al., 1998); stress response pathways, including phenylalanine ammonia lyase (Cheng et al., 2001) and amino-1-cyclopropane carboxylate synthase (Huang et al., 2001); ion and water transport, such as aquaporins (Weaver and Roberts, 1992; Johansson et al., 1996; Huang et al., 2001), calcium pump ACA2 (Hwang et al., 2000), plasma membrane proton pump (Lino et al., 1998), chloride channel (Pei et al., 1996), and the KAT1 potassium channel (Li et al., 1998; Berkowitz et al., 2000); cytoskeleton, such as actin depolymerizing factor (Allwood et al., 2001) and myosin light chain (McCurdy and Harmon, 1992); and transcription, such as a pseudoresponse regulator (Patharkar and Cushman, 2000).
Evidence for a potential function of a CDPK came from the expression of a constitutively active CDPK in maize protoplasts (Sheen, 1996). In this case, a constitutively active version of isoform AtCPK10, but not isoform CPK1 or 11, triggered a signaling pathway, resulting in expression of reporters for drought and cold stress under the control of the ABA/stress regulated promoter HVA-1. To date, there have been no published reports on the stable expression of a constitutively active CDPK in transgenic plants.
However, a plant phenotype that resulted from overexpression of a wild-type CDPK has been reported. In this case, rice plants displayed improved drought, salt, and cold stress tolerance through the overexpression of OsCDPK7 (Saijo et al., 2000). There is also strong in vivo evidence for the potential role of CDPKs in controlling a pathogen response in tobacco (Romeis et al., 2001). In this case, suppression of NtCDPK2 and NtCDPK3 by viral-induced gene silencing reduced and delayed the Cf-9/Avr9–induced hypersensitive response and blocked an expected wilting phenotype. Together, these in planta studies highlight the potential to engineer plants with altered CDPKs to improve a plant's response to biotic and abiotic stress.
ASSIGNING SPECIFICITY TO CA2+ SIGNALS
Convergence and Specificity in Plant Ca2+ Signaling
Specific responses to qualitatively or quantitatively different stimuli can be brought about by a range of mechanisms. Most obviously, differentiation gives rise to cell types that are poised to respond differently by expressing different sensing or response elements. Indeed, cell type–specific responses to drought, salt, and cold have been shown in Arabidopsis roots by the use of cell type–specific enhancer trap targeting of aequorin (Kiegle et al., 2000b). At the single cell level, the involvement of Ca2+ in stimulus–response coupling raises the problem of how a single messenger can convey information for specific responses to a wide range of different stimuli (McAinsh and Hetherington, 1998). For example, both auxin and ABA can lead to elevation of Ca2+ in stomatal guard cells (Schroeder et al., 2001). However, auxin leads to stomatal opening and ABA leads to closure. The question of specificity becomes more complex when factors such as acclimation to prior stimuli are considered. For example, previous history of drought or cold stress can significantly affect the subsequent Ca2+ response of Arabidopsis seedlings, which may involve changes in the relative contributions of different cellular Ca2+ sources to the overall Ca2+ signal (Knight et al., 1998).
Plant cells also display signaling convergence, whereby a wide range of inputs can be integrated into a smaller number of outputs. In contrast to specificity, convergence involves activation of similar downstream elements in response to different stimuli. A good example is stomatal closure in response to light, CO2, and drought, in which Ca2+ signals appear to play a key role (Schroeder et al., 2001).
Calcium Signatures
Although the number of reported stimulus-specific changes in cellular Ca2+ (Ca2+ “signatures”) in plants has steadily increased (see Evans et al., 2001; Rudd and Franklin Tong, 2001 for recent reviews), relatively few of these have been categorically assigned with specific downstream responses. Indeed, certain changes in cellular Ca2+ may reflect perturbations of Ca2+ homeostasis that do not have any specific function (Plieth, 2001). Even where it has been shown that Ca2+ elevation is essential for a specific process, a signaling role may not be clear. In pollen tubes, for example, BAPTA buffer injections that abolish the apical Ca2+ gradient also lead to growth inhibition (Miller et al., 1992). Moreover, artificial elevation of Ca2+ at one side of the pollen tube apex leads to reorientation of growth (Malho et al., 1996). However, the direct role of Ca2+ as a specific signal in directing growth has been questioned by the observation that oscillations in apical Ca2+ lag behind corresponding growth oscillations (Messerli et al., 2000). Although there is compelling evidence for the widespread interaction between Ca2+-dependent and Ca2+-independent pathways in plant signaling (e.g., Jacob et al., 1999; Knight and Knight, 2001), it is also becoming clear that specific patterns of Ca2+ elevation alone can give rise to specific responses.
However, assigning function to a particular Ca2+ signal requires monitoring the response to a stimulus during inhibition of the Ca2+ signal and imposing the Ca2+ signal in the absence of a stimulus. In higher plant cells, this is often difficult, and specific manipulation of Ca2+ signals to directly affect a downstream response has been successful in only a limited number of cell types. In whole Arabidopsis seedlings responding to ozone treatment, a biphasic Ca2+ signature was shown to correlate with increased expression of the antioxidant enzyme glutathione S-transferase (GST; Clayton et al., 1999). La3+ pretreatment resulted in abolition of the second, but not the first, component of the Ca2+ elevation and also inhibited GST gene expression. Although the involvement of different cell types with different response kinetics to ozone cannot be ruled out in this response, the results point to a different function of each phase of the Ca2+ “signature.”
Since the demonstration that stomatal guard cells could exhibit oscillations or repetitive increases in cytosolic Ca2+ (McAinsh et al., 1995), significant progress has been made in dissecting their functional significance. A key to the generation of specific cytosolic Ca2+ signals may lie in the existence of multiple Ca2+ entry and efflux pathways, which themselves may be regulated by cytosolic Ca2+ (Harper, 2001). In stomatal guard cells, several of these components have been manipulated with consequent effects on the form of stimulus-induced changes in [Ca2+]c. In Commelina communis stomatal guard cells, inhibition of Ca2+ elevation by BAPTA buffer microinjection produced almost complete inhibition of ABA-induced stomatal closure (Webb et al., 2001). Transient Ca2+ elevations can be brought about in guard cells following activation of plasma membrane Ca2+ channels by hyperpolarization (Grabov and Blatt, 1998) or H2O2 treatment (Pei et al., 2000). The effects on stomatal aperture of manipulating Ca2+ oscillations by stimulation or inhibition of Ca2+ release by cADPR or InsP3 (Leckie et al., 1998; Staxen et al., 1999) suggest a clear involvement of these messengers but also indicate a degree of redundancy in the signaling pathways. Redundancy in guard cell Ca2+ signaling is also evident from work with mutants defective in the FIERY gene that encodes an inositol phosphatase. Fiery mutants have elevated levels of InsP3 yet show apparently normal stomatal regulation, based on measurements of transpirational water loss (Xiong et al., 2001). The roles in regulation of stomatal aperture of other molecules, such as NAADP (Navazio et al., 2000) and inositol hexakisphosphate (InsP6) (Lemitri-Chlieh et al., 2000), likely to be involved in eliciting [Ca2+]cyt elevations remain to be determined.
Further evidence for the response-dependence of specific patterns of Ca2+ elevation has come from the recent discovery that sphingosine-1-phosphate (S-1-P) induces Ca2+ oscillations in guard cells (Ng et al., 2001). The frequency and amplitude of these were dependent on the concentration of applied S-1-P, with corresponding effects on the kinetics of stomatal closure.
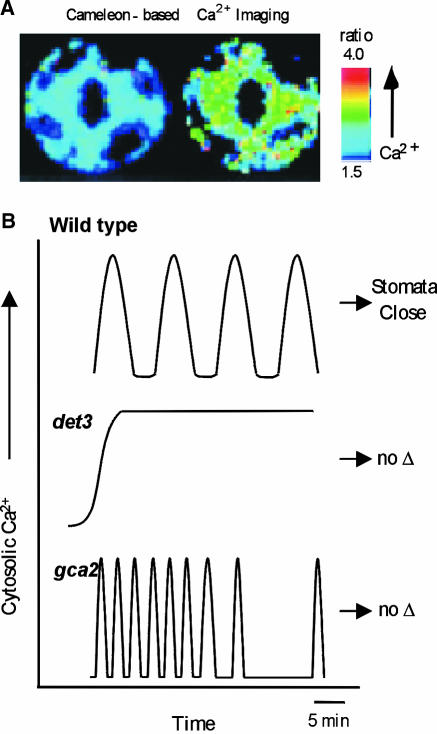
Over the past three years, further significant advances have been made in assigning specificity to Ca2+ “signatures” in guard cells by the use of ratiometic fluorescent protein Ca2+ indicators (cameleons) transformed into Arabidopsis guard cells (Allen et al., 1999). This work has combined cameleon technology with the use of Ca2+ homeostasis or signaling mutants to provide some exciting new insights. Thus, as shown in Figure 5, det3 mutants that are defective in a V-type H+-ATPase respond to elevated external Ca2+ or oxidative stress with prolonged Ca2+ elevations, unlike wild-type plants that show repetitive transient increases in Ca2+ (Allen et al., 2000). Moreover, unlike wild-type plants, stomata of the det3 mutants failed to close in response to these treatments. In contrast, cold or ABA treatments produced similar oscillatory Ca2+ increases and stomatal closure responses in det3 and wild-type plants. Significantly, imposing specific patterns of Ca2+ oscillations by repetitive membrane hyperpolarization and depolarization could induce stomatal closure in both the wild type and det3 mutants.
Figure 5.
The Use of Arabidopsis Mutants and New Imaging Techniques Leads to a Better Understanding of Calcium Signaling.
(A) Cameleon indicators expressed in guard cells allow a ratiometric measurement of cytosolic Ca2+ changes (low at left and high at right).
(B) Mutants display abnormal Ca2+ signals that fail to trigger stomatal closure (Allen et al., 2000, 2001). In wild type (top), a variety of hormone or stress treatments trigger a series of Ca2+ oscillations that are followed by a prolonged closure of the stomata. In contrast, a steady state rise in calcium was observed in det3 mutants (middle) in response to treatments such as high external Ca2+ or oxidative stress (but not ABA and cold). Treatments that failed to produce normal oscillations also failed to trigger a prolonged stomatal closure. The stomatal closure response was also disrupted in the mutant gca2 (bottom). In this case, calcium signals occurred as an abnormal “high frequency” pattern of calcium oscillations. These results provide genetic evidence to support the hypothesis that a specific frequency of Ca2+ oscillations is required for a normal stomatal closure response.
More recently, Allen et al. (2001) showed that specific patterns of Ca2+ elevation may be involved in controlling both the stomatal closure response and the final steady state stomatal aperture. Thus, in wild-type plants, elevations of cytosolic Ca2+ gave rise to “calcium-reactive” closure, but oscillations within a specific frequency range were required to program the duration of long-term closure. This work also made use of gca2 (growth controlled by abscisic acid) mutants that exhibited an increased frequency of Ca2+ oscillations in response to elevated extracellular Ca2+ or ABA compared with wild-type plants. The stomata of these mutants were unable to close in response to external Ca2+ or ABA (Figure 5). Imposing higher-frequency Ca2+ oscillations on wild-type stomata, similar to those occurring in gca2 mutants, produced only transient stomatal closure. Dissection of the Ca2+-regulated downstream elements involved in the initial and steady state closure is eagerly awaited.
Spatial Heterogeneity and Localization of Ca2+ Signals: Small Messages with Big Meanings
Although there is evidence that stomatal guard cell Ca2+ elevations can propagate across the cell as slow-moving waves of elevated Ca2+ (Grabov and Blatt, 1998; Webb et al., 2001), there is so far little evidence to suggest that spatial localization in specific regions of the cytosol plays a role in their function. In other cell types, however, Ca2+ elevations restricted to specific organelles or localized cytosolic regions may have specific signaling roles. By using nucleoplasmin-conjugated aequorin, van der Luit et al. (1999) were able to demonstrate separate nuclear and cytosolic Ca2+ elevations in tobacco seedlings in response to mechanical (wind) and cold shock, respectively. Interestingly, both types of Ca2+ signal resulted in increased expression of NpCAM1, suggesting convergence of these different Ca2+ signaling pathways. In a parallel study with tobacco suspension culture cells, Pauly et al. (2001) showed that different cytosolic and nuclear signals may be involved in discrimination between hyper- and hypo-osmotic treatments.
In embryos of the multicellular alga Fucus, different degrees of hypo-osmotic shock produced strikingly different spatial patterns of Ca2+ elevation in the rhizoid cell (Goddard et al., 2000). Mild hypo-osmotic treatments produced a sustained amplification of the Ca2+ gradient in the rhizoid apex. However, more-severe hypo-osmotic shocks produced a transient elevation of apical cytosolic Ca2+, lasting for only a few seconds, followed by a much larger elevation of Ca2+ that propagated as a bi-directional Ca2+ wave from the nuclear region. By using localized photorelease of caged InsP3 in apical or nuclear regions, Ca2+ elevations in the cell apex were shown to be involved in osmoregulation, whereas nuclear Ca2+ elevations acted to retard cell cycle progression. This work also provided clues about the mechanism of propagation of Ca2+ waves. Both apical and perinuclear Ca2+ waves propagated as elemental Ca2+ elevations characteristic of those described in a variety of animal cells (e.g., Haak et al., 2001; Wang et al., 2001). So far, no such elemental components of Ca2+ signals have been described for other plant cells. This may reflect, in part, the paucity of imaging studies of propagating Ca2+ signals in higher plants. The rapid elevations of Ca2+ in response to cold and osmotic shock in higher plant cells may provide useful experimental systems for high-resolution imaging of elemental Ca2+ elevations that may underlie the propagation of plant Ca2+ signals.
Spatially restricted Ca2+ signals also appear to underlie the response of leguminous root hairs to Nod factors. Recent work with mutants of Medicago (Wais et al., 2000) and pea (Walker et al., 2000) is beginning to suggest that patterns of Ca2+ spiking convey information that is critical for initiation of early nodulation events. In both systems, mutants that were defective in Ca2+ spiking were unable to elicit responses such as root hair deformation or expression of early nodulation (ENOD) genes. These mutants included sym8, sym10, and sym19 mutants of pea and the dmi1 and dmi2 mutants of Medicago. However, other mutants (sym2A, sym7, sym9, and sym30 of pea and dmi3 of Medicago) that were also defective in early nodulation responses did show normal Ca2+ spiking. Thus, two classes of genes (i.e., those that act to generate Ca2+ spiking and those that act downstream of Ca2+ spiking) seem to be required for the nodulation response. Although it is still not possible to distinguish between a direct role of Ca2+ in this response or a secondary effect of the nodulation process on Ca2+ homeostasis, these mutants provide essential tools for detailed dissection that should eventually allow this distinction to be made. Interestingly, certain pea nodulation mutants (sym8, sym19, and sym30) were also unable to form mycorrhizas while others (sym10) formed normal mycorrhizas. Because the sym10, sym8, and sym 19 mutants were defective in Ca2+ spiking, this could indicate that specific Ca2+ signals are exclusive to either nodulation or mycorrhiza formation, with sym10 being specifically involved in Nod factor signaling.
Finally, evidence is emerging from animal studies that stimulus-specific Ca2+ signals may occur without any detectable changes in cellular Ca2+ levels. It is well known that Ca2+ influx associated with filling of intracellular stores can occur without measurable alteration of cytosolic Ca2+ (Putney, 1999). Such capacitative Ca2+ entry is likely to be an essential process in the generation of repetitive Ca2+ elevations (Berridge, 1995). It has also been shown recently (Dolmetsch et al., 2001) that flux of Ca2+ through rat cortical neuron L-type voltage-gated Ca2+ channels that possess calmodulin binding domains can lead to nuclear cAMP response element binding protein phosphorylation via a mitogen-activated protein kinase pathway. The calmodulin binding domain was shown to be critical for conveying the Ca2+ signal to the nucleus. This exciting revelation suggests that a calmodulin binding region near the channel pore may serve not only to effect regulation of channel activity but also to relay information carried by Ca2+ influx specifically from that channel to downstream elements. It is therefore tempting to speculate a downstream signaling role for the calmodulin binding domain of CNGCs (Arazi et al., 2000). Given the variety of plant calmodulin isoforms (Snedden and Fromm, 2001), the potential for channel-specific signaling pathways becomes highly significant. Because entry through plasma membrane Ca2+-permeable channels is likely to lead to highly localized Ca2+ elevations (Marsault et al., 1997; Zou et al., 1999), a wide range of Ca2+-specific signaling processes may go undetected by current methods for monitoring cellular Ca2+.
FUTURE PROSPECTS
In most plant cells, the relative contributions of different sources (external, ER, or vacuole) of Ca2+ in the generation of specific Ca2+ signals is still not clear. Improvements in the use of targeted aequorin and cameleons, combined with improvements in spatio-temporal imaging of Ca2+ signals, will be required, together with identification at the molecular level, in plants, of the various Ca2+-permeable channels and the effects of their disruption. The range of protein-based fluorescent indicators for use in higher plants is likely to increase, providing a range of spectral properties, dynamic ranges, and kinetics for imaging Ca2+ in different environments. For example, insertions of calmodulin into green fluorescent protein derivatives, such as the newly developed “pericam” proteins, can allow fast line scan imaging of rapid, highly loclalised Ca2+ elevations (Nagai et al., 2001).
Highly significant findings on the roles of mitochondria in regulating the spatio-temporal patterns of Ca2+ signals have emerged from the use of targeted indicators in a variety of animal cells (e.g., Montero et al., 2000). Mitochondria have also been shown to play key roles in the patterning and propagation of Ca2+ waves (e.g., Haak et al., 2002). Even more surprising, by using cameleon indicators targeted to the cytosol, ER lumen, and mitochondrial matrix in HeLa cells, Arnaudeau et al. (2001) showed that the mitochondria played a critical role in recycling Ca2+ to the ER during histamine-induced Ca2+ signaling. Such approaches, together with wider application of high spatial and temporal resolution confocal and multiphoton imaging of fluorescent indicators, should go a long way toward elucidating the subcellular mechanisms that underlie the generation of specific Ca2+ signals. These approaches, completed with the wealth of mutants now available, will allow rigorous testing of hypotheses concerning the ways in which Ca2+ signals are coded and decoded.
Acknowledgments
We dedicate this review to the memory of our friend and colleague, Gethyn Allen.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.002899.
References
- Allen, G.J., Chu, S.P., Harrington, C.L., Schumacher, K., Hoffman, T., Tang, Y.Y., Grill, E., and Schroeder, J.I. (2001). A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411 1053–1057. [DOI] [PubMed] [Google Scholar]
- Allen, G.J., Chu, S.P., Schumacher, K., Shimazaki, C.T., Vafeados, D., Kemper, A., Hawke, S.D., Tallman, G., Tsien, R.Y., Harper, J.F., Chory, J., and Schroeder, J.I. (2000). Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289 2338–2342. [DOI] [PubMed] [Google Scholar]
- Allen, G.J., Kwak, J.M., Chu, S.P., Llopis, J., Tsien, R.Y., Harper, J.F., and Schroeder, J.I. (1999). Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J. 19 735–747. [DOI] [PubMed] [Google Scholar]
- Allen, G.J., and Sanders, D. (1995). Calcineurin, a type 2b protein phosphatase, modulates the Ca2+ permeable slow vacuolar ion channel of stomatal guard cells. Plant Cell 7 1473–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allwood, E.G., Smertenko, A.P., and Hussey, P.J. (2001). Phosphorylation of plant actin-depolymerising factor by calmodulin-like domain protein kinase. FEBS Lett. 499 97–100. [DOI] [PubMed] [Google Scholar]
- Anil, V.S., and Rao, K.S. (2001). Calcium-mediated signal transduction in plants: A perspective on the role of Ca2+ and CDPKs during early plant development. J. Plant Physiol. 158 1237–1256. [Google Scholar]
- Arazi, T., Kaplan, B., and Fromm, H. (2000). A high-affinity calmodulin-binding site in a tobacco plasma-membrane channel protein coincides with a characteristic element of cyclic nucleotide-binding domains. Plant Mol. Biol. 42 591–601. [DOI] [PubMed] [Google Scholar]
- Arazi, T., Sunkar, R., Kaplan, B., and Fromm, H. (1999). A tobacco plasma membrane calmodulin-binding transporter confers Ni2+ tolerance and Pb2+ hypersensitivity in transgenic plants. Plant J. 20 171–182. [DOI] [PubMed] [Google Scholar]
- Arnaudeau, S., Kelley, W.L., Walsh, J.V., and Demaurex, N. (2001). Mitochondria recycle Ca2+ to the endoplasmic reticulum and prevent the depletion of neighbouring endoplasmic reticulum regions. J. Biol. Chem. 276 29430–29439. [DOI] [PubMed] [Google Scholar]
- Axelsen, K.B., and Palmgren, M.G. (2001). Inventory of the superfamily of P-type ion pumps in Arabidopsis. Plant Physiol. 126 696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum, G., Long, J.C., Jenkins, G.I., and Trewavas, A.J. (1999). Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+. Proc. Natl. Acad. Sci. USA 96 13554–13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz, G., Zhang, X., Mercier, R., Leng, Q., and Lawton, M. (2000). Co-expression of calcium-dependent protein kinase with the inward rectified guard cell K+ channel KAT1 alters current parameters in Xenopus laevis oocytes. Plant Cell Physiol. 41 785–790. [DOI] [PubMed] [Google Scholar]
- Berridge, M.J. (1995). Capacitative calcium entry: Sifting through the evidence for CIF. Biochem. J. 314 1055–1056. [Google Scholar]
- Bewell, M.A., Maathuis, F.J.M., Allen, G.J., and Sanders, D. (1999). Calcium-induced calcium release mediated by a voltage-activated cation channel in vacuolar vesicles from red beet. FEBS Lett. 458 41–44. [DOI] [PubMed] [Google Scholar]
- Bhogal, M.S., and Colyer, J. (1998). Depletion of Ca2+ from the sarcoplasmic reticulum of cardiac muscle prompts phosphorylation of phospholamban to stimulate store refilling. Proc. Natl. Acad. Sci. USA 95 1484–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder, B.M., Harper, J.F., and Sussman, M.R. (1994). Characterization of an Arabidopsis calmodulin-like domain protein-kinase purified from Escherichia coli using an affinity sandwich technique. Biochem. US 33 2033–2041. [DOI] [PubMed] [Google Scholar]
- Blackford, S., Rea, P.A., and Sanders, D. (1990). Voltage sensitivity of H+/Ca2+ antiport in higher plant tonoplast suggests a role in vacuolar calcium accumulation. J. Biol. Chem. 265 9617–9620. [PubMed] [Google Scholar]
- Bonza, M.C., Morandini, P., Luoni, L., Geisler, M., Palmgren, M.G., and De Michelis, M.I.. (2000). At-ACA8 encodes a plasma membrane-localized calcium-ATPase of Arabidopsis with a calmodulin-binding domain at the N terminus. Plant Physiol. 123 1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler, C., Neuhaus, G., Yamagata, H., and Chua, N.-H. (1994). Cyclic-GMP and calcium mediate phytochrome transduction. Cell 77 73–81. [DOI] [PubMed] [Google Scholar]
- Camacho, P., and Lechleiter, J.D. (1993). Increased frequency of calcium waves in Xenopus laevis oocytes that express a calcium-ATPase. Science 260 226–229. [DOI] [PubMed] [Google Scholar]
- Camoni, L., Harper, J.F., and Palmgren, M.G. (1998). 14–3-3 proteins activate a plant calcium-dependent protein kinase (CDPK). FEBS Lett. 430 381–384. [DOI] [PubMed] [Google Scholar]
- Carpaneto, A., Cantu, A.M., and Gambale, F. (2001). Effects of cytoplasmic Mg2+ on slowly activating channels in isolated vacuoles of Beta vulgaris. Planta 213 457–468. [DOI] [PubMed] [Google Scholar]
- Cheng, S.H., Sheen, J., Gerrish, C., and Bolwell, G.P. (2001). Molecular identification of phenylalanine ammonia-lyase as a substrate of a specific constitutively active Arabidopsis CDPK expressed in maize protoplasts. FEBS Lett. 503 185–188. [DOI] [PubMed] [Google Scholar]
- Clayton, H., Knight, M.R., Knight, H., McAinsh, M.R., and Hetherington, A.M. (1999). Dissection of the ozone-induced calcium signature. Plant J. 17 575–579. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., Fengler, K.A., Yu, I.C., Lippok, B., Smith, R.K., and Bent, A.F. (2000). The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc. Natl. Acad. Sci. USA 97 9323–9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik, V., Davenport, R.N., and Tester, M. (2002). Non-selective cation channels in plants. Annu. Rev. Plant Mol. Biol. Plant Physiol. 53 67–107. [DOI] [PubMed] [Google Scholar]
- Demidchik, V., and Tester, M. (2002). Sodium fluxes through nonselective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiol. 128 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison, K.L., and Spalding, E.P. (2000). Glutamate-gated calcium fluxes in Arabidopsis. Plant Physiol. 124 1511–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWald, D.B., Torabinejad, J., Jones, C.A., Shope, J.C., Cangelosi, A.R., Thompson, J.E., Prestwich, G.D., and Hama, H. (2001). Rapid accumulation of phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate correlates with calcium mobilization in salt-stressed Arabidopsis. Plant Physiol. 126 759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch, R.E., Pajvani, U., Fife, K., Spotts, J.M., and Greenberg, M.E. (2001). Signaling to the nucleus by an L-type calcium channel complex through the MAP kinase pathway. Science 294 333–339. [DOI] [PubMed] [Google Scholar]
- Douglas, P., Moorhead, G., Hong, Y., Morrice, N., and MacKintosh, C. (1998). Purification of a nitrate reductase kinase from Spinacea oleracea leaves, and its identification as a calmodulin-domain protein kinase. Planta 206 435–442. [DOI] [PubMed] [Google Scholar]
- Drobak, B.K., and Watkins, P.A.C. (2000). Inositol (1,4,5) trisphosphate production in plant cells: An early response to salinity and hyperosmotic stress. FEBS Lett. 481 240–244. [DOI] [PubMed] [Google Scholar]
- Durner, J., Wendehenne, D., and Klessig, D.F. (1998). Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 95 10328–10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durr, G., Strayle, J., Plemper, R., Elbs, S., Klee, S.K., Catty, P., Wolf, D.H., and Rudolph, H.K. (1998). The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell 9 1149–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East, J.M. (2000). Sarco(endo)plasmic reticulum calcium pumps: Recent advances in our understanding of structure/function and biology. Mol. Membr. Biol. 17 189–200. [DOI] [PubMed] [Google Scholar]
- Ellard-Ivey M, Hopkins, R.B., White, T.J., and Lomax, T.L.. (1999). Cloning, expression and N-terminal myristoylation of CpCPK1, a calcium- dependent protein kinase from zucchini (Cucurbita pepo L.). Plant Mol. Biol. 39 199–208. [DOI] [PubMed] [Google Scholar]
- Evans, N.H., McAinsh, M.R., and Hetherington, A.M. (2001). Calcium oscillations in higher plants. Curr. Opin. Plant Biol. 4 415–420. [DOI] [PubMed] [Google Scholar]
- Farmer, P.K., and Choi, J.H. (1999). Calcium and phospholipid activation of a recombinant calcium-dependent protein kinase (DcCPK1) from carrot (Daucus carota L.). Biochim. Biophys. Acta 1434 6–17. [DOI] [PubMed] [Google Scholar]
- Ferrol, N., and Bennett, A.B. (1996). A single gene may encode differentially localized Ca2+-ATPases in tomato. Plant Cell 8 1159–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohnmeyer, H., Loyall, L., Blatt, M.R., and Grabov, A. (1999). A millisecond UV-B irradiation evokes prolonged elevation of cytosolic-free Ca2+ and stimulates gene expression in transgenic parsley cell cultures. Plant J. 20 109–117. [DOI] [PubMed] [Google Scholar]
- Furuichi, T., Cunningham, K.W., and Muto, S. (2001). A putative two-pore channel AtTPC1 mediates Ca2+ flux in Arabidopsis leaf cells. Plant Cell Physiol. 42 900–905. [DOI] [PubMed] [Google Scholar]
- Furumoto, T., Ogawa, N., Hata, S., and Izui, K. (1996). Plant calcium-dependent protein kinase-related kinases (CRKs) do not require calcium for their activities. FEBS Lett. 396 147–151. [DOI] [PubMed] [Google Scholar]
- Geisler, M., Axelsen, K.B., Harper, J.F., and Palmgren, M.G. (2000. a). Molecular aspects of higher plant P-type Ca(2+)-ATPases. Biochim. Biophys. Acta 1465 52–78. [DOI] [PubMed] [Google Scholar]
- Geisler, M., Frangne, N., Gomes, E., Martinoia, E., and Palmgren, M.G. (2000. b). The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol. 124 1814–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelli, A., and Blumwald, E. (1997). Hyperpolarization-activated Ca2+ permeable channels in the plasma membrane of tomato cells. J. Membr. Biol. 155 35–45. [DOI] [PubMed] [Google Scholar]
- Gelli, A., Higgins, V.J., and Blumwald, E. (1997). Activation of plant plasma membrane Ca2+permeable channels by race-specific fungal elicitors. Plant Physiol. 113 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard, H., Manison, N.F.H., Tomos, D., and Brownlee, C. (2000). Elemental propagation of calcium signals in response-specific patterns determined by environmental stimulus strength. Proc. Natl. Acad. Sci. USA 97 1932–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov, A., and Blatt, M.R. (1998). Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proc. Natl. Acad. Sci. USA 95 4778–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guern, J., Felle, H., Mathieu, Y., and Kurkdjian, A. (1991). Regulation of intracellular pH in plant-cells. Int. Rev. Cyt. 127 111–173. [Google Scholar]
- Haak, L.L., Grimaldi, M., Smaili, S.S., and Russell, J.T. (2002). Mitochondria regulate Ca2+ wave initiation and inositol trisphosphate signal transduction in oligodendrocyte progenitors. J. Biol. Chem. 80 405–415. [DOI] [PubMed] [Google Scholar]
- Haak, L.L., Song, L.S., Molinski, T.F., Pessah, I.N., Cheng, H.P., and Russell, J.T. (2001). Sparks and puffs in oligodendrocyte progenitors: Crosstalk between ryanodine receptors and inositol trisphosphate receptors. J. Neurosci. 21 3860–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter, U., Ishitani, M., and Zhu, J.K. (2000). The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc. Natl. Acad. Sci. USA 97 3735–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, D.W.A., Hills, A., Kohler, B., and Blatt, M.R. (2000). Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc. Natl. Acad. Sci. USA 97 4967–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon, A.C., Gribskov, M., and Harper, J.F. (2000). CDPKs—a kinase for every Ca2+ signal? Trends Plant Sci. 5 154–159. [DOI] [PubMed] [Google Scholar]
- Harper, J.F. (2001). Dissecting calcium oscillators in plant cells. Trends Plant Sci. 6 395–397. [DOI] [PubMed] [Google Scholar]
- Harper, J.F., Binder, B.M., and Sussman, M.R. (1993). Calcium and lipid regulation of an Arabidopsis protein-kinase expressed in Escherichia coli. Biochem. US 32 3282–3290. [DOI] [PubMed] [Google Scholar]
- Harper, J.F., Sussman, M.R., Schaller, G.E., Putnam-Evans, C., Charbonneau, H., and Harmon, A.C. (1991). A calcium-dependent protein kinase with a regulatory domain similar to calmodulin. Science 252 951–954. [DOI] [PubMed] [Google Scholar]
- Hedrich, R., and Neher, E. (1987). Cytoplasmic calcium regulates voltage dependent ion channels in plant vacuoles. Nature 329 833–836. [Google Scholar]
- Hirschi, K. (2001). Vacuolar H+/Ca2+ transport: Who's directing the traffic? Trends Plant Sci. 6 100–104. [DOI] [PubMed] [Google Scholar]
- Hirschi, K.D. (1999). Expression of Arabidopsis CAX1 in tobacco: Altered calcium homeostasis and increased stress sensitivity. Plant Cell 11 2113–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi, K.D., Korenkov, V.D., Wilganowski, N.L., and Wagner, G.J. (2000). Expression of Arabidopsis CAX2 in tobacco. Altered metal accumulation and increased manganese tolerance. Plant Physiol. 124 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi, K.D., Zhen, R.G., Cunningham, K.W., Rea, P.A., and Fink, G.R. (1996). CAX1, an H+/Ca2+ antiporter from Arabidopsis. Proc. Natl. Acad. Sci. USA 93 8782–8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, B., Ichida, A., Wang, Y., Gens, J.S., Pickard, B.G., and Harper, J.F. (1999). Identification of a calmodulin-regulated Ca2+-ATPase in the endoplasmic reticulum. Plant Physiol. 119 1165–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J.Z., Hardin, S.C., and Huber, S.C. (2001). Identification of a novel phosphorylation motif for CDPKs: Phosphorylation of synthetic peptides lacking basic residues at P-3/P-4. Arch. Biochem. Biophys. 393 61–66. [DOI] [PubMed] [Google Scholar]
- Huang, L., Berkelman, T., Franklin, A.E., and Hoffman, N.E. (1993). Characterization of a gene encoding a Ca2+-ATPase-like protein in the plastid envelope. Proc. Natl. Acad. Sci. USA 90 10066–10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, S.C., Huber, J.L., Liao, P.C., Gage, D.A., McMichael, R.W., Jr., Chourey, P.S., Hannah, L.C., and Koch, K. (1996). Phosphorylation of serine-15 of maize leaf sucrose synthase. Occurrence in vivo and possible regulatory significance. Plant Physiol. 112 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, I., Sze, H., and Harper, J.F. (2000). A calcium-dependent protein kinase can inhibit a calmodulin-stimulated Ca2+ pump (ACA2) located in the endoplasmic reticulum of Arabidopsis. Proc. Natl. Acad. Sci. USA 97 6224–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob, T., Ritchie, S., Assmann, S.M., and Gilroy, S. (1999). Abscisic acid signal transduction in guard cells is mediated by phospholipase D activity. Proc. Natl. Acad. Sci. 96 12192–12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, X.C., and Wu, W.H. (1999). Involvement of cyclic AMP in ABA- and Ca2+-mediated signal transduction of stomatal regulation in Vicia faba. Plant Cell Physiol. 40 1127–1133. [Google Scholar]
- Johansson, I., Larsson, C., Ek, B., and Kjellbom, P. (1996). The major integral proteins of spinach leaf plasma membranes are putative aquaporins and are phosphorylated in response to Ca2+ and apoplastic water potential. Plant Cell 8 1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai, M., and Muto, S. (1990). Ca2+ pump and Ca2+/H+ antiporter in plasma membrane vesicles isolated by aqueous two-phase partitioning from corn leaves. J. Membr. Biol. 114 133–142. [DOI] [PubMed] [Google Scholar]
- Kiegle, E., Gilliham, M., Haseloff, J., and Tester, M. (2000. a). Hyperpolarization-activated calcium currents found only in cells from the elongation zone of Arabidopsis thaliana roots. Plant J. 21 225–229. [DOI] [PubMed] [Google Scholar]
- Kiegle, E., Moore, C.A., Haseloff, J., Tester, M.A., and Knight, M.R. (2000. b). Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J. 23 267–278. [DOI] [PubMed] [Google Scholar]
- Kim, S.A., Kwak, J.M., Jae, S.K., Wang, M.H., and Nam, H.G. (2001). Overexpression of the AtGluR2 gene encoding an Arabidopsis homolog of mammalian glutamate receptors impairs calcium utilization and sensitivity to ionic stress in transgenic plants. Plant Cell Physiol. 42 74–84. [DOI] [PubMed] [Google Scholar]
- Klusener, B., Boheim, G., Liss, H., Engelberth, J., and Weiler, E.W. (1995). Gadolinium-sensitive, voltage-dependent calcium-release channels in the endoplasmic-reticulum of a higher-plant mechanoreceptor organ. EMBO J. 14 2708–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusener, B., and Weiler, E.W. (1999). A calcium-selective channel from root-tip endomembranes of garden cress. Plant Physiol. 119 1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, H. (2000). Calcium signaling during abiotic stress in plants. Int. Rev. Cytol. 195 269–324. [DOI] [PubMed] [Google Scholar]
- Knight, H., Brandt, S., and Knight, M.R. (1998). A history of drought stress alters calcium signalling pathways in Arabidospsis. Plant J. 16 681–687. [DOI] [PubMed] [Google Scholar]
- Knight, H., and Knight, M.R. (2001). Abiotic stress signalling pathways: Specificity and cross-talk. Trends Plant Sci. 6 262–267. [DOI] [PubMed] [Google Scholar]
- Kohler, C., Merkle, T., Roby, D., and Neuhaus, G. (2001). Developmentally regulated expression of a cyclic nucleotide-gated ion channel from Arabidopsis indicates its involvement in programmed cell death. Planta 213 327–332. [DOI] [PubMed] [Google Scholar]
- Kohler, C., and Neuhaus, G. (2000). Characterisation of calmodulin binding to cyclic nucleotide-gated ion channels from Arabidopsis thaliana. FEBS Lett. 471 133–136. [DOI] [PubMed] [Google Scholar]
- Lacombe, B., et al. (2001). The identity of plant glutamate receptors. Science 292 1486–1487. [DOI] [PubMed] [Google Scholar]
- Lechleiter, J.D., John, L.M., and Camacho, P. (1998). Ca2+ wave dispersion and spiral wave entrainment in Xenopus laevis oocytes overexpressing Ca2+ ATPases. Biophys. Chem. 72 123–129. [DOI] [PubMed] [Google Scholar]
- Leckie, C.P., McAinsh, M.R., Allen, G.J., Sanders, D., and Hetherington, A.M. (1998). Abscisic acid-induced stomatal closure mediated by cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 95 15837–15842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.Y., Yoo, B.C., and Harmon, A.C. (1998). Kinetic and calcium-binding properties of three calcium-dependent protein kinase isoenzymes from soybean. Biochemistry 37 6801–6809. [DOI] [PubMed] [Google Scholar]
- Lemitri-Chlieh, F., MacRobbie, E.A.C., and Brearley, C.A. (2000). Inositol hexakisphosphate is a physiological signal regulating K+-inward rectifying conductance in guard cells. Proc. Natl. Acad. Sci. USA 97 8687–8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng, Q., Mercier, R.W., Hua, B.G., Fromm, H., and Berkowitz, G.A. (2002). Electrophysiological analysis of cloned cyclic nucleotide-gated ion channels. Plant Physiol. 128 400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng, Q., Mercier, R.W., Yao, W.Z., and Berkowitz, G.A. (1999). Cloning and first functional characterization of a plant cyclic nucleotide-gated cation channel. Plant Physiol. 121 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Lee, Y.R., and Assmann, S.M. (1998). Guard cells possess a calcium-dependent protein kinase that phosphorylates the KAT1 potassium channel. Plant Physiol. 116 785–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, F., Cunningham, K.W., Harper, J.F., and Sze, H. (1997). ECA1 complements yeast mutants defective in Ca2+ pumps and encodes an endoplasmic reticulum-type Ca2+-ATPase in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 94 8579–8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lino, B., Baizabal-Aguirre, V.M., and de la Vara, L.E.G. (1998). The plasma-membrane H+-ATPase from beet root is inhibited by a calcium-dependent phosphorylation. Planta 204 352–359. [DOI] [PubMed] [Google Scholar]
- Liu, Z.H., Xia, M., and Poovaiah, B.W. (1998). Chimeric calcium/calmodulin-dependent protein kinase in tobacco: Differential regulation by calmodulin isoforms. Plant Mol. Biol. 38 889–897. [DOI] [PubMed] [Google Scholar]
- Lu, S.X., and Hrabak, E.M. (2002). An Arabidopsis calcium-dependent protein kinase is associated with the endoplasmic reticulum. Plant Physiol. 128 1008–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan, S. Kudla, J., Rodriguez-Concepcion, M., Yalovsky, S., and Gruissem, S. (2002). CaMs and CBLs: Calcium sensors for specific signal-response coupling in plants. Plant Cell 14 (suppl.), S389–S400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis, F.J.M., and Sanders, D. (2001). Sodium uptake in Arabidopsis roots is regulated by cyclic nucleotides. Plant Physiol. 127 1617–1625. [PMC free article] [PubMed] [Google Scholar]
- Malho, R., and Trewavas, A.J. (1996). Localized apical increases of cytosolic free calcium control pollen tube orientation. Plant Cell 8 1935–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsault, R., Murgia, M., Pozzan, T., and Rizzuto, R. (1997). Domains of high Ca2+ beneath the plasma membrane of living A7r5 cells. EMBO J. 16 1575–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, M.L., and Busconi, L. (2000). Membrane localization of a rice calcium-dependent protein kinase (CDPK) is mediated by myristoylation and palmitoylation. Plant J. 24 429–435. [DOI] [PubMed] [Google Scholar]
- Martinec, J., Feltl, T., Scanlon, C.H., Lumsden, P.J., and Machackova, I. (2000). Subcellular localization of a high affinity binding site for D-myo-inositol 1,4,5-trisphosphate from Chenopodium rubrum. Plant Physiol. 124 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser, P., et al. (2001). Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 126 1646–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh, M.R., and Hetherington, A.M. (1998). Encoding specificity in Ca2+ signalling systems. Trends Plant Sci. 3 32–36. [Google Scholar]
- McAinsh, M.R., Webb, A.A.R., Taylor, J.E., and Hetherington, A.M. (1995). Stimulus-induced oscillations in guard cell cytoplasmic free calcium. Plant Cell 7 1207–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy, D.W., and Harmon, A.C. (1992). Phosphorylation of a putative myosin light chain in Chara by calcium-dependent protein kinase. Protoplasma 171 85–88. [Google Scholar]
- Messerli, M.A., Creton, R., Jaffe, L.F., and Robinson, K.R. (2000). Periodic increases in elongation rate precede increases in cytosolic Ca2+ during pollen tube growth. Dev. Biol. 222 84–98. [DOI] [PubMed] [Google Scholar]
- Miller, D., Callaham, D.A., Gross, D.J., and Hepler, P.K. (1992). Free calcium gradient in growing pollen tubes of Lilium. J. Cell Sci. 101 7–12. [Google Scholar]
- Mogami, H., Tepikin, A.V., and Petersen, O.H. (1998). Termination of cytosolic Ca2+ signals: Ca2+ reuptake into intracellular stores is regulated by the free Ca2+ concentration in the store lumen. EMBO J. 17 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero, M., Alonso, M.T., Carnicero, E., Cuchillo-Ibanez, I., Albillos, A., Garcia, A.G., Garcia-Sancho, J., and Alvarez, J. (2000). Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat. Cell Biol. 2 57–61. [DOI] [PubMed] [Google Scholar]
- Moutinho, A., Hussey, P.J., Trewavas, A.J., and Malho, R. (2001). cAMP acts as a second messenger in pollen tube growth and reorientation. Proc. Natl. Acad. Sci. USA 98 10481–10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munne-Bosch, S., and Alegre, L. (2002). The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 21 31–57. [Google Scholar]
- Murata, Y., Pei, Z.M., Mori, I.C., and Schroeder, J. (2001). Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1–1 and abi2–1 protein phosphatase 2C mutants. Plant Cell 13 2513–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai, T., Sawano, A., Park, E.S., and Miyawaki, A. (2001). Circularly permuted green fluorescent proteins engineered to sense Ca2+. Proc. Natl. Acad. Sci. USA 98 3197–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazio, L., Bewell, M.A., Siddiqua, A., Dickinson, G.D., Galione, A., and Sanders, D. (2000). Calcium release from the endoplasmic reticulum of higher plants elicited by the NADP metabolite nicotinic acid adenine dinucleotide phosphate. Proc. Natl. Acad. Sci. USA 97 8693–8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazio, L., Mariani, P., and Sanders, D. (2001). Mobilization of Ca2+ by cyclic ADP-ribose from the endoplasmic reticulum of cauliflower florets. Plant Physiol. 125 2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton, R.P., Roef, L., Witters, E., and Van Onckelen, H. (1999). Cyclic nucleotides in higher plants: The enduring paradox. New Phytol. 143 427–455. [DOI] [PubMed] [Google Scholar]
- Ng, C.K.Y., Carr, K., McAinsh, M.R., Powell, B., and Hetherington, A.M. (2001). Drought-induced guard cell signal transduction involves sphingosine-1-phosphate. Nature 410 596–598. [DOI] [PubMed] [Google Scholar]
- Ng, C.K.Y., and Hetherington, A.M. (2001). Sphingolipid-mediated signalling in plants. Ann. Bot. London 88 957–965. [Google Scholar]
- Nurnberger, T., and Scheel, D. (2001). Signal transmission in the plant immune response. Trends Plant Sci. 6 372–379. [DOI] [PubMed] [Google Scholar]
- Patharkar, O.R., and Cushman, J.C. (2000). A stress-induced calcium-dependent protein kinase from Mesembryanthemum crystallinum phosphorylates a two-component pseudo-response regulator. Plant J. 24 679–691. [DOI] [PubMed] [Google Scholar]
- Patil, S., Takezawa, D., and Poovaiah, B.W. (1995). Chimeric plant calcium/calmodulin-dependent protein-kinase gene with a neural visinin-like calcium-binding domain. Proc. Natl. Acad. Sci. USA 92 4897–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly, N., Knight, M.R., Thuleau, P., Graziana, A., Muto, S., Ranjeva, R., and Mazars, C. (2001). The nucleus together with the cytosol generates patterns of specific cellular calcium signatures in tobacco suspension culture cells. Cell Calcium 30 413–421. [DOI] [PubMed] [Google Scholar]
- Pei, Z.M., Murata, Y., Benning, G., Thomine, S., Klusener, B., Allen, G.J., Grill, E., and Schroeder, J.I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406 731–734. [DOI] [PubMed] [Google Scholar]
- Pei, Z.M., Ward, J.M., Harper, J.F., and Schroeder, J.I. (1996). A novel chloride channel in Vicia faba guard cell vacuoles activated by the serine/threonine kinase, CDPK. EMBO J. 15 6564–6574. [PMC free article] [PubMed] [Google Scholar]
- Pei, Z.M., Ward, J.M., and Schroeder, J.I. (1999). Magnesium sensitizes slow vacuolar channels to physiological cytosolic calcium and inhibits fast vacuolar channels in fava bean guard cell vacuoles. Plant Physiol. 121 977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera, I.Y., Heilmann, I., and Boss, W.F. (1999). Transient and sustained increases in inositol 1,4,5-hisphosphate precede the differential growth response in gravistimulated maize pulvini. Proc. Natl. Acad. Sci. USA 96 5838–5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman, J.K., and Hirschi, K.D. (2001). Regulation of CAX1, an Arabidopsis Ca2+/H+ antiporter. Identification of an N-terminal autoinhibitory domain. Plant Physiol. 127 1020–1029. [PMC free article] [PubMed] [Google Scholar]
- Plieth, C. (2001). Plant calcium signaling and monitoring: Pros and cons and recent experimental approaches. Protoplasma 218 1–23. [DOI] [PubMed] [Google Scholar]
- Pottosin, I.I., Tikhonova, L.I., Hedrich, R., and Schonknecht, G. (1997). Slowly activated vacuolar channels cannot mediate Ca2+-induced release. Plant J. 12 1387–1398. [Google Scholar]
- Putney, J.W., Jr. (1999). “Kissin' cousins”: Intimate plasma membrane-ER interactions underlie capacitative calcium entry. Cell 99 5–8. [DOI] [PubMed] [Google Scholar]
- Roderick, H.L., Lechleiter, J.D., and Camacho, P. (2000). Cytosolic phosphorylation of calnexin controls intracellular Ca2+ oscillations via an interaction with SERCA2b. J. Cell Biol. 149 1235–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis, T., Ludwig, A.A., Martin, R., and Jones, J.D.G. (2001). Calcium-dependent protein kinases play an essential role in a plant defense response. EMBO J. 20 5556–5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis, T., Piedras, P., and Jones, J.D.G. (2000). Resistance gene-dependent activation of a calcium-dependent protein kinase in the plant defense response. Plant Cell 12 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd, J.J., and Franklin-Tong, V.E. (2001). Unravelling response-specificity in Ca2+ signalling pathways in plant cells. New Phytol. 151 7–33. [DOI] [PubMed] [Google Scholar]
- Saijo, Y., Hata, S., Kyozuka, J., Shimamoto, K., and Izui, K. (2000). Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J. 23 319–327. [DOI] [PubMed] [Google Scholar]
- Sanders, D., Brownlee, C., and Harper, J.F. (1999). Communicating with calcium. Plant Cell 11 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterlee, J.S., and Sussman, M.R. (1998). Unusual membrane-associated protein kinases in higher plants. J. Membr. Biol. 164 205–213. [DOI] [PubMed] [Google Scholar]
- Schaller, G.E., Harmon, A.C., and Sussman, M.R. (1992). Characterization of a calcium-dependent and lipid-dependent protein kinase associated with the plasma membrane of oat. Biochemistry 31 1721–1727. [DOI] [PubMed] [Google Scholar]
- Schroeder, J.I., Allen, G.J., Hugouvieux, V., Kwak, J., and Waner, D. (2001). Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52 627–658. [DOI] [PubMed] [Google Scholar]
- Schumaker, K.S., and Sze, H. (1986). Calcium transport into the vacuole of oat roots. Characterization of H+/Ca2+ exchange activity. J. Biol. Chem. 261 12172–12178. [PubMed] [Google Scholar]
- Shacklock, P.S., Read, N.D., and Trewavas, A.J. (1992). Cytosolic free calcium mediates red light induced photomorphogenesis. Nature 358 153–155. [Google Scholar]
- Sheen, J. (1996). Ca2+-dependent protein kinases and stress signal transduction in plants. Science 274 1900–1902. [DOI] [PubMed] [Google Scholar]
- Shigaki, T., Cheng, N.H., Pittman, J.K., and Hirschi, K. (2001). Structural determinants of Ca2+ transport in the Arabidopsis H+/Ca2+ antiporter CAX1. J. Biol. Chem. 276 43152–43159. [DOI] [PubMed] [Google Scholar]
- Snedden, W.A., and Fromm, H. (2001). Calmodulin as a versatile calcium signal transducer in plants. New Phytol. 151 35–66. [DOI] [PubMed] [Google Scholar]
- Staxen, I., Pical, C., Montogomery, L.T., Gray, J.E., Hetherington, A.M., and McAinsh, M.R. (1999). Abscisic acid induces oscillations in guard cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc. Natl. Acad. Sci. USA 96 1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah, C.C., and Sachs, M.M. (2000). Maize cap1 encodes a novel SERCA-type calcium-ATPase with a calmodulin-binding domain. J. Biol. Chem. 275 21678–21687. [DOI] [PubMed] [Google Scholar]
- Suen, K.L., and Choi, J.H. (1991). Isolation and sequence analysis of a cDNA clone for a carrot calcium-dependent protein kinase: Homology to calcium/calmodulin-dependent protein kinases and to calmodulin. Plant Mol. Biol. 17 581–590. [DOI] [PubMed] [Google Scholar]
- Sunkar, R., Kaplan, B., Bouche, N., Arazi, T., Dolev, D., Talke, I.N., Maathuis, F.J.M., Sanders, D., Bouchez, D., and Fromm, H. (2000). Expression of a truncated tobacco NtCBP4 channel in transgenic plants and disruption of the homologous Arabidopsis CNGC1 gene confer Pb2+ tolerance. Plant J. 24 533–542. [DOI] [PubMed] [Google Scholar]
- Sze, H., Liang, F., Hwang, I., Curran, A.C., and Harper, J.F. (2000). Diversity and regulation of plant Ca2+ pumps: Insights from expression in yeast. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51 433–462. [DOI] [PubMed] [Google Scholar]
- Van Breusegem, F., Vranova, E., Dat, J.F., and Inze, D. (2001). The role of active oxygen species in plant signal transduction. Plant Sci. 131 405–414. [Google Scholar]
- van den Wijngaard, P.W.J., Bunney, T.D., Roobeek, I., Chonknecht, G., and de Boer, A.H. (2001). Slow vacuolar channels from barley mesophyll cells are regulated by 14–3-3 proteins. FEBS Lett. 488 100–104. [DOI] [PubMed] [Google Scholar]
- van der Luit, A.H., Olivari, C., Haley, A., Knight, M.R., and Trewavas, A.J. (1999). Distinct calcium signaling pathways regulate calmodulin gene expression in tobacco. Plant Physiol. 121 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry, A.A., and Davies, J.M. (2000). Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc. Natl. Acad. Sci. USA 97 9801–9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volotovski, I.D., Sokolovsky, S.G., Molchan, O.V., and Knight, M.R. (1998). Second messengers mediate increases in cytosolic calcium in tobacco protoplasts. Plant Physiol. 117 1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wais, R.J., Galera, C., Oldroyd, G., Catoria, R., Penmetsa, R.V., Cook, D., Gough, C., Denarie, J., and Long, S.R. (2000). Genetic analysis of calcium spiking responses in nodulation mutants of Medicago trunculata. Proc. Natl. Acad. Sci. USA 97 13407–13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, S.A., Viprey, V., and Downie, J.A. (2000). Dissection of nodulation signaling using pea mutants defective for calcium spiking induced by Nod factors and chitin oligomers. Proc. Natl. Acad. Sci. USA 97 13413–13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S.Q., Song, L.S., Lakatta, E.G., and Cheng, H.P. (2001). Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature 410 592–596. [DOI] [PubMed] [Google Scholar]
- Ward, J.M., Pei, Z.-M., and Schroeder, J.I. (1995). Roles of ion channels in initiation of signal transduction in higher plants. Plant Cell 7 833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, J.M., and Schroeder, J.I. (1994). Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell 6 669–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver, C.D., and Roberts, D.M. (1992). Determination of the site of phosphorylation of nodulin-26 by the calcium-dependent protein-kinase from soybean nodules. Biochemistry 31 8954–8959. [DOI] [PubMed] [Google Scholar]
- Webb, A.A.R., Larman, M.G., Montgomery, L.T., Taylor, J.E., and Hetherington, A.M. (2001). The role of calcium in ABA-induced gene expression and stomatal movements. Plant J. 26 351–362. [DOI] [PubMed] [Google Scholar]
- White, P.J. (2000).Calcium channels in higher plants. Biochim. Biophys. Acta 1465 171–189. [DOI] [PubMed] [Google Scholar]
- Wood, N.T., Allan, A.C., Haley, A., Viry-Moussaid, M., and Trewavas, A.J. (2000). The characterization of differential calcium signalling in tobacco guard cells. Plant J. 24 335–344. [DOI] [PubMed] [Google Scholar]
- Wu, Y., Kuzma, J., Marechal, E., Graeff, R., Lee, H.C., Forster, R., and Chua, N.H. (1997). Abscissic acid signaling through cyclic ADP-ribose in plants. Science 278 2126–2130. [DOI] [PubMed] [Google Scholar]
- Xiong, L., Lee, B.-H., Ishitani, M., Zhang, C., and Zhu, J.-K. (2001). FIERY1 encoding and inositol polyphosphate 1-phosphatase is a negative regulator of abscisic acid and stress signaling in Arabidopsis. Genes Dev. 15 1971–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X.S., and Choi, J.H. (2001). Molecular evolution of calmodulin-like domain protein kinases (CDPKs) in plants and protists. J. Mol. Evol. 53 214–224. [DOI] [PubMed] [Google Scholar]
- Zhang, X., Zhang, L., Dong, F.C., Gao, J.F., Galbraith, D.W., and Song, C.P. (2001). Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 126 1438–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y., Kappes, B., and Franklin, R.M. (1993). Gene structure and expression of an unusual protein kinase from Plasmodium falciparum homologous at its carboxyl terminus with the EF hand calcium-binding proteins. J. Biol. Chem. 268 4347–4354. [PubMed] [Google Scholar]
- Zou, H., Lieshitz, L.M., Tuft, R.A., Fogarty, K.E., and Singer, J.J. (1999). Imaging Ca2+ entering the cytoplasm through a single opening of a plasma membrane cation channel. J. Gen. Physiol. 114 575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]