Abstract
The structures of membrane receptors mediating rapid, nongenomic actions of steroids have not been identified. We describe the cloning of a cDNA from spotted seatrout ovaries encoding a protein that satisfies the following seven criteria for its designation as a steroid membrane receptor: plausible structure, tissue specificity, cellular distribution, steroid binding, signal transduction, hormonal regulation, and biological relevance. For plausible structure, computer modeling predicts that the protein has seven transmembrane domains, typical of G protein-coupled receptors. The mRNA (4.0 kb) is only detected in the brain and reproductive tissues on Northern blots. Antisera only detect the protein (40 kDa) in plasma membranes of reproductive tissues. The recombinant protein produced in an Escherichia coli expression system has a high affinity (Kd = 30 nM), saturable, displaceable, single binding site specific for progestins. Progestins alter signal transduction pathways, activating mitogen-activated protein kinase and inhibiting adenylyl cyclase, in a transfected mammalian cell line. Inhibition of adenylyl cyclase is pertussis toxin sensitive, suggesting the receptor may be coupled to an inhibitory G protein. Progestins and gonadotropin up-regulate both mRNA and protein levels in seatrout ovaries. Changes in receptor abundance in response to hormones and at various stages of oocyte development, its probable coupling to an inhibitory G protein and inhibition of progestin induction of oocyte maturation upon microinjection of antisense oligonucleotides are consistent with the identity of the receptor as an intermediary in oocyte maturation. These characteristics suggest the fish protein is a membrane progestin receptor mediating a “nonclassical” action of progestins to induce oocyte maturation in fish.
Many physiological effects of steroid hormones are too rapid to be mediated by the classical genomic mechanism of steroid action involving activation of nuclear steroid receptors (1–3). Rapid, nongenomic steroid actions initiated on the cell surface have been described in a wide variety of animal, tissue, and cell models (4–8). Steroid membrane receptors characterized in plasma membrane fractions of many target tissues are the likely intermediaries of these nonclassical steroid actions (6, 9–11). For example, extensive studies in our laboratory have demonstrated that a progestin membrane receptor characterized in spotted seatrout (Cynoscion nebulosus) ovaries mediates the induction of oocyte meiotic maturation by the maturation-inducing steroid, 17,20β,21-trihydroxy-4-pregnen-3-one (20β-S), in this species (12–15). However, detailed knowledge of the molecular structures and mechanisms of action of steroid membrane receptors has eluded investigators, despite intensive research efforts in many laboratories over several decades to purify and sequence the receptor proteins (16–20). The minute quantities of receptor proteins present in target tissues and major losses of binding activity during solubilization and fractionation have been insurmountable problems in receptor purification. Therefore, we developed an alternative strategy, which involved a combination of protein purification, antibody screening, and molecular approaches, to clone the progestin membrane receptor (mPR) from a spotted seatrout ovarian cDNA library (15). Mice were immunized with a partially purified progestin membrane receptor fraction containing a major protein band around 40 kDa obtained by DEAE chromatography of a solubilized ovarian membrane extract (see Supporting Text and Fig. 8b, which are published as supporting information on the PNAS web site, www.pnas.org). The mouse splenocytes were fused, and hybrid cultures established. Monoclonal antibodies that recognized ovarian plasma membrane proteins in the 25- to 100-kDa range were screened for receptor recognition (specific progestin binding) by using a double-antibody receptor capture assay we developed for binding proteins, such as steroid membrane receptors, with rapid rates of dissociation (15). Positive antibodies were subsequently used to screen a seatrout ovarian expression library. This paper describes a gene and its protein identified by this approach with the characteristics of an mPR.
Materials and Methods
Cloning and Expression of a Putative mPR.
Total RNA was extracted from seatrout ovarian tissue (2.1 g) by using 30 ml of TRIzol extraction reagent (Life Technologies, Rockville, MD), and mRNA was purified with magnetic oligo(dT) particles by using Straight A's mRNA Isolation System (Novagen). First- and second-strand cDNAs were synthesized from the polyadenylated mRNA by using a TimeSaver cDNA Synthesis Kit (Amersham Pharmacia) and linked to adaptors containing an EcoRI linker and an internal NotI site. A seatrout ovarian cDNA library was constructed after ligation of synthesized cDNAs into EcoRI sites of λZAP II vectors and packaging of ligated λZAP vectors (Stratagene). Expressed fusion proteins from 300,000 phages were screened with mouse monoclonal antibodies (obtained previously) and a goat anti-mouse picoBlue immunoscreening kit (Stratagene). Positive clones were subjected to three additional rounds of screening before excision of pBluescript II SK (−) plasmid vector from λZAP II phage and purification of selected plasmids for further analysis. Sequencing was performed for both strands of the selected cDNA clones by using an automated DNA sequencer (model 310, Perkin–Elmer). Computer sequence analysis was carried out by using the Wisconsin Package GCG V.9.1 programs (Genetics Computer Group, Madison, WI) on a UNIX platform (Sun 5.3, SUN Microsystems, Mountain View, CA) and MACVECTOR V.6.1 (Oxford). A homologous search of sequences was carried out by using BLAST (National Center for Biotechnology Information, at http://www.ncbi.nlm.nih.gov/BLAST/).
Northern Blot Analysis.
Expression of the putative mPR gene in seatrout tissues was examined with Northern hybridization by using total RNA and a Northern Max Kit (Ambion, Austin, TX) following the procedures described in the paper by Zhu et al. in this issue of PNAS (21).
Membrane Preparation, Solubilization, and Receptor Binding Assays.
Membrane fractions of ovarian tissue or human MDA-MB-231 cells were obtained following procedures described (12) with few modifications. An additional centrifugation step of 500 × g for 5 min was included to remove any remaining nuclear material. The resulting supernatant was centrifuged at 20,000 × g for 20 min to obtain the plasma membrane fraction and twice at 150,000 × g for 1 h to produce a cytosolic fraction. For Western blot analyses, the plasma membrane was further purified by centrifuging the pellet three times with a sucrose pad (1.2 M sucrose) at 6,500 × g for 45 min. Treatment of resuspended membrane with 12 mM Triton X-100 in 4 vol of HA buffer (25 mM Hepes, 10 mM NaCl, 1 mM dithioerythritol, pH 7.8) for 30 min, followed by removal of detergent with polystyrene adsorbents (2:1 vol:wt; Bio-Rad SM-2) for 2 h and subsequent removal of the adsorbents by filtration (G-8 filter, Fisher), resulted in recovery of 70–80% of the original specific binding in the solubilized fraction. Solubilized receptor incubation conditions were similar to those used for the intact membrane preparation (12). The incubation (at 4°C) was terminated by adding 1 ml of ice-cold dextran-coated charcoal suspension (0.1 g of dextran T-70 and 1 g of charcoal per 100 ml of HA buffer); the reaction mixture was incubated on ice for 5 min to absorb free steroid, followed by centrifugation at 3,200 × g for 10 min at 4°C.
Western Blot Analysis and Immunocytochemistry.
Specific, high-titer rabbit polyclonal antibodies were generated against synthetic 20-oligo peptides derived from the N-terminal domain (stmPRαpAb1) and second extracellular loop (stmPRαpAb2) of the putative mPR linked to keyhole limpet hemocyanin after six intradermal injections (50–100 mg per injection in Freund's adjuvant). For Western blot analysis, solubilized membrane proteins were resolved in SDS/12% PAGE gels and transferred to a nitrocellulose membrane. The membrane was blocked with 5% nonfat milk in TBST (50 mM Tris/100 mM NaCl/0.1% Tween 20, pH 7.4) for 1 h, incubated with antibody overnight, followed by four 5-min TBST washes, incubated for 1 h at 23°C with horseradish peroxidase conjugated to goat anti-rabbit antibody (Cell Signaling, Beverly, MA), and finally washed three times for 15 min with TBST. Blots then were treated with enhanced chemiluminescence (Amersham Pharmacia) and exposed to x-ray film. Representative fixed ovarian sections (fixed in 10% buffered formalin for several days), containing fully grown follicles, were incubated with primary antiserum (stmPRαpAb1) for 18 h at 4°C before labeling by the ABC method (Vector Laboratories, Burlingame, CA).
Expression of Recombinant Protein in Escherichia coli BL21(DE3) Cells.
The putative mPR coding region was amplified by PCR from a full-length cDNA plasmid clone following protocols described in the paper by Zhu et al. (21). PCR products were purified and ligated into a pET-27b expression vector (Novagen), which was then transformed into BL21(DE3) E. coli cells. Positive clones were purified and sequenced. Plasmids without an insert and with a reversed mPR sequence insert were used as controls. Expression of recombinant protein was induced with isopropyl β-d-thiogalactoside (IPTG) at 25°C. Soluble recombinant proteins were extracted from whole-cell lysates, and aliquots (250 μg of protein) were analyzed for progestin binding, as described.
Progestin Regulation of Signal Transduction Pathways in Transfected MDA-MB-231 Cells.
The putative mPR coding region was amplified by PCR and inserted into a PBK-CMV expression vector (Stratagene). Correct insertion was verified by DNA sequencing. Human MDA-MB-231 breast carcinoma cells (American Type Culture Collection) were transfected with either mPR cDNA, an empty carrier vector, or a vector containing reversed mPR insert by using Lipofectamine (Life Technologies) following manufacturer's suggestions and grown to 80% confluence for 2–3 days in media containing 10% (vol/vol) charcoal-stripped FBS.
cAMP analysis.
Transfected MDA-MB-231 cells were washed three times with PBS buffer. Serum-free medium was added and the cells were preincubated with or without pertussis toxin for 30 min at 37°C before stimulation with steroids for 5 min. Cells were harvested and frozen at −70°C until analysis. cAMP concentrations were measured in cytosolic fractions according to the manufacturer's instructions (Biomedical Technologies, Stoughton, MA).
Mitogen-activated protein (MAP) kinase analysis.
Procedures described by Filardo et al. (22) were followed for selection and culture of cells. Cells were subsequently cultured in serum-free media for 3 days to reduce basal extracellular signal-regulated kinase (Erk)1 and Erk2 activities to minimum levels. Cells were incubated with steroids or recombinant human epidermal growth factor (EGF; Upstate Biotechnology, Lake Placid, NY), a positive control for MAP kinase activation, for 5–15 min. Cells then were harvested and lysed, and 20 μg of cytosolic protein were separated by SDS/PAGE and transferred to a nitrocellulose membrane. Activation of MAP kinase was assessed by Western blot analysis by using both phosphorylated Erk1 and Erk2 and phosphorylation-independent (i.e., total) Erk1 and Erk2 antibodies (Cell Signaling).
Microinjection of Zebrafish Oocytes with Antisense Oligonucleotides to Zebrafish mPR.
In three separate experiments, midvitellogenic follicle-enclosed oocytes with diameters of 450–500 μm were microinjected with ≈1 nl (≈2.5% of total oocyte volume) of either morpholino or phosphorothioate antisense oligonucleotides to the homologous mPR cloned from zebrafish (final concentration = 25 μM), or their respective control oligonucleotides dissolved in 60% Leibovitz L-15 made with ultra-pure water (concentration = 1 mM), or L-15 alone (controls). Follicles (10–15 follicles per 0.1 ml) were incubated at 26°C for 5–6 h in the presence of gonadotropin (hCG, 20 units per ml) to induce oocyte maturational competence (OMC; ref. 23) and incubated for a further 10 h in fresh medium containing 50 nM of the maturation-inducing steroid, 17,20β-dihydroxy-4-pregnen-3-one (17,20β-P). Oocytes were scored at the end of the incubation for disappearance of the nucleus and dissolution of yolk in the ooplasm, easily identifiable markers of oocyte maturation.
Results
Cloning and Sequence Analysis of a Putative mPR.
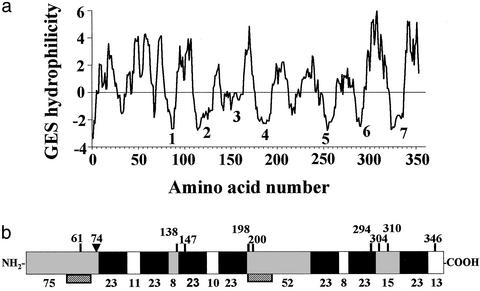
Four positive monoclonal antibodies that bound ovarian protein preparations with specific 20β-S binding activity were identified with the receptor capture assay (see Supporting Text and Table 1, which are published as supporting information on the PNAS web site; ref. 15). One cDNA clone was isolated after screening of a seatrout ovarian expression cDNA library by using one of the monoclonal antibodies (PR10-1). The 1.7-kb cDNA sequence has an ORF of 1,059 bp encoding a protein of 352 amino acids with an estimated molecular mass of 40,585 Da (GenBank accession no. AF262028). Comparison of the cDNA sequence with available sequences in the GenBank database suggests it encodes a previously uncharacterized protein. Computer analyses [DAS, HMMTOP, PREDICT PROTEIN, SOSUI (24), TMHMM, TMPRED, TOPPRED2] of the deduced amino acid sequence consistently predicted that the protein is located in the plasma membrane and has seven transmembrane domains, which is characteristic of G protein-coupled membrane receptors. A representative hydrophilicity plot is shown (Fig. 1a). The protein is rich in cysteine (13 residues, 3.68%) and leucine (41 residues, 11.6%). The cysteines could form bridges both within and among the receptor molecules. One N-linked glycosylation site at the N-terminal end and multiple phosphorylation sites are predicted by NETPHOS analysis (Fig. 1b; ref. 25).
Figure 1.
Structural analysis of deduced amino acid sequence of putative mPR gene. (a) Hydrophilicity profile according to GES method (Goldman/Engelman/Steitz, 1986). (b) Schematic diagram of encoded protein showing extracellular (gray), seven-transmembrane (solid black), and cytoplasmic (clear) domains predicted by several programs. Vertical lines indicate potential phosphorylation sites. ▾, potential N-linked glycosylation site. Numbers above: amino acids from N-terminal end; numbers below: number of amino acids in each domain. Hatched boxes: peptide sequences used to generate polyclonal antibodies.
Tissue Distribution of mRNA and Cellular Localization of the Putative mPR.
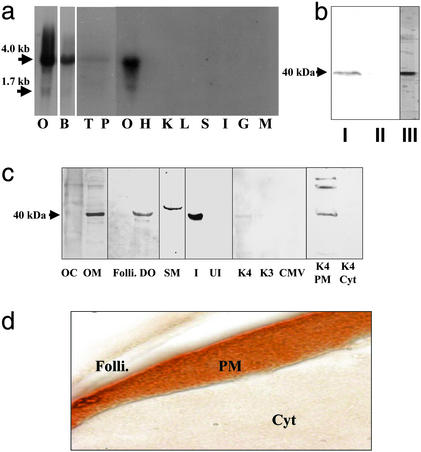
Northern blot analysis showed a major band ≈4 kb and a minor band at 1.7 kb in the ovary (Fig. 2a), as well as 4-kb bands in the brain, testis, and pituitary (Fig. 2a). No detectable signals were obtained in heart, kidney, liver, spleen, intestine, gill, and muscle (Fig. 2a), suggesting the receptor gene is expressed only in reproductive and neuroendocrine tissues. A single immunoreactive band ≈40 kDa was detected by Western blot analysis by using monoclonal antibody PR10-1 in the solubilized ovarian membrane fraction and in the purified membrane fraction 1 used to generate the monoclonal antibodies, whereas no immunoreactivity was detected in the cytosolic fraction (Fig. 2b). A protein band with a molecular mass ≈40 kDa was also detected in a solubilized oocyte membrane (OM) fraction as well as in the membranes of denuded oocytes (DO) by Western blot analysis using polyclonal antibody stmPRαAb1 (Fig. 2c). A slightly higher molecular weight band was detected in sperm membranes (SM), which possibly reflects a different posttranslational modification. No immunoreaction was obtained in cytosolic fractions of oocytes or the membranes of follicle cells (Fig. 2c). Identical results (not shown) were obtained with the stmPRαAb2 antibody. These studies show that the putative mPR is localized on oocyte and sperm plasma membranes, sites where specific mPRs have been characterized (12, 26).
Figure 2.
Tissue distribution and cellular localization of putative mPR. (a) Northern blot analysis showing mRNA expression in ovarian (O, gel loading: 1 μg) and other tissues (gel loading: 5 μg); B, brain; T, testis; P, pituitary; H, heart; K, kidney; L, liver; S, stomach; I, intestine; G, gill; M, muscle. (b) Western blot analysis of solubilized ovarian membrane proteins using monoclonal antibody PR10-1. I, plasma membrane; II, cytosol; III, partially purified membrane fraction used to generate monoclonal antibodies. (c) Cellular localization of receptor by Western blot analysis using stmPRαpAb1 antibody (gel loading: 10 μg). OC, oocyte cytosol; OM, oocyte membrane; Folli., follicle cell membrane; DO, denuded oocyte plasma membrane; SM, sperm membrane; I, recombinant protein induced by IPTG in E. coli; UI, noninduced E. coli protein; K4, membrane proteins from mPR-transfected MDA-MB-231 cells; K3, control cells transfected with vector containing reverse insert; CMV, control cells transfected with empty carrier vector. The following lanes were probed with PR10-1 antibody: K4 PM, plasma membrane from mPR transfected MDA-MB-231 cells; K4 Cyt, cytosol from mPR transfected cells. (d) Immunocytochemical localization of mPR receptor protein in a mature seatrout follicle containing a stage IV oocyte using stmPRαpAb1 antibody. Folli., follicle cells; PM, oocyte plasma membrane; Cyt, oocyte cytoplasm. (Magnification: 1 cm ≈ 20 μm.)
Strong immunoreactivity was observed only on the oocyte surface layer containing the plasma membrane of a representative fully grown seatrout oocyte under high power magnification (Fig. 2d). No specific immunoreactive staining was detected in follicle cells or in the cytoplasm of the oocyte. These results clearly demonstrate the oocyte plasma membrane localization of the receptor in seatrout.
Steroid Binding of Putative mPR Produced in E. coli.
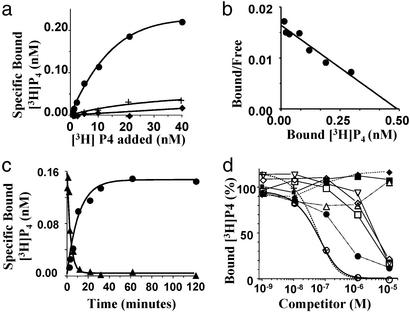
An essential criterion to meet is that steroid binding to the recombinant protein should be characteristic of steroid membrane receptors. The receptor protein should demonstrate high affinity, saturable and displaceable binding that is specific for steroids, and display rapid dissociation and association kinetics. The coding region of the receptor was inserted into a pET-27b expression vector, and soluble recombinant protein was produced in BL21 E. coli cells (lane I in Fig. 2c). Saturable, specific progesterone binding was obtained with the recombinant protein (Fig. 3a), whereas no specific progesterone binding was observed with control proteins expressed by E. coli without IPTG induction or transformed with an empty vector (Fig. 3a). Saturation analysis confirmed progestin binding to the recombinant bacterial protein was saturable (Bmax = 0.49 nM, Fig. 3b). Scatchard analysis showed the presence of a single class of high-affinity binding sites (Kd 30 nM, Fig. 3b). Progestin binding to the receptor was readily displaceable, with ≈60% of [3H]progesterone exchanged within 10 min (Fig. 3c). The kinetics of association and dissociation were rapid, with t1/2s of 7.8 and 2.2 min, respectively (Fig. 3c), which are typical for steroid membrane receptors (15). Steroid competition studies showed binding was highly specific for progesterone and 17-hydroxyprogesterone (Fig. 3d). No displacement of [3H]progesterone was observed with estradiol-17β, cortisol, and 20β-S, whereas 20β-hydroxyprogesterone, testosterone, 17,20β-dihydroxy-4-pregnen-3-one (17,20β-P) and 11-deoxycortisol showed displacement at 10- to 100-fold higher concentrations (Fig. 3d).
Figure 3.
Steroid binding characteristics of recombinant mPR protein produced in E. coli. (a) Representative plots of specific [3H]progesterone binding to protein produced with a cDNA-inserted vector with (●) or without (♦) IPTG induction, or an empty carrier vector (+, controls). (b) Scatchard analysis of specific [3H]progesterone binding. (c) Time course of association and dissociation of specific [3H]progesterone binding. (d) Competition curves of steroid binding expressed as a percentage of maximum specific [3H]progesterone binding [concentration: [3H]progesterone, 10 nM, specific activity; 65 Ci/mmol (1 Ci = 37 GBq); Amersham Pharmacia]; ○, progesterone; +, 17-hydroxyprogesterone; ●, 20β-hydroxyprogesterone; ▵, 20β-S; □, testosterone; ⋄, 17,20β-dihydroxy-4-pregnen-3-one; ▿, 11-deoxycortisol; ♦, estradiol-17β; ■, cortisol.
Alteration of Intracellular Signal Transduction Pathways.
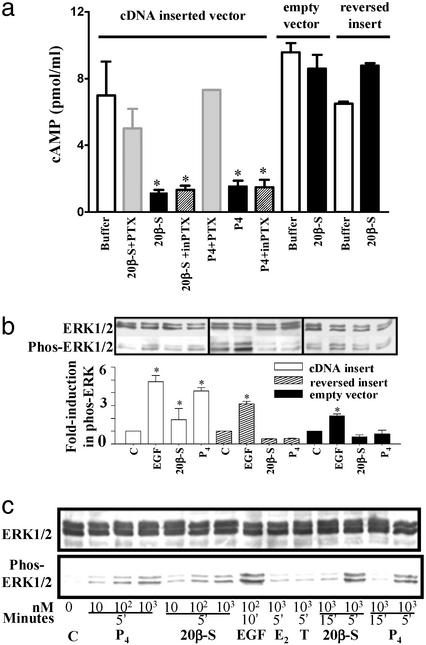
To determine whether progestin binding to the putative mPR causes rapid activation of intracellular signaling pathways, the cDNA coding region was inserted into a mammalian expression vector, PBK-CMV, and stably transfected into MDA-MB-231 breast cancer cells. The receptor protein was localized in the plasma membranes of the transfected cells (lane K4 PM in Fig. 2c) and was not detected in the cytosolic fraction (Fig. 2c, lane K4 Cyt). The presence of two higher molecular weight immunoreactive bands in the membrane fraction may be the result of aggregation of the receptor protein, because SDS-resistant oligomers are commonly observed with G protein-coupled receptors (GPCR; ref. 27). Production of cAMP was significantly reduced within 5 min of progestin addition (20β-S) in the transfected cells and was partially recovered by 30 min (results not shown). Subsequent experiments showed this inhibitory effect of progesterone and 20β-S was partially blocked when the cells were pretreated with pertussis toxin, a specific inhibitor for Gi/o proteins (Fig. 4a). In contrast, these progestins did not exert an inhibitory effect in control cells transfected with a carrier vector or a reversed vector. Erk1 and Erk2 were activated within 5 min after stimulation by progesterone and 20β-S (Fig. 4b), whereas no activation of Erk1 and Erk2 was observed in control cells transfected with an empty carrier vector or a control vector containing a reversed insert (Fig. 4b). Activation of MAP kinase by progesterone and 20β-S was rapid and transient (Fig. 4c). Significant activation occurred 5 min after addition of the hormones, with subsequent declines in phosphorylated Erk1/Erk2 protein after 15 min of exposure (Fig. 4c). MAP kinase activation by the progestins was also dose-dependent over the range of 10–1,000 nM.
Figure 4.
Inhibition of cAMP production (a) and activation of MAP kinase signaling pathway (b and c) in response to progestin hormones in MDA-MB-231 cells stably transfected with putative mPR cDNA (means ± SEM, n = 4; *, P < 0.05). (a) Cells were preexposed to activated pertussis toxin (PTX) or inactive pertussis toxin (inPTX) for 30 min at 37°C before incubation with 20β-S or progesterone for 5 min. (b) MAP kinase activity (shown as increase in phospho-Erk1/2) in cells transfected with vector containing mPR insert (white bar), controls containing reversed insert (shaded bar) or vector alone (black bar) after a 5-min stimulation with 1 μM 20β-S or progesterone (P4). C, untreated control; EGF, human epidermal growth factor (1 μM, positive control). (c) Time- and dose-dependent activation of MAP kinase (gel loading: 20 μg per lane) in transfected cells treated with 20β-S, P4, estradiol-17β (E2), or testosterone (T).
In addition to demonstrating activation of intracellular signaling pathways, the cAMP experiments showing a decrease in intracellular cAMP levels soon after addition of the progestin hormones to the transfected cells suggest the receptor activates an inhibitory G protein. Subsequent experiments demonstrating that the inhibitory effect was reversed by preincubation with an inhibitor of Gi/o proteins, pertussis toxin, are also consistent with this suggestion.
Hormonal Regulation.
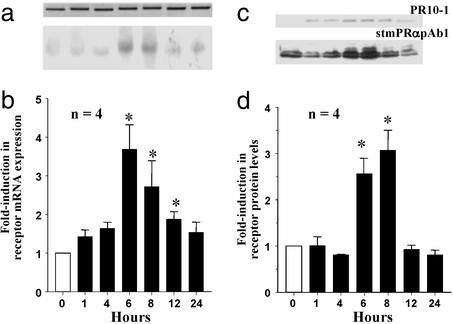
Both the putative membrane receptor mRNA and protein were up-regulated in ovarian tissues after incubation with 20β-S. Receptor mRNA and protein levels were significantly increased in ovarian tissues incubated with 20β-S (290 nM, Fig. 5) for 6–8 h. The mRNA and protein levels subsequently decreased after an 8- to 12-h incubation. (Fig. 5). No changes in receptor mRNA or protein expression were observed in control tissues incubated without hormones (data not shown). hCG treatment (15 units per ml of hCG) also caused an up-regulation of receptor protein levels (Fig. 6b).
Figure 5.
Time course of changes in putative mPR mRNA (a and b) and protein levels (c and d) in ovarian fragments after in vitro treatment with 300 nM 20β-S, relative to control (0 h) values (mean ± SEM, n = 4; *, P < 0.05). (a) Representative Northern blot (gel loading: 1 μg). (Lower) Putative mPR mRNA. (Upper) Corresponding β-actin mRNA. (b) Relative levels of mPR mRNA. (c) Representative Western blot analyses using PR10-1 and stmPRαAb1 antibodies (gel loading: 10 μg). (d) Relative levels of mPR protein.
Figure 6.
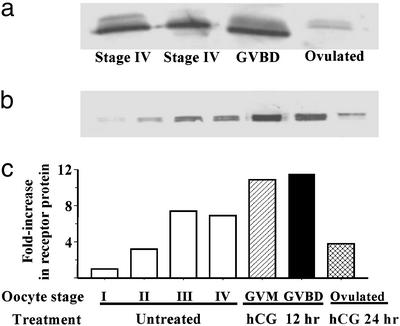
Changes in putative mPR protein levels in oocytes at various stages of development and final maturation detected by Western blot analysis with stmPRαAb1 antibody (gel loading: membrane proteins from five oocytes per lane). (a) In wild fish captured on their spawning grounds. (b) After incubation in vitro in the presence or absence of hCG. I, vitellogenic oocyte, 200–300 μm (diameter); II, 300–375 μm; III, 375–440 μm; IV, full-grown oocyte, 430–500 μm; GVM, germinal vesicle migration stage of final maturation; GVBD, germinal vesicle breakdown stage of final maturation. (c) mPR protein levels expressed relative to stage I oocyte values.
Biological Relevance.
Receptor protein levels in fish captured on their spawning ground were lower in fully grown oocytes compared with those at a later stage of maturation undergoing germinal vesicle breakdown (GVBD, threefold increase) and were lowest in ovulated oocytes (Fig. 6a). A similar pattern of receptor changes was observed during oocyte maturation in laboratory studies. A rapid increase in receptor levels occurred in fully grown (stage IV) oocytes during hCG-induced oocyte maturation (GVM and GVBD), followed by a dramatic decline in receptor levels in ovulated oocytes (Fig. 6 b and c). Receptor protein levels were low in the smallest early stage vitellogenic oocytes and showed a size-dependent increase in later vitellogenic stage oocytes (Fig. 6 b and c). These results further indicate an intimate relationship between the putative mPR and oocyte maturation.
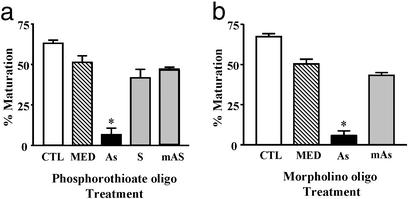
Antisense studies could not be performed with seatrout oocytes because of their fragility. Therefore, the homologous mPR gene in zebrafish (mPRα; ref. 21) was cloned for antisense microinjection studies because their oocytes tolerate these procedures. The nucleotide sequence of the zebrafish cDNA was 73% identical with that of seatrout, whereas the deduced amino acid sequence displayed 80% identity. Microinjection of zebrafish oocytes with zebrafish mPRα (21) antisense consistently blocked maturation of oocytes in vitro in response to the maturation-inducing steroid in zebrafish, 17,20β-P. The percentage of oocytes that matured was significantly lower in the mPRα antisense groups (6.5%, phosphorothioated antisense in Fig. 7a; 5.8%, morpholino antisense in Fig. 7b) than in the control groups (38.5%, phosphorothioated sense in Fig. 7a; 51.4%, morpholino mis-antisense in Fig. 7b). These results from the antisense experiments provide more direct evidence of an involvement of the putative mPR in progestin induction of oocyte maturation in teleosts.
Figure 7.
Effects of microinjection of zebrafish oocytes with zebrafish antisense oligonucleotides on their subsequent maturation in response to 17,20β-P (mean ± SEM, n = 4; *, P < 0.05). (a) Phosphorothioate antisense experiment. CTL, control uninjected oocytes; MED, injected with media alone; As, zebrafish mPR α-antisense; S, mPR α-sense; mAS, mPR α-mis-antisense. (b) Morpholino antisense experiment.
Discussion
These results provide clear evidence that the hitherto unknown gene isolated from spotted seatrout ovaries encodes a steroid membrane receptor. The following seven criteria were met for its designation as a steroid membrane receptor: structural plausibility, tissue specificity, plasma membrane localization, characteristic steroid binding, activation of signal transduction pathways, hormonal regulation, and biological relevance. In particular, the presence of high affinity, saturable, displaceable, single binding sites specific for a single class of steroids on the recombinant protein are defining characteristics of steroid receptors. To our knowledge, this is the first gene and its protein unrelated to steroid nuclear receptors that has been shown to fulfill all of the criteria for steroid receptor designation.
In addition, substantial evidence was obtained that the receptor mediates progestin induction of oocyte meiotic maturation in spotted seatrout. Firstly, the tissue and cellular localization of the protein and mRNA are consistent with its proposed function as the membrane receptor regulating gamete maturation in this species. The mRNA is only detected in reproductive endocrine and brain tissues, and the protein is localized on the plasma membrane of oocytes and sperm, where progestin membrane receptors have been fully characterized in seatrout (12, 15, 26). Moreover, the patterns of protein changes suggest an involvement of the receptor in oocyte maturation. The dramatic increase in receptor protein levels in oocytes undergoing meiotic maturation and the subsequent decrease in protein levels in ovulated oocytes a few hours later closely parallel the changes in progestin membrane receptor-binding activity observed previously in seatrout oocytes during this period (12, 13). Earlier structure/activity studies had established that the progestin membrane receptor is an intermediary in the maturation-inducing steroid initiation of oocyte maturation in this species (14). In addition, the finding that the protein is up-regulated by hCG supports a physiological role for the receptor in oocyte maturation. Both the receptor protein and its mRNA are significantly elevated in seatrout ovaries after 8-h incubation with 15 units hCG, similar to the time-course (6–9 h) for hCG up-regulation of progestin membrane receptor binding activity (13). Up-regulation of the progestin membrane receptor is associated with the development of the ability of oocytes to become responsive to progestins (OMC) and complete meiotic maturation (13). Interestingly, hCG-induced increases in receptor protein levels in the present study also coincided with the development of OMC. Finally, the up-regulation of the receptor mRNA by 20β-S suggests a potential role for the nuclear progestin receptor in the development of OMC, which could also partially explain some recent results obtained with Xenopus (discussed in ref. 15).
Direct evidence for a role of the mPR in the induction of oocyte maturation was obtained from microinjection studies with zebrafish oocytes. It was clearly demonstrated that microinjection with two types of mPR antisense oligonucleotides directed against the zebrafish homolog (zebrafish α; ref. 21) prevented oocyte maturation in response to the progestin. However, injections with control oligonucleotides did not influence oocyte maturation. Finally, the finding that the seatrout progestin receptor activates a pertussis toxin–sensitive inhibitory G protein is entirely consistent with its proposed role as the receptor mediating progestin induction of oocyte maturation in fishes. It is well established that progestin induction of oocyte maturation in fish and amphibians involves a decrease in intracellular cAMP (15, 20, 28). Moreover, we have shown that microinjection of pertussis toxin blocks maturation of seatrout oocytes as well as those in a closely related perciform species, Atlantic croaker, in response to the hormone (15). A pertussis toxin-sensitive G protein has also been implicated in the progestin induction of oocyte maturation in rainbow trout (28).
The results of progestin specificity studies in the different expression systems are somewhat equivocal. The fact that both 20β-S and progesterone alter the activities of adenylyl cyclase and MAP kinase in human breast cancer cells transfected with the receptor cDNA indicates it recognizes both progestins, whereas the recombinant protein produced in E. coli only binds progesterone. Taken together, these findings suggest deficiencies in the E. coli expression system for receptor protein production, possibly related to the lack of glycosylation of the recombinant protein or other posttranslational modifications.
Progestin induction of oocyte maturation is inhibited in the presence of pertussis toxin, suggesting that the receptor mediating oocyte maturation activates an inhibitory G protein (15, 28) and, therefore, may be a GPCR. The structural analysis data for the receptor protein are in accordance with this suggestion. The presence of seven transmembrane domains in the receptor protein suggests it may be a GPCR. Moreover, the molecular mass of the progestin receptor protein (40 kDa) is within the expected range for GPCRs (29). Finally, the signal transduction experiments indicate the receptor activates an inhibitory G protein, which also suggests it may be a GPCR. If additional research confirms that the seatrout and zebrafish progestin membrane receptor proteins we have discovered are GPCRs, they are not closely related to the other GPCR subclasses identified to date (30) and most likely represent a separate subclass of this receptor type. Recent studies suggest widespread involvement of G proteins in nonclassical steroid actions (31–33). Inhibitory G proteins have also been implicated in the signal transduction of rapid, nongenomic glucocorticoid, estrogen, and androgen actions in a variety of cell types (34–36). Future investigations on the structural homology of the GPCRs mediating these nonprogestin steroid actions with the seatrout progestin receptor should provide insights into the evolution of this type of steroid receptor. GPCRs represent one of the largest and possibly most ancient classes of receptors, responding to a diverse array of signaling molecules (37). The preliminary finding that steroids, a major class of signaling molecules, may also act via this mechanism, provides a further indication of the ubiquity of this signaling system and its probable ancient origins (38).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant ESO4214 and Environmental Protection Agency Science to Achieve Results Grant R-82902401 (to P.T.), National Science Foundation Grant IBN-9980353 (to P.T. and Y.Z.), and funds from University of Texas at Austin and private donors.
Abbreviations
- 20β-S
17,20β,21-trihydroxy-4-pregnen-3-one
- mPR
membrane progestin receptor
- IPTG
isopropyl β-d-thiogalactoside
- Erk
extracellular signal-regulated kinase
- MAP
mitogen-activated protein
- hCG
human chorionic gonadotropin
- GPCR
G protein-coupled receptor
- OMC
oocyte maturational competence
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF2062028).
See commentary on page 2168.
References
- 1.Yang J, Serres C, Philibert D, Robel P, Baulieu E-E, Jouannet P. Proc Natl Acad Sci USA. 1994;91:529–533. doi: 10.1073/pnas.91.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lieberherr M, Grosse B. J Biol Chem. 1994;269:7217–7223. [PubMed] [Google Scholar]
- 3.Gu Q, Korach K S, Moss R L. Endocrinology. 1999;140:660–666. doi: 10.1210/endo.140.2.6500. [DOI] [PubMed] [Google Scholar]
- 4.Pietras R, Szego C. Nature. 1975;253:357–359. doi: 10.1038/253357a0. [DOI] [PubMed] [Google Scholar]
- 5.Qui J, Lou G, Huang Y, Lou J, Chen Z. Endocrinology. 1998;139:5103–5108. doi: 10.1210/endo.139.12.6376. [DOI] [PubMed] [Google Scholar]
- 6.Revelli A, Massobrio M, Tesarik J. Endocr Rev. 1998;19:3–17. doi: 10.1210/edrv.19.1.0322. [DOI] [PubMed] [Google Scholar]
- 7.Watson C S, Gametchu B. Proc Soc Exp Biol Med. 1999;220:9–19. doi: 10.1046/j.1525-1373.1999.d01-2.x. [DOI] [PubMed] [Google Scholar]
- 8.Orchinik M, Murray T F, Moore F L. Science. 1991;252:1848–1851. doi: 10.1126/science.2063198. [DOI] [PubMed] [Google Scholar]
- 9.Falkenstein E, Tillman H-S, Christ M, Feuring M, Wehling M. Pharmacol Rev. 2000;52:513–555. [PubMed] [Google Scholar]
- 10.Maller J L. Proc Natl Acad Sci USA. 2001;98:8–10. doi: 10.1073/pnas.98.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loomis A K, Thomas P. Biol Reprod. 2000;62:995–1004. doi: 10.1095/biolreprod62.4.995. [DOI] [PubMed] [Google Scholar]
- 12.Patino R, Thomas P. Gen Comp Endocrinol. 1990;78:204–217. doi: 10.1016/0016-6480(90)90007-9. [DOI] [PubMed] [Google Scholar]
- 13.Thomas P, Pinter J, Das S. Biol Reprod. 2001;64:21–29. doi: 10.1095/biolreprod64.1.21. [DOI] [PubMed] [Google Scholar]
- 14.Thomas P, Das S. Biol Reprod. 1997;57:999–1007. doi: 10.1095/biolreprod57.5.999. [DOI] [PubMed] [Google Scholar]
- 15.Thomas P, Zhu Y, Pace M. Steroids. 2002;67:511–517. doi: 10.1016/s0039-128x(01)00180-5. [DOI] [PubMed] [Google Scholar]
- 16.Evans S J, Moore F L, Murray T F. J Steroid Biochem Mol Biol. 1998;67:1–8. doi: 10.1016/s0960-0760(98)00070-3. [DOI] [PubMed] [Google Scholar]
- 17.Nemere I, Dormanen M C, Hammond M W, Okamura W H, Norman A W. J Biol Chem. 1994;269:23750–23756. [PubMed] [Google Scholar]
- 18.Evans S J, Murray T F, Moore F L. J Steroid Biochem Mol Biol. 2000;72:209–221. doi: 10.1016/s0960-0760(00)00031-5. [DOI] [PubMed] [Google Scholar]
- 19.Sadler S E, Maller J L. J Biol Chem. 1982;257:355–361. [PubMed] [Google Scholar]
- 20.Maller J L. Biol Cell. 1998;90:453–460. [PubMed] [Google Scholar]
- 21.Zhu Y, Bond J, Thomas P. Proc Natl Acad Sci USA. 2003;100:2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filardo E J, Quinn J A, Bland K I, Frackelton A R. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 23.Pang Y, Ge W. Biol Reprod. 2002;66:259–265. doi: 10.1095/biolreprod66.2.259. [DOI] [PubMed] [Google Scholar]
- 24.Hirokawa A, Boon-Chieng S, Mitaku S. Bioinformatics. 1998;14:378–379. doi: 10.1093/bioinformatics/14.4.378. [DOI] [PubMed] [Google Scholar]
- 25.Blom N, Gammeltoft S, Brunak S. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 26.Thomas P, Breckenridge-Miller D, Detweiler C. Fish Physiol Biochem. 1997;17:109–117. [Google Scholar]
- 27.Overton M C, Blumer K J. Curr Biol. 2000;10:341–344. doi: 10.1016/s0960-9822(00)00386-9. [DOI] [PubMed] [Google Scholar]
- 28.Yoshikuni M, Nagahama Y. Dev Biol. 1994;16:615–622. doi: 10.1006/dbio.1994.1341. [DOI] [PubMed] [Google Scholar]
- 29.Reilander H, Reinhart C, Szmolenszky A. In: G-Protein-Coupled Receptors. Haga T, Berstein G, editors. Boca Raton, FL: CRC; 2000. pp. 281–322. [Google Scholar]
- 30.Wess J. Pharmacol Ther. 1998;80:231–264. doi: 10.1016/s0163-7258(98)00030-8. [DOI] [PubMed] [Google Scholar]
- 31.Gu Q, Moss R L. J Physiol. 1998;506:745–754. doi: 10.1111/j.1469-7793.1998.745bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y Z, Qiu J. Mol Cell Biol Res Commun. 1999;2:145–149. doi: 10.1006/mcbr.1999.0163. [DOI] [PubMed] [Google Scholar]
- 33.Lutz L B, Kim B, Jahani D, Hammes S R. J Biol Chem. 2000;275:41512–41520. doi: 10.1074/jbc.M006757200. [DOI] [PubMed] [Google Scholar]
- 34.Benten W P M, Lieberherr M, Stamm O, Wrehlke C, Guo Z, Wunderlich F. Mol Biol Cell. 1999;10:3113–3123. doi: 10.1091/mbc.10.10.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benten W P M, Stephan C, Lieberherr M, Wunderlich F. Endocrinology. 2001;142:1669–1677. doi: 10.1210/endo.142.4.8094. [DOI] [PubMed] [Google Scholar]
- 36.Orchinik M, Murray T F, Franklin P H, Moore F L. Proc Natl Acad Sci USA. 1992;89:3830–3834. doi: 10.1073/pnas.89.9.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bockaert J, Pin J P. EMBO J. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarty D R, Chory J. Cell. 2000;103:201–209. doi: 10.1016/s0092-8674(00)00113-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.