Abstract
Astrocytes are coupled to one another by gap junction channels that allow the diffusion of ions and small molecules throughout the interconnected syncytium. In astrocytes, gap junctions are believed to participate in spatial buffering removing the focal excess of potassium resultant from intense neuronal activity by current loops through the syncytium and are also implicated in the propagation of astrocytic calcium waves, a form of extraneuronal signaling. Gap junctions can be modulated by several factors, including elevation of extracellular potassium concentration. Because K+ elevation is a component of spinal cord injury, we evaluated the extent to which cultured spinal cord astrocytes is affected by K+levels and obtained evidence suggesting that a Ca2+–calmodulin (CaM) protein kinase is involved in the K+-induced increased coupling. Exposure of astrocytes to high K+ solutions induced a dose-dependent increase in dye coupling; such increased coupling was greatly attenuated by reducing extracellular Ca2+concentration, prevented by nifedipine, and potentiated by Bay-K-8644. These results indicate that K+-induced increased coupling is mediated by a signaling pathway that is dependent on the influx of Ca2+ through L-type Ca2+ channels. Evidence supporting the participation of the CaM kinase pathway on K+-induced increased coupling was obtained in experiments showing that calmidazolium and KN-93 totally prevented the increase in dye and electrical coupling induced by high K+ solutions. Because no changes in connexin43 expression levels or distribution were observed in astrocytes exposed to high K+ solutions, we propose that the increased junctional communication is related to an increased number of active channels within gap junction plaques.
Keywords: glia, potassium, dye coupling, junctional conductance, Ca2+–calmodulin, connexin, Lucifer yellow spread
Diverse forms of CNS pathophysiology have common components, including increased extracellular K+ attributable to hyperexcitability and/or cell death. In mammalian CNS, extracellular K+ concentration may increase from a resting value of 3 to 5–15 mm during intense neuronal activity or epileptiform bursting (Somjen, 1979) and up to 30–80 mm during hypoxia and anoxia, hypoglycemia, and spreading depression (Astrup and Norberg, 1976; Blank and Kirshner, 1977;Nicholson and Kraig, 1981).
Astrocytes have long been implicated in potassium clearance from the extracellular space, removing excess K+ by current loops through the syncytium (Kuffler et al., 1966; Orkand et al., 1966; Chen and Nicholson, 2000; Walz, 2000). One feature of astrocytes that enhances their role in spatial buffering is the presence of gap junction channels, which provides electrotonic and ionic continuity among the spatially extended astrocytes necessary for the spread of current to nondepolarized regions of the syncytium (Gardner-Medwin, 1983a,b). Both in situ and in vitro experiments have shown that astrocytes are extensively coupled to one another (Dermietzel et al., 1991; Ransom, 1995), with connexin43 (Cx43) and Cx30 being the main gap junction proteins at the appositional membranes (Dermietzel et al., 2000; Nagy and Rash, 2000;Scemes et al., 2000).
Gap junction permeability is modulated by several factors, including high levels of extracellular K+ (for review, see Giaume and Venance, 1996), which has been reported to induce an increase in the strength of coupling between astrocytes (Enkvist and McCarthy, 1994). Although very little is known about the mechanisms by which high levels of K+induce the increase in astrocytic coupling, it has been proposed that it may be either related to a direct effect of membrane potential on gap junction conductance (Enkvist and McCarthy, 1994) or to the effect of intracellular pH shifts on junctional conductance resulting from depolarization-induced cytoplasmic alkalinization (Pappas and Ransom, 1994). Furthermore, alterations in junctional coupling attributable to Cx43 phosphorylation have been associated with activation of several distinct protein kinases (Godwin et al., 1993;Moreno et al., 1994a,b; Warn-Cramer et al., 1996, 1998; Saez et al., 1997); in this regard, it has been shown recently that Cx43 phosphorylation states are altered in rat brain slices after exposure to high K+ solutions (Nagy and Li, 2000) and in spinal cord after sciatic nerve stimulation (Li and Nagy, 2000).
Because K+ elevation is an essential early component of spinal cord injury, we have evaluated the extent to which spinal cord astrocytes undergo K+-induced increase in gap junction-mediated intercellular communication and obtained evidence suggesting that the Ca2+-calmodulin protein (CaM) kinase signaling pathway is involved in the K+-induced increased coupling. For these studies, we measured the degree of dye and electrical coupling and evaluated whether agents blocking the CaM kinase pathway affected K+-induced increased coupling. Furthermore, Western blot analysis and immunocytochemistry studies were performed to evaluate whether K+-induced increased coupling was related to changes in Cx43 expression levels and/or distribution.
MATERIALS AND METHODS
Astrocyte cultures. Primary cultures of spinal cord astrocytes derived from neonatal mice (C57BL/6; Charles River Laboratories, Wilmington, MA) were prepared as described previously (Scemes et al., 2000). Briefly, after 10–20 min digestion of spinal cords with 0.25% collagenase (Sigma, St. Louis, MO) in Dulbecco's PBS (Life Technologies), cells were grown for 2–3 weeks in DMEM (Cellgro, Herndon, VA) containing 5% fetal bovine serum (Gemini Bio-Products, Woodland, CA) and 1% penicillin–streptomycin (Cellgro). Approximately 90–95% of the cells were glial fibrillary acid protein (GFAP) immunopositive.
High K+solution treatments. Confluent cultures of spinal cord astrocytes were exposed to high K+ solutions (10, 25, and 50 mm; prepared in a Dulbecco's-based saline; see below) for 30 min, at 37°C, and the degree of coupling was compared with that of cells maintained for the same period of time in control Dulbecco's saline (5.4 mmK+). The high K+ solutions were prepared from a standard Dulbecco's-based saline (in mm: 100 NaCl, 5.4 KCl, 1.4 CaCl2, 0.4 MgSO4, 44 NaHCO3, 0.9 NaH2PO4, and 25 glucose, pH 7.4) by replacing NaCl with equivalent amounts of KCl to maintain iso-osmolality. In a set of experiments, low (100 μm) Ca2+ high K+ solutions were used. Bay-K-8644 (Calbiochem-Novabiochem Corp., La Jolla, CA), nifedipine, carbenoxolone, calmidazolium chloride, and KN-93 (all from Sigma) were used to evaluate their effects on K+-induced changes in coupling.
Dye coupling. The scrape loading technique (el-Fouly et al., 1987) was used to evaluate the degree of gap junctional communication. After exposure to high K+ solutions, spinal cord astrocytes plated in 35 mm dishes to confluency were bathed in PBS, pH 7.4, containing 0.5 mg/ml Lucifer yellow (LY) (Sigma). Using a razor blade, one or more scrapes per dish allowed the dye to enter the damaged cells. Five minutes after scraping, preparations were washed five to six times with PBS and then fixed in 4%p-formaldehyde (Sigma) and photographed using a Nikon (Tokyo, Japan) inverted microscope equipped with an FITC filter set to determine the extent of LY spread from the scrape edge to adjacent cells. The images were acquired with a SPOT-RT digital camera (Diagnostic Instruments, Sterling Heights, MI) and the optical density profile of LY obtained using Scion NIH Image software was used to calculate the extent of LY spread (see Fig. 1). Values of LY spread were obtained by averaging the maximal distances at which LY fluorescence could be detected (see Fig. 1A,d1, d2); at least four measurements of LY spread were obtained along each single scrape line in a minimum of two independent experiments. Fractional changes in LY spread were calculated as [(dtest/dcontrol) − 1].
Fig. 1.

Time course of changes in dye coupling induced by high K+ solution. A, Fluorescence image of LY spread between spinal cord astrocytes obtained using the scrape loading technique. The densitometric profile of LY fluorescence obtained using Scion NIH Image software is shown on theright. The distances d1 andd2 were measured as that over which LY spread from the scrape to adjacent cells; for each experiment, changes in LY spread induced by high K+ solutions were normalized against the distance of LY spread obtained under control conditions, and the mean values were expressed as fractional changes [(dtest/dcontrol) − 1] in LY spread. B, Time course of the fractional changes in LY spread between confluent cultures of spinal cord astrocytes exposed for 5, 15, 30, and 45 min to 25 mmK+ solutions. Note that, after 30 min exposure to 25 mm K+ solution, no additional increase in dye coupling was observed (baseline corresponds to the extent of LY spread between astrocytes bathed in solution containing 5.4 mmK+). C, Time course of fractional changes in LY spread observed in spinal cord astrocytes when reexposed to control (5.4 mm K+) solution after 30 min exposure to high (25 mm) K+solution. Note that dye coupling between astrocytes returned to levels similar to those observed under control (untreated) conditions within 3 hr. The bar histograms correspond to the mean ± SE values ofN (in parentheses) measurements of LY spread obtained from two independent experiments. *p < 0.05; **p < 0.001 (one-way ANOVA, followed by Student–Newman–Keuls test).
Electrical coupling. Junctional conductance between pairs of spinal cord astrocytes was measured using the dual whole-cell voltage-clamp technique (Spray et al., 1981; Srinivas et al., 1999). Freshly dissociated pairs of astrocytes were voltage clamped at holding potentials of 0 mV; small (±10 mV) and brief (100 msec duration) command steps (ΔV) were presented to one cell using pClamp6 software (Axon Instruments, Foster City, CA). Junctional currents (Ij) were recorded in the cell clamped at 0 mV; junctional conductance (gj) was calculated as −Ij/ΔV (Spray et al., 1981). Patch pipettes were filled with (in mm): 130 CsCl, 10 EGTA, 0.5 CaCl2, 3 MgATP, 2 Na2ATP, and 10 HEPES, pH 7.2. Junctional conductance of cell pairs preexposed to 25 mmK+ in the absence and presence of 100 μm KN-93 were recorded, and values were compared with cells maintained in control Dulbecco's-based saline.
Intracellular Ca2+ levels.Changes in cytosolic Ca2+ levels induced by high K+ solutions were measured as described previously (Scemes et al., 2000). Briefly, spinal cord astrocytes were loaded with 10 μm fura-2 AM (Molecular Probes, Eugene, OR) for 45 min at 37°C, washed with PBS, pH 7.4, and viewed on a Zeiss (Oberkochen, Germany) epifluorescence microscope using a Nikon UV-transparent (fluor) 40× objective. The ratio of fura-2 fluorescence emitted at two excitation wavelengths (340 and 380 nm) was obtained using a combined computerized system of optical filter wheel (Sutter Instruments, Burlingame, CA) and a shutter (Uniblitz, Rochester, NY) driven by an OEI computer (Universal Imaging Corp., West Chester, PA). The ratio images acquired with a CCD camera (Quantex) were analyzed using Metafluor Imaging software (Universal Imaging Corp.). Ca2+ levels were obtained as described previously by measuring the fluorescence ratio values during excitation at 340 and 380 nm from regions of interest after correction using calibration equation (Scemes et al., 2000). Changes in intracellular Ca2+ levels (peak − basal) induced by high K+ solutions were evaluated immediately after the addition of 10, 25, and 50 mm K+ in the absence and presence of nifedipine, Bay-K-8644, calmidazolium, and KN-93.
Western blots. Cell lysates obtained from confluent cultures of spinal cord astrocytes untreated and treated for 30 min with high K+ solutions (10, 25, and 50 mm K+) were electrophoresed in 10% SDS-polyacrylamide gels (Bio-Rad, Hercules, CA), and the separated proteins were transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, NH). The membranes were blotted for 1 hr at room temperature using anti-Cx43 18-A polyclonal antibodies (1:5000; a gift from Dr. E. L. Hertzberg, Albert Einstein College of Medicine, Bronx, NY) and then with horseradish peroxidase-conjugated anti-rabbit IgG (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA). Detection was performed on x-ray film (Fuji Photo Film Co. Ltd, Tokyo, Japan) after incubation of the membranes with enhanced chemiluminescence reagents (ECL; Amersham Pharmacia Biotech, Piscataway, NJ).
Immunocytochemistry. Spinal cord astrocytes plated on glass coverslips (24 × 60 mm) and treated with high K+ solutions were immunostained with anti-Cx43 polyclonal antibodies (Zymed, San Francisco, CA) after the LY scrape loading procedure. After fixation withp-formaldehyde, cells were washed in PBS for 15 min at room temperature and then permeabilized with 70% ethanol for 20 min at −20°C. After the 30 min exposure to 0.1% bovine serum albumin (Sigma), primary antibodies specific for Cx43 were applied at 1:3000 dilution. Goat anti-rabbit Alexa 594-conjugated IgG secondary antibodies (1:1250; Molecular Probes, Eugene, OR) were applied for 1 hr at room temperature, and cells were counterstained with 4,6-diamidino-2-phenylindole (DAPI) (1:500,000; Sigma) to visualize nuclei.
Statistical analysis. Statistical significance was evaluated from ANOVA, followed by the Student–Newman–Keuls test. Data are represented as mean ± SE of N dye spread measurements obtained from n independent experiments.
RESULTS
High extracellular [K+] induces increased dye coupling in a dose-dependent manner
To establish the time necessary for high K+ solutions to induce changes in dye coupling, confluent cultures of spinal cord astrocytes were exposed to 25 mm K+ for different periods of time, and the scrape loading technique (el-Fouly et al., 1987) was used to evaluate the degree of dye coupling (Fig.1A). After 5, 15, 30, and 45 min exposure of confluent cultures of spinal cord astrocytes to 25 mm K+ solution, a significant increase in LY spread (0.14 ± 0.06 above baseline) was observed at 5 min after exposure, attaining a plateau (0.26 ± 0.1 above baseline) at ∼30 min (Fig. 1B); interestingly, this 26% increase in dye coupling observed after 30 min exposure to 25 mm K+solution was maintained for 1.5 hr after reexposure of cultured spinal cord astrocytes to control (5.4 mmK+) solution, returning to basal levels only after 3 hr (Fig. 1C).
Based on these results, all other experiments were performed in cultured spinal cord exposed for 30 min to solutions containing different K+ concentrations. After 30 min exposure of confluent cultures of spinal cord astrocytes to solutions containing 10, 25, and 50 mmK+, LY spread was increased by 0.16 ± 0.05-fold, 0.27 ± 0.06-fold, and 0.58 ± 0.07-fold, respectively, in relation to cultures bathed in control (5.4 mm K+) solution (Fig.2A,B). [Comparison with results obtained using the dual whole-cell voltage-clamp technique (see Fig. 4B) indicates that the 0.27-fold increase in dye spread is equivalent to a fourfold increase in junctional conductance.] To evaluate whether changes in the extent of LY spread between spinal cord astrocytes exposed to high K+ solution were related to the diffusion of the dye through gap junction channels or through nonjunctional channels, confluent cultures of spinal cord astrocytes were exposed for 15 min to 100 μm carbenoxolone (a gap junction channel blocker; Davidson and Baumgarten, 1988) and then exposed to high K+ solutions containing carbenoxolone. As shown in Figure 2B, carbenoxolone (100 μm) totally prevented LY spread between astrocytes that were exposed for 30 min to control or to high K+ solutions; in these cases, LY was restricted to the scraped cells without spreading to adjacent astrocytes (Fig. 2A, right panels). In terms of the extent of LY spread between astrocytes, the gap junction channel blocker reduced the distance of dye diffusion by ∼60% when compared with control, untreated cultures (Fig.2B). Representative examples of LY spread between confluent cultures of spinal cord astrocytes exposed to 5.4 mm K+ (control) and to 50 mm K+solutions in the absence and presence of 100 μmcarbenoxolone are shown in Figure 2A.
Fig. 2.

Dose-dependent increase in dye coupling induced by high K+ solutions. A, Representative fluorescence images of LY spread between spinal cord astrocytes exposed to 5.4 mm K+(control) and to 50 mmK+ in the absence and presence of 100 μm carbenoxolone. B, Fractional changes in LY spread between spinal cord astrocytes exposed for 30 min to 10, 25, and 50 mmK+ in the absence (first 3 bars) and presence of 100 μm carbenoxolone (last 3 bars). Note that, when bathed in control (5.4 mm) K+ solution, LY spread between astrocytes was totally prevented by carbenoxolone (gap junction channel blocker); such dye coupling blockade is represented in the graph as a negative value, which in this case corresponds to a decrease of 0.65-fold in LY spread in relation to control conditions (baseline). For each experiment, changes in LY spread induced by high K+ solutions were normalized against the distance of LY spread obtained under control conditions (baseline corresponds to the extent of LY spread between astrocytes bathed in solution containing 5.4 mmK+ and 1.4 mmCa2+) (for details, see Materials and Methods and Fig. 1A). The bar histograms correspond to the mean ± SE values of N (inparentheses) measurements of LY spread obtained from three independent experiments. *p < 0.05; **p < 0.001 (one-way ANOVA, followed by Student–Newman–Keuls test).
Fig. 4.
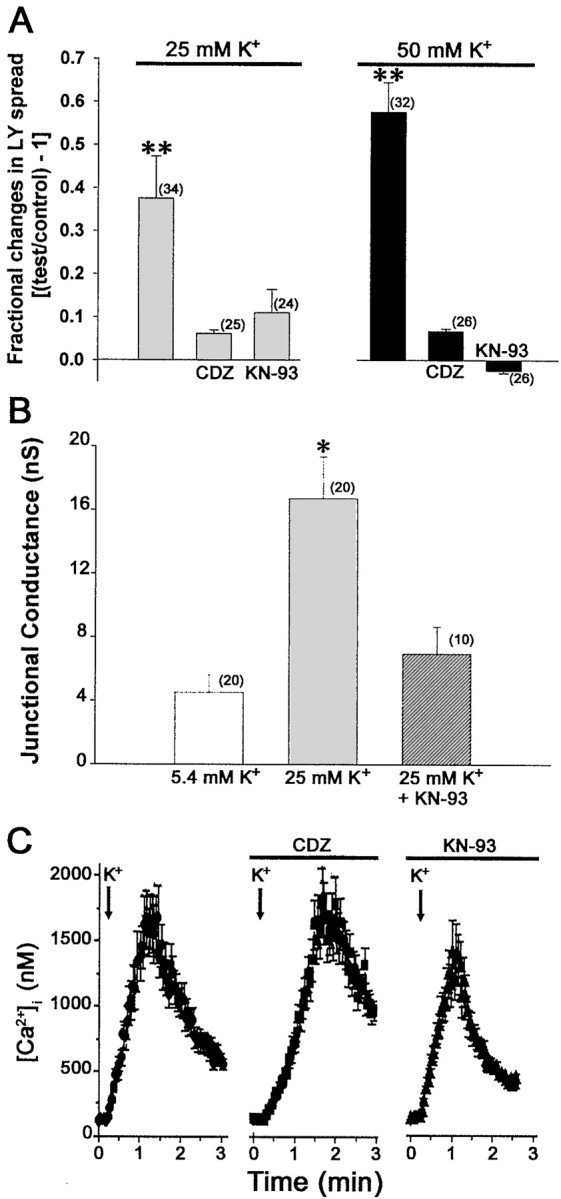
Involvement of CaM-kinase pathway in K+-induced increase in junctional communication. A, Fractional changes in LY spread between spinal cord astrocytes induced by 25 and 50 mmK+ in the absence and presence of 10 μm calmidazolium (CDZ) and 100 μm KN-93. Both the calmodulin antagonist and the CaM kinase inhibitor prevented the K+-induced increase in dye coupling. The bar histograms correspond to the mean ± SE values of N (in parentheses) measurements of LY spread obtained from three independent experiments. *p < 0.05; **p < 0.001 (one-way ANOVA, followed by Student–Newman–Keuls test).B, Mean values of junctional conductance recorded from pairs of spinal cord astrocytes under control conditions (5.4 mm K+ solution; white bar) and after 30 min exposure to 25 mmK+ in the absence (light gray bar) and presence (dark gray bar) of 100 μm KN-93. Note that KN-93 prevented the increase in electrical coupling induced by 30 min exposure to 25 mm K+. C, Intracellular Ca2+ levels recorded from fura-2 AM-loaded spinal cord astrocytes exposed to 50 mmK+ before and after preexposure to 10 μm calmidazolium (CDZ) and to 100 μm KN-93. Addition of calmidazolium or KN-93 to spinal cord astrocytes bathed in normal 5.4 mmK+ solution did not affect intracellular calcium levels and did not alter the amplitudes of the Ca2+responses induced by 50 mm K+; eachpoint in the graphs represents the mean ± SE values of intracellular calcium levels obtained from 70 cells.
These data showing that K+ induces, in a dose-dependent manner, a gap junction-mediated increase in LY spread between spinal cord astrocytes is in agreement with previous work showing increased dye coupling after exposure of glial cells to high K+ solutions (Enkvist and McCarthy, 1994;Marrero and Orkand, 1996).
It has been proposed that K+-induced increased coupling in astrocytes was attributable to a direct effect of membrane depolarization on gap junction channels (Enkvist and McCarthy, 1994). However, the conductance of gap junction channels (gj) are mainly dependent on transjunctional voltage with little dependence on absolute membrane potential (Vm) (Barrio et al., 2000); even in cases in which a degree of dependence ofgj onVm has been demonstrated, membrane depolarization was shown to decrease rather than increasegj in cells expressing Cx43 (Revilla et al., 2000).
To pursue the mechanism by which high K+induced the increase in dye coupling, we evaluated whether a Ca2+-dependent signaling pathway was involved.
K+-induced increase in coupling is related to Ca2+ influx through L-type Ca2+ channels
Because astrocyte resting membrane potential is mainly governed by the K+ equilibrium potential (Walz and Hertz, 1983; Walz et al., 1984), changes in extracellular K+ concentration lead to activation of several types of ion channels, including voltage-gated Ca2+ channels (MacVicar, 1984; MacVicar et al., 1991; Duffy and MacVicar, 1994; Westenbroek et al., 1998). We evaluated in two different sets of experiments the extent to which changes in cytosolic Ca2+ contributed to the increase in dye coupling by manipulating the amount of Ca2+ influx. In the first set of experiments, changes in intracellular Ca2+levels induced by high K+ (normal Ca2+) solutions were evaluated in fura-2 AM-loaded astrocytes in the presence and absence of an L-type Ca2+ channel blocker (50 μmnifedipine) and in the presence of an agent that prolongs L-type Ca2+ channel open time (1 μmBay-K-8644); experiments were also performed on fura-2 AM-loaded cells exposed to high K+ but low Ca2+ solutions. In the second set of experiments, the degree of dye coupling induced by high K+ solutions was evaluated using the scrape loading technique in confluent cultures of spinal cord astrocytes exposed to normal Ca2+solutions containing the L-type Ca2+channel blocker nifedipine (50 μm) or the L-type Ca2+ channel opener Bay-K-8644 (1 μm); the degree of dye coupling was also evaluated in cultures exposed to low Ca2+ but high K+ solutions.
Changes in cytosolic Ca2+ levels induced by high K+ solutions
When in low (100 μm) Ca2+ solution, fura-2 AM-loaded astrocytes responded to bath application of 10, 25, and 50 mmK+ by increasing cytosolic Ca2+ levels in a dose-dependent manner (Fig. 3A, first row); the amplitudes of these Ca2+responses were enhanced by exposing the cells to high K+ solutions containing 1.4 mm (normal) Ca2+(Fig. 3A, second row). The L-type Ca2+ channel opener Bay-K-8644 potentiated and the L-type Ca2+ channel blocker nifedipine greatly attenuated the amplitude of Ca2+ transients induced by high K+ solutions (Fig. 3A,last two rows); no changes in basal cytosolic calcium levels were observed when Bay-K-8644 or nifedipine were applied to cultures bathed in control (5.4 mmK+) solutions.
Fig. 3.
Contribution of cytosolic Ca2+ transients to K+-mediated increase in dye coupling. A, Changes in intracellular Ca2+ levels induced by high K+solutions (from left to right, 10, 25, and 50 mm) recorded from fura-2 AM-loaded spinal cord astrocytes bathed in low Ca2+ and in normal Ca2+ solutions (in the absence and presence of 1 μm Bay-K-8644 and 50 μm nifedipine). Note that the amplitudes of intracellular Ca2+ transients obtained in response to bath application of high K+solutions were attenuated by decreasing the level of extracellular Ca2+ to 100 μm (compare thefirst 2 rows) and by 50 μm nifedipine (last row) and potentiated by 1 μmBay-K-8644 (third row); each point in the graphs represents the mean ± SE values of intracellular calcium levels obtained from 60 cells. B, Correlation between changes in membrane potential and in cytosolic Ca2+levels induced by high K+ solutions; the degree of membrane depolarization was calculated according to Ransom and Goldring (1973). Note that the amplitude of the Ca2+transients is proportional to membrane depolarization and dependent on the amount of extracellular [Ca2+] and potentiated by Bay-K-8644. C, Fractional changes of LY spread between spinal cord astrocytes exposed for 30 min to high K+ (10, 25, and 50 mm) solutions under conditions in which calcium influx was manipulated by exposing the cells to low Ca2+ solution (first set of bars) or to solutions containing 1.4 mm Ca2+ in the absence (second set of bars) and in the presence of 1 μm Bay-K-8644 (third set ofbars) and 50 μm nifedipine (last set of bars); values were normalized against the extent of LY spread between astrocytes bathed in solution containing 5.4 mm K+ and 1.4 mm Ca2+ (baseline). Bay-K-8644 potentiated the effects of 10 and 25 mmK+ solutions, whereas low extracellular Ca2+ attenuated and nifedipine prevented the K+-induced increase in dye coupling. The bar histograms correspond to the mean ± SE values of N(in parentheses) measurements of LY spread obtained from three independent experiments. *p < 0.05; **p < 0.001 (one-way ANOVA, followed by Student–Newman–Keuls test). D, Graph showing the relationship between intracellular calcium levels and dye coupling obtained from data displayed in parts A andC. Note the direct relationship between the amplitude of cytosolic calcium transients and the degree of LY spread obtained from cultured astrocytes exposed to K+ solutions.
These data showing that the amplitude of Ca2+ responses of astrocytes exposed to high K+ solutions is dependent on the extracellular Ca2+ concentration and that this amplitude is modulated by two agents affecting voltage-gated Ca2+ channels indicate that, under these conditions, changes in cytosolic Ca2+levels is mainly related to the influx of Ca2+ through L-type Ca2+ channels (but see Carmignoto et al., 1998).
Considering an intracellular K+concentration of 200 mm and a 38 mV change in astrocytic membrane potential per 10-fold change in extracellular K+ concentration (Ransom and Goldring, 1973), we estimated that membrane potential would be depolarized by 11, 26, and 37 mV by exposing the cells to 10, 25, and 50 mmK+ solutions, respectively [average resting membrane potential of cultured astrocytes of approximately −60 mV (McKhann et al., 1997)]; Figure 3B shows the correlation between the degree of calculated membrane depolarization and the measured amplitude of Ca2+ responses induced by the high K+ solutions.
Relationship between cytosolic Ca2+ levels and dye coupling
When exposed to high K+ but low Ca2+ solutions, LY spread between confluent cultures of spinal cord astrocytes increased 0.16 ± 0.04-fold and 0.30 ± 0.04-fold after 30 min exposure to 25 and 50 mm K+, respectively (Fig.3C); low Ca2+ solution containing 10 mm K+, however, did not induce a measurable increase in dye coupling (Fig.3C). The increments in dye coupling induced by the two higher K+–low Ca2+ solutions were significantly lower than those observed in high K+–normal Ca2+ solutions (Fig. 3C). When bathed in normal Ca2+ solutions, Bay-K-8644 potentiated and nifedipine attenuated the K+-induced increase in dye coupling between spinal cord astrocytes (Fig. 3C). In the presence of the L-type Ca2+ channel opener, LY spread between spinal cord astrocytes exposed to 10, 25, and 50 mm K+ was increased, respectively, by 0.35 ± 0.05-fold, 0.54 ± 0.05-fold, and 0.51 ± 0.05-fold when compared with cultures exposed to normal K+ solutions in the absence of Bay-K-8644 (Fig. 3C); nifedipine (50 μm; L-type Ca2+ channel blocker) totally prevented the K+ induced increase in dye coupling (Fig. 3C). No significant changes in dye coupling were observed between astrocytes exposed to control (5.4 mm K+) solution in the absence or presence of Bay-K-8644 or nifedipine.
Although elevation of intracellular Ca2+levels has been long considered to close gap junction channels (Loewenstein, 1981) (for discussion, see Spray and Scemes, 1998), we observed a direct linear correlation between the amplitude of cytosolic calcium transients (at least up to 0.5 μm above baseline) and the extent of dye coupling when spinal cord astrocytes were exposed to high K+ solutions (Fig. 3D). It should be pointed out that because these Ca2+ transients precede the high K+-induced steady-state increase in dye coupling by 15–30 min (compare Figs. 1C, 3A), it is likely that changes in dye coupling are not directly linked to changes in cytosolic Ca2+ levels but are related to downstream events that are dependent on transient changes in Ca2+ levels. Thus, the results presented here clearly indicate that K+-induced increase in dye coupling is mediated by a signaling pathway dependent on the influx of Ca2+ through L-type Ca2+ channels that are opened by membrane depolarization.
CaM kinase pathway mediates increased dye coupling induced by high K+ solutions
CaM protein kinase II is widely distributed in neuronal and non-neuronal tissues and is involved in a variety of Ca2+-mediated events (Colbran and Soderling, 1990), including regulation of astrocytic cytoskeletal proteins vimentin and glial fibrillary acidic protein (Yano et al., 1994). Furthermore, CaM kinase II enhances chemical synaptic transmission and gap junctional conductance between Mauthner cells in the goldfish CNS (Pereda et al., 1998).
To evaluate whether a Ca2+/calmodulin-dependent protein kinase signaling pathway was involved in the increase in coupling observed in spinal cord astrocytes exposed to high K+solutions, cultures were exposed to 10 μm calmidazolium (a calmodulin antagonist) and to 100 μm KN-93 (broad-spectrum inhibitor of CaM kinases) before the addition of high K+–normal Ca2+ solutions, and scrape loading and dual whole-cell voltage-clamp techniques were used to measure the degree of coupling. Preincubation of confluent cultures with calmidazolium or with KN-93 totally prevented the increase in dye coupling induced by 25 and 50 mmK+ solutions (Fig.4A). (Exposure of confluent cultures to KN-93, for 30 min under control conditions, caused a 10% decrease in dye coupling, whereas the preexposure to calmidazolium was totally inert.) Dual whole-cell voltage-clamp recordings on isolated pairs of spinal cord astrocytes pretreated for 30 min with 25 mm K+solution before and after pretreatment with KN-93 indicated that the fourfold increase in junctional conductance (from 4 to 17 nS) (Fig.4B) induced by the high K+ solution was prevented by the CaM kinase inhibitor KN-93 (Fig. 4B).
It has been reported recently that calmidazolium may reduce and KN-93 may increase Ca2+ influx (Sunagawa et al., 1999; Bhatt et al., 2000; Harper and Daly, 2000; Jan and Tseng, 2000), thus potentially misleading the interpretation of results implying the participation of a CaM kinase signaling pathway in a particular event. Therefore, we evaluated whether these two compounds affected calcium influx induced by high K+ solutions in fura-2 AM-loaded spinal cord astrocytes. Bath application of 100 μm calmidazolium or 10 μm KN-93 did not affect basal cytosolic Ca2+ levels of spinal cord astrocytes bathed in control (5.4 mmK+) solution; furthermore, Ca2+ influx induced by 50 mmK+ solution was not prevented when cultures were preincubated with calmidazolium and was not potentiated by the preexposure of cells to KN-93 (Fig. 4C). Together, these results provide additional support for the hypothesis that a CaM kinase pathway is involved in the increase in coupling induced by exposure of astrocytes to high K+solutions.
Connexin43 expression levels and distribution are not altered after exposure to high K+ solutions
Cultured spinal cord astrocytes from neonatal mice express multiple connexins, with Cx43 contributing ∼70% of total junctional conductance (Scemes et al., 2000). Because gap junctions formed by the other connexins Cx45, Cx40, and Cx26 are not permeable to the negatively charged LY (Veenstra et al., 1994; Beblo et al., 1995; Beblo and Veenstra, 1997; Wang and Veenstra, 1997), it is more likely that Cx43 mediates the K+-induced increased coupling observed here to occur between spinal cord astrocytes. Although selective permeability of gap junctions formed by the other astrocytic connexin Cx30 is not known, it is unlikely that this connexin contributes to the increased coupling; Cx30 has been reported to be highly expressed in adult murine brain, and such late onset in Cx30 expression can also be observed in vitro, being detected only 1 month after plating (Kunzelmann et al., 1999). Given that we used 2- to 3-week-old cultures from neonatal mice, it is unlikely that Cx30 would significantly contribute to the increased coupling induced by high K+ solutions.
To evaluate whether the increase in coupling observed in spinal cord astrocytes treated with high K+ solutions was related to increased expression levels and/or to changes in Cx43 distribution that might have resulted from the activation of CaM kinase signaling pathway, Western blot analysis and immunocytochemistry were performed in spinal cord astrocyte cultures exposed to high K+ solutions. No significant changes in protein levels or in Cx43 distribution were observed in cultured spinal cord astrocytes treated with high K+solutions (Fig.5A,B). Based on this result, it is therefore likely that K+-induced increased coupling is related to changes in the number of open gap junction channels between cells rather than to incorporation of newly synthesized protein or redistribution of preexisting Cx43 within cells.
Fig. 5.

Expression levels and distribution of Cx43 in spinal cord astrocytes exposed to high K+solutions. A, Mean values of Cx43 expression levels obtained from Western blot analyses of confluent cultures of spinal cord astrocytes exposed for 30 min to 10, 25, and 50 mm K+ solutions. No significant difference in Cx43 expression levels was observed under high K+ conditions. Cx43 expression levels were quantified using Scion NIH Image software and normalized against control (first bar); mean ± SE values were obtained from six independent experiments. An example of Cx43 immunoblot is shown on the top of the bar histograms; the arrows indicate the phosphorylated (top 2 arrows) and the nonphosphorylated (bottom arrow) Cx43 isoforms. B, C, Fluorescence images obtained from confluent culture of spinal cord astrocytes under control condition (B) and after 30 min exposure to 50 mm K+ solution (C) showing the extent of LY (green) spread from the scrape to adjacent cells; after the scrape loading, the cells were immunostained with Cx43 antibodies (red) and with the nuclear stain DAPI (blue). Note that exposure of spinal cord astrocytes to high K+ solutions did not alter Cx43 distribution. Images were obtained with a Nikon 100× oil immersion objective using the SPOT-RT digital camera. Scale bar, 16 μm.
DISCUSSION
It is shown here that gap junctional communication between cultured spinal cord astrocytes is increased after exposure to high K+ solutions and that this increased coupling is mediated by CaM protein kinase, most likely CaM kinase II. This kinase is known to regulate through phosphorylation the activity of proteins involved in a variety of cellular processes in response to elevation of cytosolic Ca2+ induced by membrane receptor activation and by depolarizing agents (high K+) (Fukunaga et al., 1992; Lorca et al., 1993; Braun and Schulman, 1995; Soderling, 1995). After binding of Ca2+–calmodulin, the autophosphorylated CaM kinase II can maintain an autonomous Ca2+-independent activity for a prolonged period of time after CaM dissociation from the autophosphorylated subunit (Fukunaga et al., 1996; Soderling, 2000). The magnitude of this autonomous activity, and thus the duration of its effect, is dependent on the amplitude, frequency, and duration of cytosolic Ca2+ elevation (De Koninck and Schulman, 1998).
Evidence favoring the participation of CaM kinases in the K+-induced increased coupling obtained here includes (1) the direct relationship between the amplitude of Ca2+ transients and the degree of dye coupling, (2) the blockade of K+-induced increased coupling by the calmodulin antagonist calmidazolium and by the inhibitor of CaM kinases KN-93, and (3) the long-term increase in coupling that outlasts the stimulus (Fig. 1C). Although presently available inhibitors cannot distinguish which CaM kinases isoforms are involved in this process (KN-93 is a general inhibitor of CaM kinases; Soderling 2000), it seems likely that CaM kinase II is involved in the increased coupling observed in spinal cord astrocytes treated with high K+ solutions; CaM kinase II has long been identified in astrocytes (Bronstein et al., 1988), and 10 distinct CaM kinase II isoforms were immunolocalized in different subcellular compartments (Takeuchi et al., 2000).
Very few studies have examined the role of CaM kinase II in astrocyte physiology or in gap junctional communication. However, in rat cortical astrocytes, CaM kinase II has been shown to be involved in the regulation of cytoskeletal proteins (vimentin and GFAP) after glutamate receptor stimulation (Yano et al., 1994), and in the goldfish mixed synapses, CaM kinase II activation was reported to mediate the enhancement of both gap junctional and glutamatergic transmission that occurs during long-term potentiation (Pereda et al., 1998).
Alteration in junctional coupling is to some extent correlated with phosphorylation of gap junction proteins (connexins), which can occur on multiples sites as a result of activity of various protein kinases (Saez et al., 1998; Cooper et al., 2000). For instance, activation of protein kinase C (PKC), which phosphorylates Cx43 at serine 368 (Lampe et al., 2000), decreases junctional conductance and dye transfer between cells (Kwak and Jongsma, 1996; Lampe et al., 2000), whereas activation of protein kinase A (PKA) upregulates junctional communication (Burghardt et al., 1995; Chanson et al., 1996; Darrow et al., 1996; Matesic et al., 1996; Paulson et al., 2000). Although it has been reported that CaM kinase II phosphorylates connexin32 at serine residues different from the sites phosphorylated by cAMP-dependent protein kinase (PKA) or PKC (Saez et al., 1990), no functional studies were performed to evaluate effects of CaM kinase phosphorylation on the degree of coupling. Interestingly, however, chemical gating of Cx32 has been proposed to be regulated by the direct binding of CaM to Cx32 N terminus (Peracchia et al., 2000).
Although biochemical evidence for the direct interaction between Cx43 and CaM kinase II is beyond the scope of the present study, we hypothesize that K+-induced increased coupling is effected by CaM kinase II phosphorylation of the C terminus of Cx43. Prediction of serine and threonine residues of Cx43 protein that would be potential phosphorylation sites for CaM kinase II (Phospho-2 software; see www.cbs.dtu.dk; Blom et al., 1999) has identified four putative CaM kinase II consensus sequences (R-X-X-S/T-X) in the C terminus of mouse Cx43: S296, S365, S369, and S373. Interestingly, it was reported recently, by the use of three different Cx43 antibodies recognizing different epitopes in the Cx43 C terminus, that exposure of brain slices to 15 mmK+ prevented detection of the phosphorylated 43 kDa band of Cx43 by an antibody whose epitope spans amino acids 360–376 but did not prevent detection by Cx43 antibodies recognizing amino acids 241–260 and 346–360 (Nagy and Li, 2000); the authors suggested that such epitope masking of Cx43 might result from conformational changes attributable to phosphorylation at or near the region 360–376, which contains three of the four putative phosphorylation sites for CaM kinase II.
Given that we did not observe any change in expression levels or distribution of Cx43 in spinal cord astrocytes treated with high K+ solutions, we suggest that CaM kinase-mediated increased coupling might be attributable to an increased number of active channels within gap junction plaques. Using Cx43 fused to a green fluorescence protein to evaluate the relationship between Cx43 distribution and electrical coupling, Bukauskas et al. (2000) estimated that only a small fraction (10–20%) of channels are active in average-sized gap junction plaques in transfected cell pairs (0.5 μm diameter containing ∼2000 channels). To account for the fourfold increase in electrical coupling measured in our experiments (from 4 to 17 nS) after 30 min exposure of spinal cord astrocytes to 25 mm K+, fewer than 200 additional Cx43 channels would need to be activated, representing an additional 10% of the channels within a plaque of this size (single-channel conductance of gap junction channels formed by Cx43 of 70–90 pS) (Moreno et al., 1994a,b).
In conclusion, the present work shows that K+-induced increase in coupling between spinal cord astrocytes is mediated by the CaM kinase pathway; this increased coupling, which is shown here to have a fast onset (minutes) and to persist for long periods of time (hours), is expected to affect K+ buffering power of astrocytes, expanding the distance to which ions and small molecules can diffuse through the interconnected syncytium. Long-term increases in gap junction-mediated coupling (LINC), in which the functional changes outlast the stimulus, have been reported for both neurons (Pereda et al., 1998) and astrocytes (this work). Although LINC may have different consequences in these two cell types (presumably increasing synchronization in neurons and extending the volume of effective intercellular space in astrocytes), in both cases, LINC is expected to provide a degree of plasticity in intercellular signaling.
Footnotes
This work was supported by Christopher Reeves Paralysis Foundation Grant SBI-9802-2 (to E.S.), by National Institutes of Health Grant NS-34931 (to D.C.S.), and by an American Heart Association Heritage Postdoctoral Fellowship (to M.S.). We are grateful to Drs. A. E. Pereda and R. Dermietzel for their suggestions and helpful discussions.
Correspondence should be addressed to Dr. Eliana Scemes, Department of Neuroscience, Kennedy Center, Room 924, Albert Einstein College of Medicine, Bronx, NY 10461. E-mail: scemes@aecom.yu.edu.
REFERENCES
- 1.Astrup J, Norberg K. Potassium activity in cerebral cortex in rats during progressive severe hypoglycemia. Brain Res. 1976;103:418–423. doi: 10.1016/0006-8993(76)90817-9. [DOI] [PubMed] [Google Scholar]
- 2.Barrio LC, Revilla A, Gomez-Hernandez JM, de Miguel M, Gonzalez D. Membrane potential dependence of gap junctions in vertebrates. In: Peracchia C, editor. Gap junctions: molecular basis of cell communication in health and disease. Academic; San Diego: 2000. pp. 175–188. [Google Scholar]
- 3.Beblo DA, Veenstra RD. Monovalent cation permeation through the connexin40 gap junction channel, Cs+, Rb+, K+, Li+, TEA, TMA, TBA, and effects of anions Br−, Cl−, F−, acetate, aspartate, glutamate, and NO3. J Gen Physiol. 1997;109:509–522. doi: 10.1085/jgp.109.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beblo DA, Wang HZ, Beyer EC, Westphale EM, Veenstra RD. Unique conductance, gating and selective permeability properties of gap junction channels formed by connexin40. Circ Res. 1995;77:813–822. doi: 10.1161/01.res.77.4.813. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt HS, Conner BP, Prasanna G, Yorio T, Easom RA. Dependence of insulin secretion from permeabilized pancreatic β-cells on activation of Ca2+/calmodulin-dependent protein kinase II. A re-evaluation of inhibitor studies. Biochem Pharmacol. 2000;60:1655–1663. doi: 10.1016/s0006-2952(00)00483-4. [DOI] [PubMed] [Google Scholar]
- 6.Blank WF, Jr, Kirshner HS. The kinetics of extracellular potassium changes during hypoxia and anoxia in the cat cerebral cortex. Brain Res. 1977;123:113–124. doi: 10.1016/0006-8993(77)90646-1. [DOI] [PubMed] [Google Scholar]
- 7.Blom N, Gammeltoft S, Brunak S. Sequence- and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 8.Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu Rev Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- 9.Bronstein J, Nishimura R, Lasher R, Cole R, de Villes J, Farber D, Wasterlain C. Calmodulin kinase II in pure cultured astrocytes. J Neurochem. 1988;50:45–49. doi: 10.1111/j.1471-4159.1988.tb13227.x. [DOI] [PubMed] [Google Scholar]
- 10.Bukauskas FF, Jordan K, Bukauskiene A, Bennett MVL, Lampe PD, Laird DW, Verselis VK. Clustering of connexin43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells. Proc Natl Acad Sci USA. 2000;97:2556–2561. doi: 10.1073/pnas.050588497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burghardt RC, Barhoumi R, Sewwall TC, Bowen JA. Cyclic AMP induces rapid increase in gap junction permeability and changes in the cellular distribution of connexin43. J Membr Biol. 1995;148:243–253. doi: 10.1007/BF00235042. [DOI] [PubMed] [Google Scholar]
- 12.Carmignoto G, Pasti L, Pozzan T. On the role of voltage-dependent calcium channels in calcium signaling of astrocytes in situ. J Neurosci. 1998;18:4637–4645. doi: 10.1523/JNEUROSCI.18-12-04637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chanson M, White MM, Garber SS. cAMP promotes gap junctional coupling in T84 cells. Am J Physiol. 1996;271:C533–C539. doi: 10.1152/ajpcell.1996.271.2.C533. [DOI] [PubMed] [Google Scholar]
- 14.Chen KC, Nicholson C. Spatial buffering of potassium ions in brain space. Biophys J. 2000;78:2776–2797. doi: 10.1016/S0006-3495(00)76822-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colbran RJ, Soderling T. Calcium/calmodulin-dependent protein kinase II. Curr Top Cell Regul. 1990;31:181–221. doi: 10.1016/b978-0-12-152831-7.50007-x. [DOI] [PubMed] [Google Scholar]
- 16.Cooper CD, Solan JL, Dolejsi MK, Lampe PD. Analysis of connexin phosphorylation sites. Methods. 2000;20:196–204. doi: 10.1006/meth.1999.0937. [DOI] [PubMed] [Google Scholar]
- 17.Darrow BJ, Fast VG, Kleber AG, Beyer EC, Saffitz JE. Functional and structural assessment of intercellular communication. Increased conduction velocity and enhanced connexin expression in dibutyryl cAMP-treated cultured cardiac myocytes. Circ Res. 1996;79:174–183. doi: 10.1161/01.res.79.2.174. [DOI] [PubMed] [Google Scholar]
- 18.Davidson JS, Baumgarten IM. Glycyrrhetinic acid derivates: a novel class of inhibitors of gap junctional intercellular communication. Structure-activity relationships. J Pharmacol Exp Ther. 1988;246:1104–1107. [PubMed] [Google Scholar]
- 19.De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 20.Dermietzel R, Hetrtzberg EL, Kessler JA, Spray DC. Gap junctions between cultured astrocytes: immunocytochemical, molecular, and electrophysiological analysis. J Neurosci. 1991;11:1421–1432. doi: 10.1523/JNEUROSCI.11-05-01421.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dermietzel R, Gao Y, Scemes E, Vieira D, Urban M, Kremer M, Bennett MVL, Spray DC. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res Rev. 2000;32:45–56. doi: 10.1016/s0165-0173(99)00067-3. [DOI] [PubMed] [Google Scholar]
- 22.Duffy S, MacVicar BA. Potassium-dependent calcium influx in acutely isolated hippocampal astrocytes. Neuroscience. 1994;61:51–61. doi: 10.1016/0306-4522(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 23.El-Fouly MH, Trosko JE, Chang CC. Scrape-loading and dye transfer: a rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res. 1987;168:422–430. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]
- 24.Enkvist MO, McCarthy KD. Astroglial gap junction communication is increased by treatment with either glutamate or high K+ concentration. J Neurochem. 1994;62:489–495. doi: 10.1046/j.1471-4159.1994.62020489.x. [DOI] [PubMed] [Google Scholar]
- 25.Fukunaga K, Soderling TR, Miyamoto E. Activation of Ca2+/calmodulin-dependent protein kinase II and protein kinase C by glutamate in cultured rat hippocampal neuron. J Biol Chem. 1992;267:527–533. [PubMed] [Google Scholar]
- 26.Fukunaga K, Muller D, Miyamoto E. CaM kinase II in long-term potentiation. Neurochem Int. 1996;28:343–358. doi: 10.1016/0197-0186(95)00097-6. [DOI] [PubMed] [Google Scholar]
- 27.Gardner-Medwin AR. A study of the mechanisms by which potassium moves through brain tissue in rat. J Physiol (Lond) 1983a;335:353–374. doi: 10.1113/jphysiol.1983.sp014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner-Medwin AR. Analysis of potassium dynamics in mammalian brain tissue. J Physiol (Lond) 1983b;335:393–426. doi: 10.1113/jphysiol.1983.sp014541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giaume C, Venance L. Characterization and regulation of gap junction channels in cultured astrocytes. In: Spray DC, Dermietzel R, editors. Gap junctions in the nervous system. Landes; Austin, TX: 1996. pp. 136–157. [Google Scholar]
- 30.Godwin AJ, Green LM, Walsh MP, McDonald JR, Walsh DA, Fletcher WH. In situ regulation of cell-cell communication by the cAMP-dependent protein kinase and protein kinase C. Mol Cell Biochem. 1993;127–128:293–307. doi: 10.1007/BF01076779. [DOI] [PubMed] [Google Scholar]
- 31.Harper JL, Daly JW. Effect of calmidazolium analogs on calcium influx in HL-60 cells. Biochem Pharmacol. 2000;60:317–324. doi: 10.1016/s0006-2952(00)00349-x. [DOI] [PubMed] [Google Scholar]
- 32.Jan CR, Tseng CJ. Calmidazolium-induced rises in cytosolic calcium concentrations in Madin–Darby Canine Kidney cells. Toxicol Appl Pharmacol. 2000;162:142–150. doi: 10.1006/taap.1999.8844. [DOI] [PubMed] [Google Scholar]
- 33.Kuffler SW, Nicholls JG, Orkand RK. Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29:768–787. doi: 10.1152/jn.1966.29.4.768. [DOI] [PubMed] [Google Scholar]
- 34.Kunzelmann P, Schroder W, Traub O, Steinhauser C, Dermietzel R, Willecke K. Late onset and increasing expression of gap junction protein connexin30 in adult murine brain and long-term cultured astrocytes. Glia. 1999;25:111–119. doi: 10.1002/(sici)1098-1136(19990115)25:2<111::aid-glia2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 35.Kwak BR, Jongsma HJ. Regulation of cardiac gap junctional channel permeability and conductance by several phosphorylating conditions. Mol Cell Biochem. 1996;157:93–99. doi: 10.1007/BF00227885. [DOI] [PubMed] [Google Scholar]
- 36.Lampe PD, TenBrock EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine 368 by protein kinase C regulates gap junctional communication. J Cell Biol. 2000;149:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li WE, Nagy JI. Activation of fibers in rat sciatic nerve alters the phosphorylation state of connexin43 at astrocytic gap junctions in spinal cord: evidence for junctional regulation by neuronal-glial interactions. Neuroscience. 2000;97:113–123. doi: 10.1016/s0306-4522(00)00032-4. [DOI] [PubMed] [Google Scholar]
- 38.Loewenstein WR. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981;61:829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- 39.Lorca T, Cruzalegui FH, Fesquet D, Cavadore JC, Mery J, Means A, Doree M. Calmodulin-dependent protein kinase II mediates inactivation of MPF and CSF upon fertilization of Xenopus eggs. Nature. 1993;366:270–273. doi: 10.1038/366270a0. [DOI] [PubMed] [Google Scholar]
- 40.MacVicar BA. Voltage-dependent Ca2+ channels in glial cells. Science. 1984;266:1345–1347. doi: 10.1126/science.6095454. [DOI] [PubMed] [Google Scholar]
- 41.MacVicar BA, Hochman D, Delay MJ, Weiss S. Modulation of intracellular Ca2+ in cultured astrocytes by influx through voltage activated Ca2+ channels. Glia. 1991;4:448–455. doi: 10.1002/glia.440040504. [DOI] [PubMed] [Google Scholar]
- 42.Marrero H, Orkand RK. Nerve impulses increase glial intercellular permeability. Glia. 1996;16:285–289. doi: 10.1002/(SICI)1098-1136(199603)16:3<285::AID-GLIA11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 43.Matesic DF, Hayashi T, Trosko JE, Germak JA. Upregulation of gap junctional intercellular communication in immortalized gonadotropin-releasing hormone neurons by stimulation of the cyclic AMP pathway. Neuroendocrinology. 1996;64:286–297. doi: 10.1159/000127130. [DOI] [PubMed] [Google Scholar]
- 44.McKhann GM, D'Ambrosio R, Janigro D. Heterogeneity of astrocyte resting membrane potentials and intercellular coupling revealed by whole-cell and gramicidin-perforated patch recordings from cultured neocortical and hippocampal slice astrocytes. J Neurosci. 1997;17:6850–6863. doi: 10.1523/JNEUROSCI.17-18-06850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreno AP, Rook MB, Fishman GI, Spray DC. Gap junction channels: distinct voltage-sensitive and -insensitive conductance states. Biophys J. 1994a;67:113–119. doi: 10.1016/S0006-3495(94)80460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moreno AP, Saez JC, Fishman GI, Spray DC. Human connexin43 gap junction channels: regulation of unitary conductance by phosphorylation. Circ Res. 1994b;74:1050–1057. doi: 10.1161/01.res.74.6.1050. [DOI] [PubMed] [Google Scholar]
- 47.Nagy JI, Li WE. A brain slice model for in vitro analyses of astrocytic gap junction and connexin43 regulation: actions of ischemia, glutamate and elevated potassium. Eur J Neurosci. 2000;12:4567–4572. [PubMed] [Google Scholar]
- 48.Nagy JI, Rash JE. Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res Rev. 2000;32:29–44. doi: 10.1016/s0165-0173(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 49.Nicholson C, Kraig RP. The behavior of extracellular ions during spreading depression. In: Zeuthen T, editor. The application of ion-selective microelectrodes. Elsevier; Amsterdam: 1981. pp. 217–238. [Google Scholar]
- 50.Orkand RK, Nicholls JG, Kuffler SW. Effects of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29:788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- 51.Pappas CA, Ransom BR. Depolarization-induced alkalinization (DIA) in rat hippocampal astrocytes. J Neurophysiol. 1994;72:2816–2826. doi: 10.1152/jn.1994.72.6.2816. [DOI] [PubMed] [Google Scholar]
- 52.Paulson AF, Lampe PD, Meyer RA, TenBroek E, Atkinson MM, Walseth TF, Johnson RG. Cyclic AMP and LDL trigger a rapid enhancement in gap junction assembly through a stimulation of connexin trafficking. J Cell Sci. 2000;113:3037–3049. doi: 10.1242/jcs.113.17.3037. [DOI] [PubMed] [Google Scholar]
- 53.Peracchia C, Sotkis A, Wang XG, Peracchia LL, Persechini A. Calmodulin directly gates gap junction channels. J Biol Chem. 2000;275:26220–26224. doi: 10.1074/jbc.M004007200. [DOI] [PubMed] [Google Scholar]
- 54.Pereda AE, Bell TD, Chang BH, Czernik AJ, Nairn AC, Soderling TR, Faber DS. Ca2+/calmodulin-dependent kinase II mediates simultaneous enhancement of gap junctional conductance and glutamatergic transmission. Proc Natl Acad Sci USA. 1998;95:13272–13277. doi: 10.1073/pnas.95.22.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ransom BC. Gap junctions. In: Kettenmann H, Ransom BC, editors. Neuroglia. Oxford UP; Oxford: 1995. pp. 299–318. [Google Scholar]
- 56.Ransom BR, Goldring S. Ionic determinants of membrane potential of cells presumed to be glia in cerebral cortex of cat. J Neurophysiol. 1973;36:855–868. doi: 10.1152/jn.1973.36.5.855. [DOI] [PubMed] [Google Scholar]
- 57.Revilla A, Bennett MV, Barrio LC. Molecular determinants of membrane potential dependence in vertebrate gap junction channels. Proc Natl Acad Sci USA. 2000;97:14760–14765. doi: 10.1073/pnas.97.26.14760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saez JC, Nairn AC, Czernik AJ, Spray DC, Hertzberg EL, Greengard P, Bennett MVL. Phosphorylation of connexin32, a hepatocyte gap-junction protein, by cAMP-dependent protein kinase, protein kinase C and Ca2+/calmodulin-dependent protein kinase II. Eur J Biochem. 1990;192:263–273. doi: 10.1111/j.1432-1033.1990.tb19223.x. [DOI] [PubMed] [Google Scholar]
- 59.Saez JC, Nairn AC, Czernik AJ, Fishman GI, Spray DC, Hertzberg EL. Phosphorylation of connexin43 and the regulation of neonatal rat cardiac myocyte gap junctions. J Mol Cell Cardiol. 1997;29:2131–2145. doi: 10.1006/jmcc.1997.0447. [DOI] [PubMed] [Google Scholar]
- 60.Saez JC, Martinez AD, Branes MC, Gonzalez HE. Regulation of gap junctions by protein phosphorylation. Braz J Med Biol Res. 1998;31:593–600. doi: 10.1590/s0100-879x1998000500001. [DOI] [PubMed] [Google Scholar]
- 61.Scemes E, Suadicani SO, Spray DC. Intercellular communication in spinal cord astrocytes: fine tuning between gap junctions and P2 nucleotide receptors in calcium wave propagation. J Neurosci. 2000;20:1435–1445. doi: 10.1523/JNEUROSCI.20-04-01435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soderling TR. Calcium-dependent protein kinases in learning and memory. Adv Second Messenger Phosphoprotein Res. 1995;30:175–189. doi: 10.1016/s1040-7952(05)80007-2. [DOI] [PubMed] [Google Scholar]
- 63.Soderling TR. CaM-kinases: modulators of synaptic plasticity. Curr Opin Neurobiol. 2000;10:375–380. doi: 10.1016/s0959-4388(00)00090-8. [DOI] [PubMed] [Google Scholar]
- 64.Somjen GG. Extracellular potassium in the mammalian central nervous system. Annu Rev Physiol. 1979;41:159–177. doi: 10.1146/annurev.ph.41.030179.001111. [DOI] [PubMed] [Google Scholar]
- 65.Spray DC, Scemes E. Effects of intracellular pH (and Ca2+) on gap junction channels. In: Kaila K, Ransom BR, editors. pH and brain function. Wiley; New York: 1998. pp. 477–489. [Google Scholar]
- 66.Spray DC, Harris AL, Bennett MV. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981;77:77–83. doi: 10.1085/jgp.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Srinivas M, Costa M, Gao Y, Fort A, Fishman GI, Spray DC. Voltage dependence of macroscopic and unitary currents of gap junction channels formed by mouse connexin50 expressed in rat neuroblastoma cells. J Physiol (Lond) 1999;517:673–689. doi: 10.1111/j.1469-7793.1999.0673s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sunagawa M, Yokoshiki H, Seki T, Nakamura M, Laber P, Sperelakis N. Direct block of Ca2+ channels by calmidazolium in cultured vascular smooth muscle cells. J Cardiovasc Pharmacol. 1999;34:488–496. doi: 10.1097/00005344-199910000-00003. [DOI] [PubMed] [Google Scholar]
- 69.Takeuchi Y, Yamamoto H, Fukunaga K, Miyakawa T, Miyamoto E. Identification of the isoforms of Ca2+/Calcmodulin-dependent protein kinase II in rat astrocytes and their subcellular localization. J Neurochem. 2000;74:2557–2567. doi: 10.1046/j.1471-4159.2000.0742557.x. [DOI] [PubMed] [Google Scholar]
- 70.Veenstra RD, Wang HZ, Beyer EC, Brink PR. Selective dye and ionic permeability of gap junction channels formed by connexin45. Circ Res. 1994;75:483–490. doi: 10.1161/01.res.75.3.483. [DOI] [PubMed] [Google Scholar]
- 71.Walz W. Role of astrocytes in the clearance of excess extracellular potassium. Neurochem Int. 2000;36:291–300. doi: 10.1016/s0197-0186(99)00137-0. [DOI] [PubMed] [Google Scholar]
- 72.Walz W, Hertz L. Comparison between fluxes of potassium and chloride in astrocytes in primary cultures. Brain Res. 1983;277:321–328. doi: 10.1016/0006-8993(83)90940-x. [DOI] [PubMed] [Google Scholar]
- 73.Walz W, Wuttke W, Hertz L. Astrocytes in primary cultures: membrane potential characteristics reveal exclusive potassium conductance and potassium accumulator properties. Brain Res. 1984;292:367–374. doi: 10.1016/0006-8993(84)90772-8. [DOI] [PubMed] [Google Scholar]
- 74.Wang HZ, Veenstra RD. Monovalent ion selectivity sequences of rat connexin43 gap junction channel. J Gen Physiol. 1997;109:491–507. doi: 10.1085/jgp.109.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Warn-Cramer BJ, Lampe PD, Kurata WE, Kanemitsu MY, Loo LW, Eckhart W, Lau AF. Characterization of the mitogen-activated protein kinase phosphorylation sites on the connexin43 gap junction protein. J Biol Chem. 1996;271:3779–3786. doi: 10.1074/jbc.271.7.3779. [DOI] [PubMed] [Google Scholar]
- 76.Westenbroek RE, Bausch SB, Lin RC, Franck JE, Noebels JL, Caterall WA. Upregulation of L-type Ca2+ channels in reactive astrocytes after brain injury, hypomyelination, and ischemia. J Neurosci. 1998;18:2321–2334. doi: 10.1523/JNEUROSCI.18-07-02321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yano S, Fukunaga K, Ushio Y, Miyamoto E. Activation of Ca2+/calmodulin-dependent protein kinase II and phosphorylation of intermediate filament proteins by stimulation of glutamate receptors in cultured cortical astrocytes. J Biol Chem. 1994;269:5428–5439. [PubMed] [Google Scholar]



