Abstract
The conductance, gj, of many gap junctions depends on voltage between the coupled cells (transjunctional voltage, Vj) with little effect of the absolute membrane potential (Vm) in the two cells; others show combined Vj and Vm dependence. We examined the molecular determinants of Vm dependence by using rat connexin 43 expressed in paired Xenopus oocytes. These junctions have, in addition to Vj dependence, Vm dependence such that equal depolarization of both cells decreases gj. The dependence of gj on Vm was abolished by truncation of the C-terminal domain (CT) at residue 242 but not at 257. There are two charged residues between 242 and 257. In full-length Cx43, mutations neutralizing either one of these charges, Arg243Gln and Asp245Gln, decreased and increased Vm dependence, respectively, suggesting that these residues are part of the Vm sensor. Mutating both residues together abolished Vm dependence, although there is no net change in charge. The neutralizing mutations, together or separately, had no effect on Vj dependence. Thus, the voltage sensors must differ. However, Vj gating was somewhat modulated by Vm, and Vm gating was reduced when the Vj gate was closed. These data suggest that the two forms of voltage dependence are mediated by separate but interacting domains.
Gap junction channels are unique among ionic channels in that they span two cell membranes. They are composed of two hemichannels, one in each membrane, that are tightly docked head to head to form a pore that directly connects the cytoplasm of two cells (1). In vertebrates, gap junctions are formed by connexins (Cx), a gene family of protein subunits (2). Given their architecture, gap junction channels are subject to the influence of two types of voltage, that between the two cell interiors termed transjunctional voltage (Vj), and that between the interior and exterior or the membrane potential (Vm), which can differ between the cells and thus along the lumen of the channel connecting the cells. Many gap junctions are sensitive to Vj with little effect of Vm, whereas others show combined Vj and Vm dependence. The eponymous gap is accessible to small ions and because there is negligible leak through the channel wall, the potential in the gap is likely to be close to that in the bathing medium (3). Vm dependence of junctional conductance (gj) was initially described in invertebrate (deuterostome) gap junctions (4–7), which are formed by innexins, a family of proteins unrelated to connexins (8). More recently, gj dependence of Vm has also been demonstrated in junctions formed of vertebrate connexins in exogenous expression systems (9–11) as well as in native cells (12, 13). Junctions comprised of different connexin isoforms have divergent properties of Vm dependence, varying in their polarity of closing, voltage sensitivity, and kinetics (14).
Vj dependence of vertebrate gap junctions has been extensively analyzed by site-directed mutagenesis (15–18), but the molecular basis of Vm gating remains unknown. There is increasing evidence that separate gates mediate Vm and Vj dependence, a hypothesis supported by the striking differences in the gating induced by the two types of potential (14) and by the divergent evolution of their properties among Cx45 junctions in different vertebrate classes (10). Because the junctional conductance of rat Cx43 channels shows sensitivity to Vm as well as Vj, we chose to study the molecular determinants for Vm regulation in these channels. Cx43 is perhaps the most abundant gap junction protein and is widely distributed in vertebrate cells and tissues (19), being a major component of intercellular channels between cardiac myocytes, astrocytes, and uterine smooth muscle cells. In the present study, we have identified two charged amino acids located at the most proximal region of the carboxyl-terminal domain (CT) that are likely to be an integral part of the Vm sensor. Mutations of these residues altered Vm dependence markedly but had no effect on Vj dependence. From these and previously reported data, a model was derived with fast and slow Vj gates and a Vm gate in each hemichannel.
Materials and Methods
Mutagenesis and Channel Expression in Paired Oocytes.
The mutants (G242stop, R243Q, and D245Q) were constructed by PCR mutagenesis of rat Cx43 cDNA, as previously described for rat Cx43 truncated at position 257 (S257stop; ref. 18), using the following primers: G242stop, sense 5′-TGATATCCGAGCTGTCGATTATGGAGGAGA-3′ that created a new EcoRV site, and antisense 5′-CTTCACGCGATCCTTAACGCCTTTGAAGAA-3′; R243Q, sense 5′-GATCCTTACCACGCCACCACTGGCCC-3′, and antisense 5′-CGATTGTCCCTTCTTCACGCGATCCTTAAC-3′, which in combination created a new BamHI site; D245Q, sense 5′-AGATCTCAGCCTTACCACGCCACCACTGGC-3′, and antisense 5′-TCTTCCCTTCACGCGATCCTTAACGCC T-3′, which introduced a new BglII site. The mutant carrying the double R243Q and D245Q substitution was obtained by using the D245Q mutant as template and the following primers: sense 5′-AGATCTCAGCCTTACCACGCCACCACTGGC-3′ and antisense 5′-TTGTCCCTTCACGCGATCCTTAACGCCT-3′, which destroyed the BglII site created in the D245Q construct. The cRNA synthesis and purification were performed as previously described (18). Oocytes were removed from the ovaries of Xenopus laevis (African Xenopus Facility, South Africa) under anesthesia and prepared as described (13).
Electrophysiology and Data Analysis.
The macroscopic junctional conductance (gj) between the paired oocytes injected with cRNAs (0.1–0.5 μg/μl; 50 nl/oocyte) and an antisense directed against Xenopus Cx38 mRNA (10 ng/oocyte; ref. 20) were measured with the dual voltage clamp technique (21). The influence of Vm on gj was explored by applying equal displacements of the membrane potential in both cells of a pair (V1 and V2) while gj was monitored by test Vj pulses (10 mV, 500 ms, 1 Hz), too small and brief to induce changes in gj. The Vj pulses were created by voltage steps in cell 1, and defined as Vj = V1 − V2, whereas currents injected in cell 2 to hold its potential constant were equal in magnitude and opposite in sign to the currents flowing through the junctional channels (Ij). Voltages and currents in cell 1 and 2 are displayed positive up so that Ij in cell 2 is downward for positive Vj in Figs. 1 and 2. Because total current in cell 2 was the sum of junctional (Ij) and sometimes slowly changing nonjunctional (Inj) currents induced by the simultaneous application of the Vm step and the Vj pulses, the junctional current in Fig. 3C was calculated as Ij = I2(Vj = 10 mV) − I2(Vj = 0) measured at the center of each Vj pulse and at the center of the next interpulse interval. For this figure, Ij is plotted positive up. gj was calculated as Ij/Vj. Influence of Vj on gj (Fig. 3B) was characterized as in Revilla et al. (18). Stimulation and data collection were performed as previously described (10, 18).
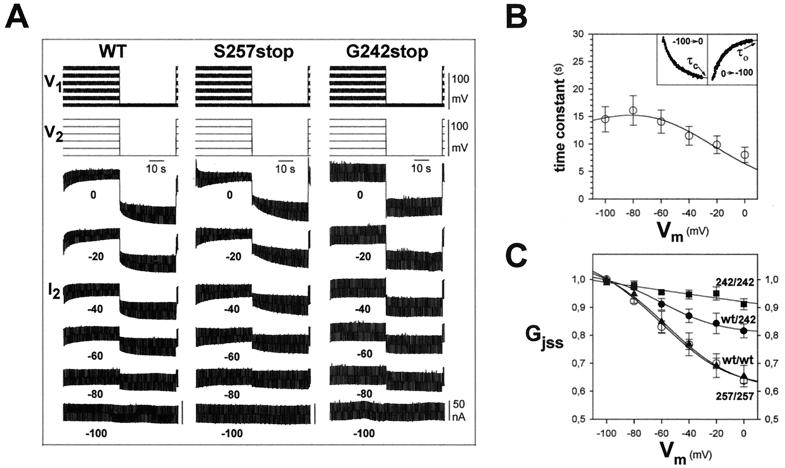
Figure 1.
Localization of the Vm determinants in the CT domain of rat Cx43. WT and the Cx43 channels truncated at positions S257stop and G242stop were expressed in Xenopus laevis oocytes pairs. (A) Sample records of nonjunctional and junctional currents (I2) induced by the equal displacement of membrane potentials in the two cells (V1 and V2; steps of 40 s from −100 to 0 mV in increments of 20 mV, returning to −100 mV for 40 s between each step). The junctional currents (Ij) measured in oocyte 2 after application of small Vj pulses in oocyte 1 (+10 mV, 500 ms, 1 Hz). For WT (left) and S257stop (center) junctions, Ij decreased on depolarization, more rapidly and to progressively lower steady state values as more positive potentials were achieved. Ij recovered to its initial values on returning to −100 mV. The mutation G242stop largely abolished the dependence of junctional conductance (gj) on Vm (right). (B) Vm dependence of the time constant of decrease in gj. The time course of relaxations to Gjss was well fit by single exponentials (Inset) with long time constants that were shorter at more positive voltages. The smooth curve is derived from calculations assuming a single Boltzmann model. (C) Graphs of Gjss/Vm relations where Gjss is normalized to the value at −100 mV. The smooth curves are derived from fits to the square of a Boltzmann relation based on a model of two independent gates in series, one in each hemichannel (except for WT-G242stop, for which a single Boltzmann and gate were assumed). The parameters are given in Table 1. The Gjss of WT (○) and truncated S257stop (▴) junctions decreased by ≈50% when Vm increased from −100 to 0 mV. Gjss of truncated G242stop junctions (■) was quite insensitive to Vm. The Gjss/Vm curve of hybrid WT-G242stop junctions (●) was in between those of the homotypic junctions of the component combinations, suggesting that only the Vm gate of WT hemichannels contributed to their Vm dependence. Each point in B represents mean values (±SEM) of six pairs.
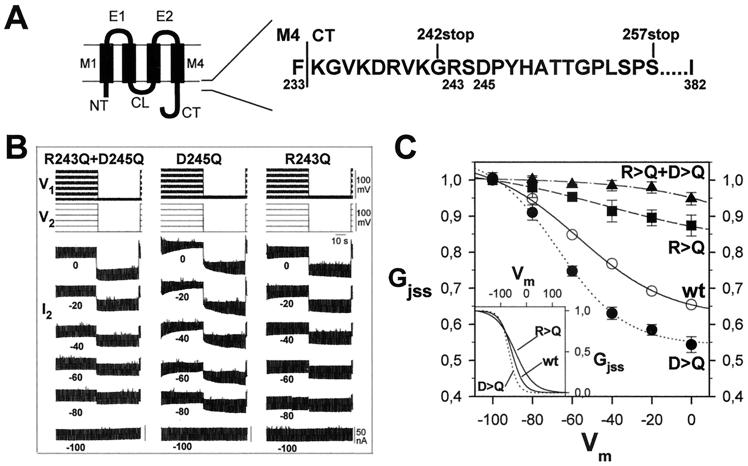
Figure 2.
Effects of mutations R243Q and D245Q on Vm gating. (A) Diagram of the topology of connexins (left). There are four transmembrane domains (M1–M4), two extracellular (E1, E2), cytoplasmic N- and C-terminal (NT, CT), and a cytoplasmic loop (CL). Amino acid sequence of the CT at its border with M4 (right) showing sites of the truncation mutants. The region between residues 242 and 257 contains one positively charged residue, Arg-243, and one negatively charged residue, Asp-245. (B) Sample records of nonjunctional and junctional currents as in Fig. 1 for mutants carrying single and double neutralizations (R243Q+D245Q, left; D245Q, center; and R243Q, right). The double mutation virtually abolished Vm sensitivity. The single mutations, D245Q and R243Q, increased and reduced dependence of gj on Vm, respectively. (C) Graphs of Gjss/Vm relations. The curves are fits of the squared Boltzmann relations with parameters of Table 1. The gj of junctions carrying the double R243Q+D245Q neutralization (▴) was not Vm sensitive. The single mutations, R243Q (■, broken line) and D245Q (●, dotted line), altered in opposite directions the steepness of Vm gating relative to WT (○, continuous line). The mutations also produced corresponding changes in V0, the voltage for half change in the Vm sensitive component, relative to WT (Inset). Each point in C represents mean values (±SEM) of six pairs.
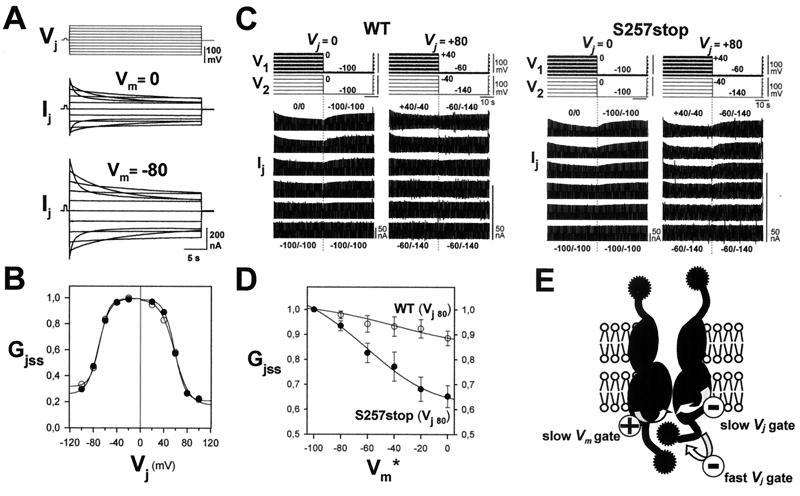
Figure 3.
Interactions between Vj and Vm gating. (A) Effect of Vm on Vj dependence. Records of junctional currents (Ij) elicited by the same Vj steps (±100 mV by 20 mV increments and 30 s duration) applied at two holding potentials (Vm = 0 and −80 mV). The Vm difference changed junctional conductance from gj(0 mV) = 2.72 μS to gj (−80 mV) = 4.80 μS, but the junctional currents declined for increasing positive and negative Vj steps with similar characteristics (positive Ij are shown upward). (B) Plots of Gjss/Vj relations normalized to gj at 0 (○) and −80 mV (●) were essentially superimposable, indicating that Vj gating was little affected by Vm. (C) Effect of Vj on Vm dependence. Sample records of junctional currents (Ij) after subtraction of nonjunctional currents evoked by the same Vm protocol as in Fig. 1 for Vj = 0 (left) and Vj = +80 mV (right) in pairs expressing WT and truncated S257stop junctions. Conductances at the two holding potential were gj(Vj = 0) = 8.31 and gj(Vj = 80) = 1.57 μS for WT junctions and gj(Vj = 0) = 10.81 to gj(Vj = 80) = 1.27 μS for S257stop junctions. With Vj = 0 mV, equal depolarization of both cells equally decreased gj of WT and of S257stop junctions. However, when gj was reduced at Vj = +80 mV, Vm sensitivity of WT junctions was markedly reduced, whereas sensitivity of S257stop junctions in which the fast Vj gate was removed (18) was little affected. For comparison, current gains are increased for the Vj = +80 mV records. (D) Gjss/Vm relations of WT (○) and truncated S257stop (●) junctions for Vj = +80 where Gj is normalized to the value at Vm*. The curves are fits of the squared Boltzmann relations with parameters of Table 1. Each point represents mean values (±SEM) of four pairs. (E) Gating model for the combined Vj and Vm dependence of Cx43 junctions. See Discussion.
Steady state gj values (gjss) measured at the end of Vj pulses were normalized relative to the gj value for brief Vj prepulses (10 mV), and the Gjss/Vj relation was modeled according to the two-state Boltzmann model (21). In the Vm protocols, gjss was normalized to its value at a holding potential of −100 mV to give Gjss, which was plotted as a function of Vm. To analyze the Vm data, we assumed two independent Vj gates in series, one per hemichannel (5, 10, 14). We also assumed that each gate could be either open or completely closed and that for each gate the steady state probability that it was open, poss, was given by a Boltzmann relation of the form:
Poss = {(Pomax − Pomin)/(1 + exp[A(Vm − V0)])} + Pomin
where Pomax and Pomin are the maximum and minimum values of Po, V0 is the voltage at which Poss = (Pomax − Pomin)/2 + PominP, A [A = nq/kT] is a constant that expresses the voltage sensitivity in terms of gating charge, the equivalent number (n) of electron charges (q) moving through the entire membrane voltage, and kT has its usual significance. Junctions with a single functional Vm gate on one side, i.e., junctions formed of wild-type (WT) Cx43 on one side and a truncation mutant lacking Vm dependence on the other side are described below. For these junctions, Po times N, the number of channels, times γ, the single channel conductance, gives gj, and after normalization:
Gjss = {(Gjmax − Gjmin)/(1 + exp[A(Vm − V0)])} + Gjmin
where Gjss is the normalized steady state value as a function of Vm, Gjmax is the maximum steady state conductance, and Gjmin is the residual conductance or Vm insensitive component. The same form of the equation applies to the situation where the channel closes to a substate, γs, rather than to a non-zero Po. Parameters of voltage dependence were determined by treating Gjmax, Gjmin, A, and V0 as free parameters and applying an iterative procedure of fitting (10). The time course of gj transitions was fitted with exponentials with R > 0.999 by using CLAMPFIT (pCLAMP, Axon, CA) and the τ/Vm relation calculated from the equations derived from first-order kinetics (22).
The model differs somewhat for channels that have two functional Vm gates and zero conductance if one or both of the Vm gates is closed, i.e., the closed gates do not have a finite residual conductance (we will justify this assumption in the presentation of Fig. 3). In this case, the open probability will be given by the square of Boltzmann relation for the single gate:
Po = {(Pomax − Pomin)/(1 + exp[A(Vm − V0)]) + Pomin}2
where Po is the steady state open probability of the channel, and Pomax and Pomin are the limiting values of the open probabilities for the two series gates. If Pomax approaches 1, Pomin will be given by (Gjmin/Gjmax)1/2.
Results
Localization of Molecular Determinants for Vm Gating in the Cx43 Molecule.
Rat connexin 43 junctions exhibit combined membrane potential (Vm) and transjunctional voltage (Vj) dependence of junctional conductance, gj (9, 18). The Vm dependence of gj was explored independently of Vj dependence by applying equal displacements of the membrane potential in two cells of a coupled pair. With Vj = 0, increasing depolarizing steps applied to both cells induced progressive reduction in gj. On returning to the holding potential of −100 mV, gj recovered its initial value (Fig. 1A WT). The steady state Gj/Vm curve was well described by the square of a Boltzmann relation (Fig. 1C, Table 1), a calculation based on the assumption that there are two identical but independent Vm gates in series, one per hemichannel. Over the range of membrane potentials explored, Gjss changed ≈80% of the difference between the extrapolated values of Gjmax and Gjmin. Decay and recovery of gj in response to application of Vm were monotonic and approximately exponential, with time constants of several seconds (Fig. 1B). We did not attempt to fit the time course data with a squared Boltzmann relation.
Table 1.
Boltzmann parameters for Vm-dependence of WT and mutant RC×43 junctions
| A, mV−1 | n, 20°C | V0, mV | Gjmax | Gjmin | Pomin | |
|---|---|---|---|---|---|---|
| WT/WT (Vj = 0)* | 0.043 | 1.09 | −54 | 1.03 | 0.78 | 0.61 |
| WT/WT (Vj = 80)* | 0.031 | 0.79 | −38 | 1.01 | 0.92 | 0.85 |
| S257stop/S257stop (Vj = 0)* | 0.041 | 1.06 | −59 | 1.04 | 0.79 | 0.62 |
| S257stop/S257stop (Vj = 80)* | 0.037 | 0.94 | −62 | 1.05 | 0.77 | 0.59 |
| G242stop/G242stop | NE | NE | NE | NE | NE | NE |
| G242stop/WT† | 0.046 | 1.16 | −60 | 1.02 | 0.81 | — |
| R243Q + D245Q/R243Q + D245Q* | NE | NE | NE | NE | NE | NE |
| R243Q/R243Q* | 0.027 | 0.69 | +11 | 1.04 | 0.91 | 0.83 |
| D245Q/D245Q* | 0.059 | 1.48 | −64 | 1.04 | 0.41 | 0.64 |
Gjss/Vm relations of homotypic junctions were described by the square of a Boltzmann relation, based on a model of two independent Vm gates in series, one in each hemichannel, where each gate could be either open or completely closed.
The Gjss/Vm relation of the heterotypic G242stop-WT junctions was fit by a single Boltzmann relation, assuming that only the gate of the WT hemichannels was contributing to the Vm sensitivity; the calculated parameters were close to those of the WT gate obtained by fitting a squared Boltzmann. NE, not estimated; the changes in Gjss were too small to allow accurate determination of the parameters. However, the smooth curves for these junctions in Figs. 1–3 were obtained by the same fitting procedures as for the more sensitive junctions.
It has been previously reported that the gj transitions induced by Vj in RCx43 junctions have fast and slow components, the fast component being larger at larger Vj (≈80% at Vj = −100 mV). Truncation of the CT domain by the mutation S257stop alters the transitions; there is a single component of decay in gj with a time constant somewhat shorter than that of the slow component of WT junctions (18). Vm dependence of Gjss of S257stop–S257stop junctions was virtually identical to that of the WT channels (Fig. 1A S257stop and 1C; Table 1), indicating that residues 257–382 of the CT domain do not participate in Vm regulation. However, shortening of the CT to the minimum length that still retains channel-forming ability (23) by the mutation G242stop almost completely abolished gj dependence on Vm (Fig. 1A G242stop) whereas Vj gating did not change significantly. The conductance of hybrid junctions comprised of G242stop hemichannels on one side and WT hemichannels in the other cell was Vm sensitive, but the sensitivity of Gjss was intermediate between those of the respective homotypic junctions (Fig. 1C). The Gjss/Vm relation for G242stop-WT junctions was well fit by a single Boltzmann function with parameters close to those used in the squared Boltzmann relation to describe the behavior of homotypic WT junctions (Table 1). This result suggests that the Vm dependence of the hybrid junctions was due to the single Vm gate contributed by the WT hemichannels, a conclusion previously reported for other hybrid junctions (10, 14). Taken together, these results strongly suggest that the CT region between 242–257 contains residues required for Vm dependence of RCx43 junctions.
Identification of Residues that Participate in Vm Sensing.
The loss of Vm sensitivity caused by the truncation of the CT domain at position 242 but not at position 257 may be due to loss of charged residues that contain or contribute to the Vm sensor. Remarkably, there are only two charged residues in the primary sequence of RCx43 in this region, positively charged Arg-243 and negatively charged Asp-245 (Fig. 2A). To investigate the role of these charges, we engineered full-length Cx43 in which one or both of these amino acids were replaced by glutamine, a polar uncharged residue. The double neutralization, R243Q and D245Q, virtually abolished Vm dependence (Fig. 2B R243Q+D245Q and 2C), whereas the D245Q mutation increased sensitivity and the R243Q mutation decreased it, but not to the extent of the double mutation (Figs. 2B R243Q and D245Q and 2C). These data indicate that Arg-243 and Asp-245 are required for Cx43 channels to sense Vm. The single neutralization of the positively charged residue, R243Q, reduced Vm dependence and decreased the equivalent gating charge (Table 1). Conversely, neutralization of the negatively charged residue (D245Q) increased Vm dependence and increased the equivalent gating charge by a similar amount. The R243Q mutation moved V0 in the positive direction whereas the D245Q mutation shifted V0 to a more negative value, as appropriate for the changes in gating charge (Fig. 2C Inset). The single and double neutralizations had little if any effect on Vj dependence of gj (illustrated in supplementary text and supplementary Fig. 4, which are published as supplemental data on the PNAS web site, www.pnas.org).
Interactions Between Vm Gating and Vj Gating.
Because truncation at residue 257 affects Vj sensitivity (18), and truncation at nearby residue 242 blocks Vm sensitivity with no further change in Vj sensitivity, we investigated whether Vj and Vm gating interact. At Vm values of −80 and 0 mV at which Gjss differed almost 2-fold, Vj gating was virtually the same (Fig. 3 A and B). The kinetics was not distinguishably different, and the steady state Gjss/Vj relations, normalized to the different values of gj at Vj = 0, also were little affected. These data indicate that Vj gating operates independently of Vm gating. Previously, mouse Cx45 junctions were shown to have very similar Vj gating at different Vm values that changed gj on depolarization by a factor of 2.5 (10).
We also studied Vm gating in the presence of a fixed, non-zero Vj. Cx43 junctions are particularly suitable for this kind of analysis because the residual conductance or Vj-insensitive component is large enough to allow estimation of Vm effects at large Vj's. For the example of Fig. 3, we defined Vm* = (V1 + V2)/2 and examined Vm* dependence for Vj = +80 mV from Vm* = −100 mV to 0 mV. A Vj of +80 mV at Vm* = −100 mV, i.e., V1 = −60 mV and V2 = −140 mV, decreased conductance to a value very close to the Vj-insensitive component (Fig. 3B). Equal depolarization of both cells induced an additional reduction in gj, but the sensitivity to Vm was reduced somewhat more than would result from block of a single Vm gate (Figs. 3C WT and 3D). Thus, the presence of a large Vj appears to interfere with operation of both Vm gates. To explore this interaction further, we carried out the same experiment evaluating the effect of a maintained Vj on Vm dependence in S257stop–S257stop junctions, which lack fast Vj gating (18). Furthermore, Gjmin was markedly smaller for these mutant junctions than for wild type, 0.16 vs. 0.36 respectively for Vj < 0 and 0.13 vs. 0.24 for Vj > 0. The Gjss/Vm relation was little affected by this mutation (Figs. 1A S257stop and 1C; Fig. 3C S257stop left). Moreover, when gj was decreased by a Vj of +80 mV, the fractional change in gj was the same as in the absence of Vj gradient (Figs. 3C S257stop right and 3D). Thus, truncation at 257, which abolished the fast Vj gating, restored Vm gating in the presence of Vj.
Discussion
Structure–Function Analysis of Vm Dependence of Cx43 Channels.
We identified two charged residues, Arg-242 and Asp-245, that are likely to be an integral part of the Vm sensor and that are located in the (cytoplasmic) CT domain close to its junction with M4, the fourth transmembrane domain. Mutations of these residues that altered Vm gating did not have any influence on Vj gating, and the S257stop truncation that greatly modified Vj gating did not affect Vm sensitivity. Taken together along with the difference in residual conductance, Gjmin, the results indicate that Vm and Vj regulation of Cx43 junctional conductance is mediated by separate but interacting domains.
Truncation by the mutation G242stop greatly reduced Vm sensitivity whereas truncation by S257stop had little effect on this property. Only two of the residues between positions 242 and 257 are charged, Arg-243 and Asp-245. When the acidic residue was replaced by Gln, a neutral amino acid, the Gjss/Vm relation was increased in steepness and V0 was shifted in the negative direction, suggesting that D245 is part of the Vm sensor. When the basic residue was replaced by Gln (R243Q), the Gjss/Vm relation was decreased in steepness and V0 was shifted in the positive direction, suggesting that R243 is also part of the Vm sensor and that it moves in the same direction as D245 during Vm gating. However, the effects of the two mutations did not summate, and the double mutation, which is electrically neutral, virtually abolished Vm dependence. Apparently the double mutation caused structural changes that prevented Vm gating. Although the anatomical symmetry of gap junctions suggests that there are two Vm gates in series, a single Boltzmann relation fit the Gjss/Vm relation about as well as the squared Boltzmann relation (with different parameters). However, the heterotypic junctions WT-G242stop would be expected to have only one Vm sensitive gate, and the single Boltzmann with the same parameters as for the squared Boltzmann applied to WT junctions gave an excellent fit. This observation is strong evidence for series Vm gates and applicability of the squared Boltzmann to WT junctions.
A Novel Gating Model for Connexin Channels with Combined Vj and Vm Dependence.
A model for the actions of Vj and Vm on Cx43 channels can be derived from data obtained in this paper and a previous analysis of the Vj dependence (18). We hypothesize that each hemichannel contains a set of three gates, one fast gate and one slow gate under Vj control and one slow gate regulated by Vm (Fig. 3D). The three gates appear to involve different regions of the connexin molecule, because they can be modified independently by different mutations and their residual conductances are different. The distal part of CT domain is involved in the fast Vj gating process, because deletion of the 257–382 region modified the time course of gj transitions from a two-component decay to a single-component with a slow time constant. Block of the fast component by the 257 truncation and the asymmetry of the Gjss/Vj relation in heterotypic WT-S257stop junctions suggest that there are two Vj gating mechanisms within the hemichannel, each operating with the same negative polarity of closing, defined as relative negativity in cytoplasmic side (18). The distal end of the CT may move to partially occlude the channel during fast Vj gating as has been proposed for pH gating (24). Interestingly, attachment of aequorin (25) or of enhanced green fluorescent protein (EGFP; ref. 26) to the CT of a full-length Cx43 blocks fast Vj gating. The mutations Arg-243 and Asp-245 affecting Vm gating also are located in the CT but more proximal to the region required for fast Vj gating. In the absence of Vj gradients, Cx43 conductance is exclusively dependent on Vm, which induces closure by membrane potential depolarization. When the CT domain was truncated at position 242, fast Vj and Vm gating were blocked, but the slow Vj gating seen in the S257stop mutant was little affected. In Cx43 junctions, the sensor for the slow mechanism of Vj gating may correspond to those initially proposed for Cx32 and Cx26 junctions, where the charged amino acids located in the NT domain and at the beginning of the first extracellular loop determine the polarity of closing of the channels (15, 17).
In this model, Gj should be governed by the combined action of three gating mechanisms according to their kinetic properties and their polarity of closing. Moreover, the effects on conductance will be also determined by the nature of interactions between the gating mechanisms, which will depend critically on their structural basis and where they are located with respect to each other and to the channel lumen. Interactions could be allosteric or could result from changes in the local electric field by the gating mechanisms. The fast and slow Vj gates may interact electrically. At large Vj, the fast Vj component dominates the conductance change of WT junctions, and Gjmin was larger than in S257stop junctions. This result suggests that the fast gate has a larger residual conductance and prevents closure of the slow gate. That the time constants for the 257 truncation mutant are somewhat shorter than for the slow component in the WT may result from a larger voltage across the sensor in the absence of the fast gating mechanism. Operating in a different time regime, a large value of Vj that closes most Vj gates modifies Vm gating, and it appears that the Vm gate does not operate when the Vj gate is closed (Fig. 3 C and D). (See supplemental material for further discussion of Vj–Vm interactions.)
Single channel analysis of the gating mechanisms should reveal the classes of gating events, including their probabilities, residual conductances, and intervening states. Vj induces fast transitions between fully or partially open states and residual states of low conductance (27). In addition, slow transitions to a fully closed state have been recorded at larger Vj in WT junctions (28). These two kinds of transition presumably underlie the two types of Vj sensitivity considered here. In Cx43-EGFP junctions, which lack fast Vj gating, fast transitions to a residual open state are not present, and channels close fully by slow transitions (26). The events in Vm gating have not yet been observed at the single channel level, but the ability of Vm to further reduce the Vj-insensitive component of macroscopic conductance is an indication that Vm gating mediates transitions between open and fully closed states of channels. Full closure of the channels is also suggested by the fact that the Vj gating properties of Cx43 (Fig. 3 A and B) do not change significantly at different membrane potentials although the Vm induces large variations in macroscopic junctional conductance. If Vm gates closed to a subconductance state, one would be expected that the voltage across the Vj gates would change affecting kinetics and steady state values.
The model presented here may be applicable in whole or in part to other Vm- and Vj-sensitive gap junctions of vertebrates (11, 14). The dependence on Vm in some vertebrate junctions is sufficiently great that intercellular coupling could be directly regulated by the membrane potential, a parameter that is always present and that varies, particularly in excitable cells. Dynamic changes of coupling coefficient in phase with membrane potential oscillations occur in pancreatic β-cells (29), which may indicate that the conductance of those junctions is Vm dependent. Vj gating would regulate coupling when membrane potentials differed, as in asynchronously active excitable cells.
Supplementary Material
Acknowledgments
We thank E. C. Beyer for providing rat Cx43 cDNA. We gratefully acknowledge Rosa Barquero for technical assistance. This work was supported by a grant of the European Community (QLG1-CT-1999-00516 to L.C.B.). M.V.L.B. is supported by National Institutes of Health Grant NS-07512 and is the Sylvia and Robert S. Olnick Professor of Neuroscience.
Abbreviations
- Cx
connexin
- gj
junctional conductance
- Vm
membrane potential
- Vj
transjunctional voltage
- CT
carboxyl-terminal domain
- WT
wild type
References
- 1.Unger V M, Kumar N M, Gilula N B, Yeager M. Science. 1999;283:1176–1180. doi: 10.1126/science.283.5405.1176. [DOI] [PubMed] [Google Scholar]
- 2.Bruzzone R, White T W, Paul D L. Eur J Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- 3.Bennett M V L, Verselis V K. Semin Cell Biol. 1992;3:29–47. doi: 10.1016/s1043-4682(10)80006-6. [DOI] [PubMed] [Google Scholar]
- 4.Obaid A L, Socolar S J, Rose B. J Membr Biol. 1983;73:69–89. doi: 10.1007/BF01870342. [DOI] [PubMed] [Google Scholar]
- 5.Verselis V K, Bennett M V L, Bargiello T A. Biophys J. 1991;59:114–126. doi: 10.1016/S0006-3495(91)82204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukauskas F F, Kempf C, Weingart R. J Physiol (London) 1992;448:321–337. doi: 10.1113/jphysiol.1992.sp019044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Churchill D, Caveney S. J Membr Biol. 1993;135:165–180. doi: 10.1007/BF00231442. [DOI] [PubMed] [Google Scholar]
- 8.Phelan P, Stebbing L A, Baines R A, Bacon J P, Davies J A, Ford C. Nature (London) 1998;391:181–185. doi: 10.1038/34426. [DOI] [PubMed] [Google Scholar]
- 9.White T W, Bruzzone R, Wolfram S, Paul D L, Goodenough D A. J Cell Biol. 1994;125:879–892. doi: 10.1083/jcb.125.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrio L C, Capel J, Jarillo J A, Castro C, Revilla A. Biophys J. 1997;73:757–769. doi: 10.1016/S0006-3495(97)78108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manthey D, Bukauskas F, Lee C G, Kozak C A, Willecke K. J Biol Chem. 1999;274:14716–14723. doi: 10.1074/jbc.274.21.14716. [DOI] [PubMed] [Google Scholar]
- 12.Verselis V K, Bargiello T A, Rubin J B, Bennett M V L. Prog Cell Res. 1993;3:97–104. [Google Scholar]
- 13.Zhao H B, Santos-Sacchi J. J Membr Biol. 2000;175:17–24. doi: 10.1007/s002320001051. [DOI] [PubMed] [Google Scholar]
- 14.Barrio L C, Revilla A, Gómez-Hernández J M, de Miguel M, González D. In: Gap Junctions: Molecular Basis of Cell Communication in Health and Disease. Peracchia C, editor. San Diego: Academic; 2000. pp. 175–188. [Google Scholar]
- 15.Verselis V K, Ginter C S, Bargiello T A. Nature (London) 1994;368:348–351. doi: 10.1038/368348a0. [DOI] [PubMed] [Google Scholar]
- 16.Ri Y, Ballesteros J A, Abrams C K, Oh S, Verselis V K, Weinstein H, Bargiello T A. Biophys J. 1999;76:2887–2898. doi: 10.1016/S0006-3495(99)77444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh S, Rubin J B, Bennett M V L, Verselis V K, Bargiello T A. J Gen Physiol. 1999;114:339–364. doi: 10.1085/jgp.114.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Revilla A, Castro C, Barrio L C. Biophys J. 1999;77:1374–1383. doi: 10.1016/S0006-3495(99)76986-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beyer E C, Paul D L, Goodenough D A. J Cell Biol. 1987;105:2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrio L C, Suchyna T, Bargiello T A, Xu L X, Roginski R S, Bennett M V L, Nicholson B J. Proc Natl Acad Sci USA. 1991;88:8410–8414. doi: 10.1073/pnas.88.19.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spray D C, Harris A L, Bennett M V L. J Gen Physiol. 1981;77:77–93. doi: 10.1085/jgp.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris A L, Spray D C, Bennett M V L. J Gen Physiol. 1981;77:95–117. doi: 10.1085/jgp.77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunham B, Liu S, Taffet S, Trabda-Janik E, Delmar M, Tetryshyn R, Zheng S, Perzova R, Vallano M L. Circ Res. 1992;70:1233–1243. doi: 10.1161/01.res.70.6.1233. [DOI] [PubMed] [Google Scholar]
- 24.Morley G E, Taffet S M, Delmar M. Biophys J. 1996;70:1294–1302. doi: 10.1016/S0006-3495(96)79686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin P E M, George C H, Castro C, Kendall J M, Capel J, Campbell A K, Revilla A, Barrio L C, Evans W H. J Biol Chem. 1998;273:1719–1726. doi: 10.1074/jbc.273.3.1719. [DOI] [PubMed] [Google Scholar]
- 26.Bukauskas F F, Jordan K, Bukauskiene A, Bennett M V L, Lampe P D, Laird D W, Verselis V K. Proc Natl Acad Sci USA. 2000;97:2556–2561. doi: 10.1073/pnas.050588497. . (First Published March 7, 2000; 10.1073/pnas.050588497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreno A P, Rook M B, Spray D C. Biophys J. 1994;67:113–119. doi: 10.1016/S0006-3495(94)80460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banach K, Weingart R. Pflügers Arch Eur J Physiol. 2000;439:248–250. doi: 10.1007/s004249900182. [DOI] [PubMed] [Google Scholar]
- 29.Andreu A, Soria B, Sanchez-Andres J V. J Physiol (London) 1997;498:753–761. doi: 10.1113/jphysiol.1997.sp021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.