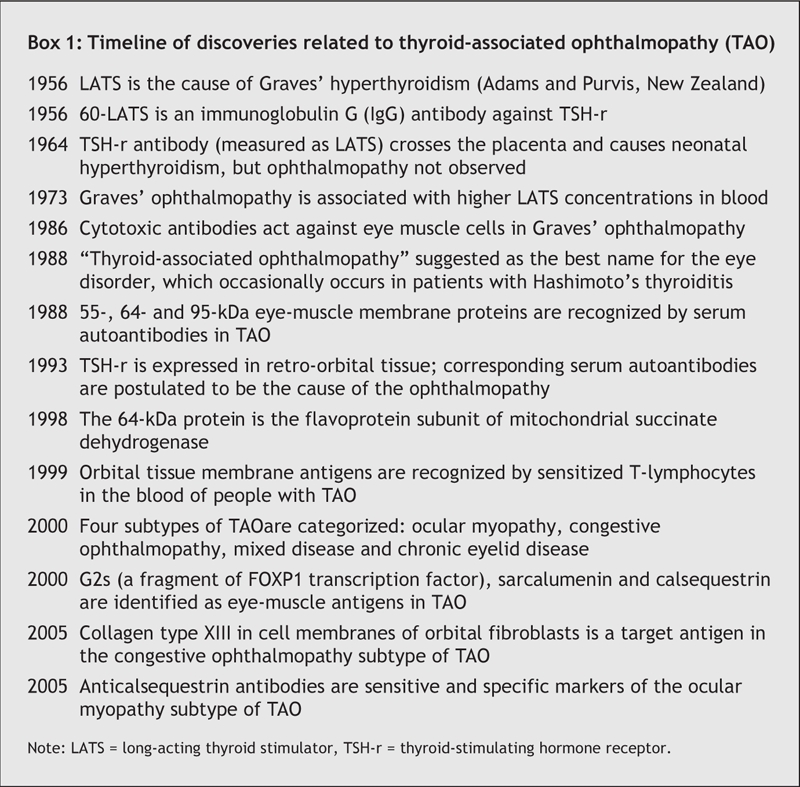
The ophthalmopathy associated with Graves' hyperthyroidism and Hashimoto's thyroiditis is an autoimmune disorder of the extraocular muscle and the surrounding orbital connective tissue (OCT) and fat (Box 1). Its most appropriate name is thyroid-associated ophthalmopathy (TAO).
Box 1.
Several theories exist to explain the disorder's unusual association with thyroid autoimmunity. Most of them favour a role of autoimmunity against a thyroid-stimulating hormone receptor (TSH-r)–like protein in the orbital preadipocyte and, possibly, extraocular muscle fibre. This is still merely hypothetical, for several reasons: TSH-r–stimulating antibody levels do not correlate with the severity and activity of the eye signs; newborns with thyrotoxicosis do not have the ophthalmopathy, even when the mother has eye signs; and ophthalmopathy has not been convincingly produced in animals immunized with the TSH-r protein. Moreover, a recent study1 found that mRNA for TSH-r was not upregulated in the orbital fat of patients with Graves' ophthalmopathy compared with that in control subjects.
One observation in favour of the theory of a primary role of autoimmunity against TSH-r is the generalized connective-tissue disorder in Graves' disease: autoimmunity against TSH-r, expressed in fat and connective tissue, could explain the development of pretibial myxedema, acropachy and the OCT component of TAO. In addition, all patients with Graves' ophthalmopathy test positive for serum TSH-r antibodies, at least when they are hyperthyroid. Proponents of the so-called TSH-r hypothesis point out that eye muscles are 30% of OCT by volume, and propose that the eye-muscle damage is secondary to antibody targeting of TSH-r in OCT.
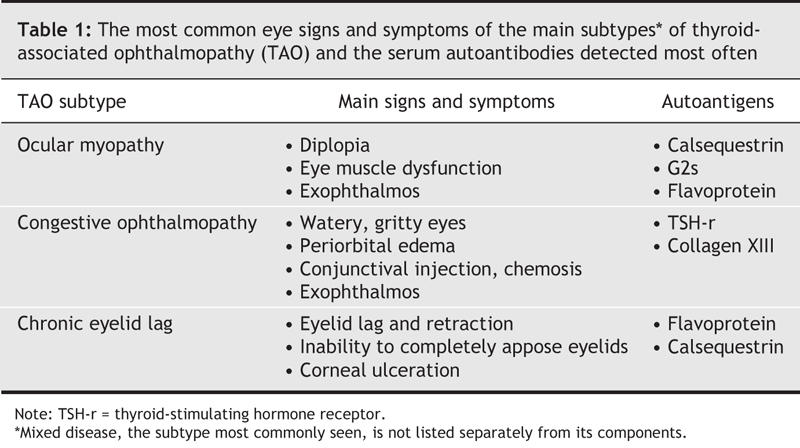
An alternative view is that the eye-muscle and OCT–fat reactions are both autoimmune disorders that can occur alone or together in patients with thyroid autoimmunity, manifesting as 3 subtypes of TAO: ocular myopathy, congestive ophthalmopathy or mixed disease. In ocular myopathy, double vision, reduced eye movement and increased volumes of eye muscle (seen with orbital imaging techniques) result from damage to the eye muscles. In congestive ophthalmopathy, eye swelling, redness, chemosis and increased tearing are caused by inflammation in the periorbital tissues. Mixed disease is the most common manifestation of TAO. Chronic eyelid lag, occurring alone or with other features of TAO, may be a fourth subtype (Table 1). The notion of subtypes of Graves' ophthalmopathy was first raised by Solovyeva2 and has been borne out by our extensive clinical studies.
Table 1
In support of the “eye-muscle hypothesis,” we have identified several autoantigens in eye muscle; namely, the flavoprotein (Fp) subunit of mitochondrial succinate dehydrogenase, G2s, sarcalumenin and the calcium-binding protein calsequestrin (Fig. 1). The corresponding autoantibodies are associated with eye-muscle dysfunction in patients with TAO. There have been both criticism of the putative role of these eye-muscle antibodies in the pathogenesis of the ophthalmopathy and arguments in favour of such a role for them.
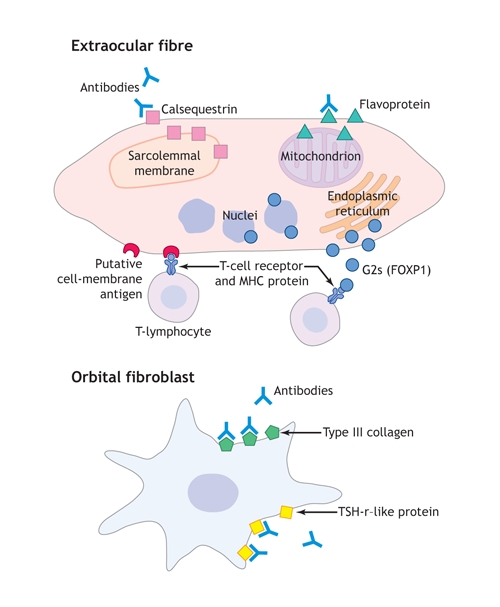
Fig. 1: Eye-muscle and orbital fibroblast proteins recognized by T-lymphocytes or antibodies in thyroid-associated ophthalmopathy (TAO). Our working hypothesis is that the ocular myopathy subtype of TAO results from T-lymphocyte–mediated targeting of eye-muscle fibre; serum antibodies against G2s protein, calsequestrin, flavoprotein and sarcalumenin are secondary to their release after fibre necrosis; and the congestive ophthalmopathy subtype of TAO results from reaction against TSH-r or collagen XIII in fibroblast cell membranes, which leads to fibroblast stimulation and excess production of collagen and glycosaminoglycans. MHC = major histocompatibility complex, TSH-r = thyroid-stimulating hormone receptor.. Photo by: Lianne Friesen and Nicholas Woolridge
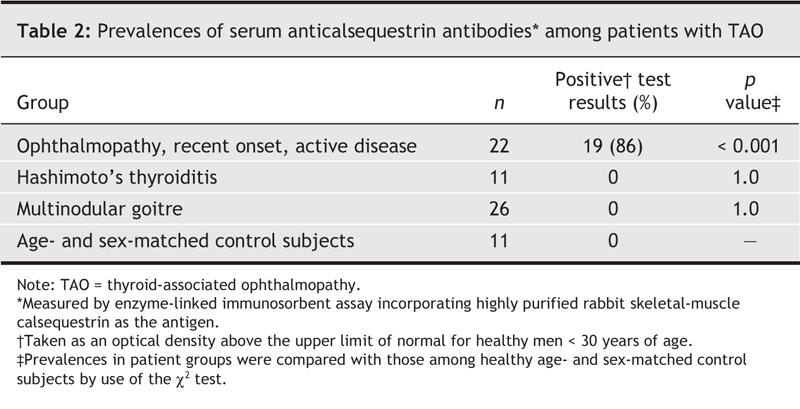
First, because the antigens are not specific to eye muscle, the rarity of systemic myopathy among patients with Graves' disease is difficult to explain. This is true, except that we have shown that the antibodies that target calsequestrin, which is expressed 4.8 times more in eye muscle than in other skeletal muscle,3 are sensitive markers of recent-onset ophthalmopathy, detected in 86% of patients with active eye signs and symptoms (Table 2) and in the great majority of patients with recent-onset ocular myopathy who have been tested so far (n = 30).
Table 2
Second, since the candidate antigens are all located inside muscle fibres, they would not encounter antibodies or T-lymphocytes until the cell walls are breached by some other reaction. Although Fp and G2s are indeed intracellular proteins, mRNA for calsquestrin is distributed throughout the cell (including the cell membrane) in the myotube stage of differentiation, where in some situations it could be targeted.
Third, because the antibodies are detected in 5%–10% of healthy subjects and patients who do not have ophthalmopathy, the clinical utility of the tests is limited to serial studies of patients with Graves' hyperthyroidism. Just the same, anticalsequestrin antibodies are detected neither among patients with Hashimoto's thyroiditis or multinodular goitre, nor among people without thyroid problems, and therefore may be specific markers of the ocular myopathy subtype of TAO.
Taking into account all the evidence, the development of the eye-muscle component of TAO may be best explained by cytotoxic T-lymphocyte targeting of a cell-membrane antigen; the eye-muscle antibodies that we identify may be secondary. On the other hand, antibodies targeting the TSH-r in OCT and fat cells may trigger periorbital inflammation. Recent studies suggest that type XIII collagen, which is expressed in the cell membranes of fibroblasts, may also be a target in the fat and OCT of patients with active congestive ophthalmopathy (Fig. 1).4
The development of the eye-muscle component of TAO can be best explained by T-cell targeting of an eye-muscle membrane antigen, whereas reactivity against TSH-r in the orbital fibroblast may be the key abnormality that leads to the congestive ophthalmopathy subtype, as well as other features of connective-tissue disorder in Graves' disease. The most specific antigen marker for the eye-muscle component of TAO appears to be calsequestrin. The availability of testing for antibodies to anticalsequestrin as a clinical aid for TAO may lead to a better understanding of the reaction of eye muscle and its relation to reactions in orbital connective and thyroid tissue.
Junichi Tani Jack R. Wall Department of Medicine University of Sydney New South Wales, Australia
Footnotes
This article has been peer reviewed.
Competing interests: None declared.
REFERENCES
- 1.Kumar S, Leontovich A, Coenen MJ, et al. Gene expression profiling of orbital adipose tissue from patients with Graves' ophthalmopathy: a potential role for secreted frizzle-related protein-1 in orbital adipogenesis. J Clin Endocrinol Metab 2005;90:4730-5. [DOI] [PMC free article] [PubMed]
- 2.Solovyeva TP. Endocrine ophthalmopathies: problems of rational classification. Orbit 1989;8:193-8.
- 3.Porter JD, Khanna S, Kaminski HJ, et al. Extraocular muscle is defined by a fundamentally distinct gene expression profile. Proc Natl Acad Sci U S A 2001;98:12062-7. [DOI] [PMC free article] [PubMed]
- 4.De Bellis A, Coronella C, Sansone D, et al. Serum antibodies to collagen XIII: a further good marker of active Graves' ophthalmopathy. Clin Endocrinol (Oxf) 2005;62:24-9. [DOI] [PubMed]


