Abstract
Mice lacking both of the best-known platelet ligands, von Willebrand factor and fibrinogen, can still form occlusive thrombi in injured arterioles. The platelets of these animals accumulate excessive amounts of fibronectin (FN). These observations led us to examine the contribution of plasma FN (pFN) to thrombus formation. Inactivation of the FN gene in FN conditional knockout mice reduced pFN levels to <2% and platelet FN to ≈20% of the levels in similarly treated control mice. The mice were then observed in a model of arterial injury to evaluate their capacity to form thrombi. The deficiency of pFN did not affect the initial platelet adhesion, but a delay of several minutes in thrombus formation was observed in the arterioles of pFN-deficient mice as compared with control mice. The thrombi that formed in the absence of pFN were stably anchored to the vessel wall but continuously shed platelets or small platelet clumps, thus slowing their growth significantly; the platelet/platelet cohesion was apparently diminished. Consequently the occlusion of pFN-deficient vessels was delayed, with the majority of vessels remaining patent at the end of the 40-min observation period. We conclude that, in addition to von Willebrand factor and fibrinogen, FN plays a significant role in thrombus initiation, growth, and stability at arterial shear rates and that deficiency in each of the three platelet ligands has its own specific impact on platelet plug formation.
Von Willebrand factor (vWF) and fibrinogen are generally considered the most important ligands supporting platelet thrombus formation and thrombus growth to an occlusive size. In an intravital microscopy model of arteriolar injury induced by ferric chloride (1, 2), we tested the importance of these ligands in vivo. Animals deficient in both vWF and fibrinogen showed delayed thrombogenesis but, surprisingly, occlusive thrombi still formed in 73% of the injured arterioles either at the site of injury or downstream (2). Mice deficient only in fibrinogen actually showed no apparent defect in thrombus growth, except in stability of large thrombi, suggesting an important role of fibrinogen/fibrin in stably anchoring the thrombus to the vessel wall (2). Similar observations were obtained in a perfusion chamber at high shear by using blood from afibrinogenemic patients (3, 4). In contrast, analyses in the same thrombosis model of mice deficient in β3 integrins show a complete absence of thrombus formation (unpublished data). Thus there appears to be another prominent ligand(s) of β3 integrins beside vWF and fibrinogen that mediates thrombus growth.
When we examined platelets deficient in fibrinogen (2), we found that the fibronectin (FN) content of these platelets was >3-fold increased over WT platelets, suggesting that FN might be implicated in thrombus formation in the absence of fibrinogen and perhaps even when fibrinogen is present. In contrast, the levels of other adhesion molecules interacting in vitro with platelets, thrombospondin 1 and vitronectin, were not elevated in the fibrinogen- or vWF/fibrinogen-deficient platelets (2). We have examined mice deficient in thrombospondin 1 (5) in our thrombosis model and found no obvious defects (H.N., unpublished observations). Mice deficient in vitronectin have been reported to display enhanced thrombosis (6) but also decreased stability of the occlusive thrombi (7). Thus the role of vitronectin in thrombosis needs further clarification.
Early studies of potential involvement of FN in platelet adhesion and/or aggregation were suggestive but inconclusive (reviewed in refs. 8 and 9). Plasma FN (pFN) is present at ≈0.5 μM, corresponding to 300 μg/ml in humans and an even higher concentration in mice (580 μg/ml) (10). FN becomes incorporated into blood clots and FN is a known ligand of αIIbβ3 integrin. Platelet α-granules contain FN that is released on platelet activation and some is retained at the platelet surface (8, 9). These data all suggest that FN could play a role in platelet thrombosis. This possibility has been difficult to evaluate in the in vivo situation because ablation of the FN gene causes embryonic lethality (10). However, the recent generation of FN conditional knockout mice (11) allowed us to address the role of pFN in platelet plug formation in vivo and to show that FN is indeed an important factor in this process.
Materials and Methods
Mice.
Mice with part of the FN gene flanked by LoxP sites and containing the Mx-cre transgene have been described (11). The mice were rederived in the Department of Comparative Medicine facility at the Massachusetts Institute of Technology and shown to be free of pathogens, and a colony of FNflox/FNflox;Mx-cre+/− mice was established. The experimental mice were littermates obtained from intercrosses of these mice. Platelet donors were adult mice (>5 weeks old) that were injected i.p. three times with 250 μg of poly inosine-poly cytosine (polyI-polyC) (Sigma), with 2 days between injections. Blood was collected at least 3 days after the final injection. Recipient mice were young mice injected when they were 19, 21, and 23 days old with 200 μg of polyI-polyC. Intravital microscopy was performed on animals 25–28 days old, weighing 13–18 g. All animals were treated with polyI-polyC, and their pFN levels were determined. The experimental procedures were performed at the Center for Blood Research and the Massachusetts Institute of Technology and approved by the respective Animal Care and Use Committees.
Genotyping.
Genotyping was performed before experiments so that recipient and donor mice could be matched. DNA from mouse tail biopsies was amplified by PCR using AACATGCTTCATCGTCGG (cre forward)/TTCGGATCATCAGCTACACC (cre reverse) or GTACTGTCCCATATA AGCCTCTG (FN forward)/CTGAGCATCTTGAGTGGATGGGA (FN reverse) as primers.
Intravital Microscopy.
Intravital microscopy was performed and data were analyzed as described (2, 12). Briefly, blood was collected by inserting a heparin-coated glass capillary tube (Drummond Scientific, Broomall, PA), cut to a length of 1.5 cm, into the retroorbital vein of platelet donor mice at least 5 weeks old. The animals were anesthetized to prevent pain and discomfort and killed at the end of the procedure. Mouse platelets were isolated from platelet-rich plasma by gel filtration on a Sepharose 2B column and were labeled fluorescently with calcein acetoxymethyl ester (Molecular Probes). This procedure does not activate α-granule release because purified platelets do not stain for P-selectin. Recipient mice (25–28 days old) were injected in the lateral tail vein with fluorescently labeled platelets (5 × 106/g) of matching genotype. The mice were anesthetized, and the mesentery was exteriorized through a midline abdominal incision. Arterioles were visualized with a Zeiss Axiovert 135-inverted microscope (×32, 0.4 numerical aperture; Zeiss) and behavior of the fluorescent platelets was recorded on videotape. FeCl3 (30 μl of a 250 mM solution) was applied topically to a section (≈2–5 mm in length) of an arteriole, which induced vessel injury and denudation of the endothelium (2, 13). Vessels were monitored for 40 min after injury or until full occlusion occurred (blood flow stopped) and lasted for >10 s. In all experiments, one arteriole was chosen in each mouse. Shear rate was determined by the centerline erythrocyte velocity and the diameter of the vessel as described (1).
Detection of FN.
Detection of FN was by Western blotting of 1–4 × 107 platelets or 0.1–1.0 μl of plasma separated on reducing SDS-polyacrylamide gels. After transfer to nitrocellulose, FN was detected by using a rabbit polyclonal antiserum (297.1) against rat FN diluted 1:2,000 followed by chemiluminescence or 125I-labeled goat anti-rabbit IgG (Amersham Pharmacia). Plasma and platelet samples were analyzed in parallel with serial dilutions of WT plasma to quantitate the relative levels of FN present by using a PhosphorImager.
Statistical Analysis.
Data are presented as mean ± SEM. Statistical significance was assessed by unpaired Student's t test.
Results and Discussion
Induction of FN Gene Inactivation.
All mice were treated with polyI-polyC to induce the endogenous production of interferons and thus activate the Mx promoter and inactivate the FN gene in animals containing the Mx-Cre transgene. The efficiency of depletion of pFN was determined in each animal. pFN was very effectively eliminated in the older donor mice that were cre-positive and was below the level of detection in the plasma of these mice as observed (11). In mice lacking the Mx-cre transgene, pFN levels were indistinguishable from WT. The FN excision was slightly less efficient in the very young recipient mice but in all mice used in our study pFN was <2% of WT levels in the plasma (Fig. 1). Mx-cre-induced reduction of platelet FN was somewhat less effective, as reported (11). In our experiments, residual platelet FN in the recipient mice was typically ≈20% of WT levels. Because platelet FN represents only ≈1% of the level of pFN (8, 9) the overall depletion of FN in the blood of Mx-cre+ mice was >97%.
Figure 1.
Determination of FN levels in plasma of FNflox/flox mice after treatment with polyI-polyC. Shown are two dilutions of plasma from two pairs of young recipient mice with or without the Mx-cre transgene, compared with a serial dilution of plasma from WT mice (Right) untreated with polyI-polyC. Note that the cre− mice are indistinguishable from WT, whereas the cre+ mice showed very low levels of FN. Quantitation of such gels showed that the signal in 1:50 dilution of WT plasma was stronger than that of undiluted cre+ samples. Thus the cre+ mice had levels of fibronection <2% of WT.
Intravital Microscopy.
Platelets were purified from donor mice, fluorescently labeled, and injected via the tail vein into recipient mice of the same genotype. Fluorescent platelets represented 5–10% of the endogenous platelets. The mice were then prepared for intravital microscopy. We analyzed platelet thrombus formation at arterial shear rates as this provides stringent conditions under which an adhesion deficit should become more apparent (Fig. 2). The shear rates in the arterioles measured before injury were similar for the two genotypes (1,161 ± 65 s−1 for pFN-positive mice and 1,244 ± 64 s−1 for pFN-deficient animals, P = 0.38). Ferric chloride was used to induce the arteriolar injury. Ferric chloride generates free radicals (13) that seriously damage the endothelium and expose subendothelial matrix to the blood stream within 2 min of application (2). The intravital microscopic observations were recorded and data were analyzed by an observer blinded to the genotype of the animals.
Figure 2.
Thrombus growth in the presence or absence of pFN. Times after FeCl3-induced injury are indicated in bold. In the control mouse (Upper) and the pFN-deficient mouse (Lower), single fluorescent platelets are seen to adhere in the arterioles at 3 min after injury. Whereas in the control vessel large stable thrombi grew (15 min), leading to occlusion at 22 min, only very small thrombi were apparent at 15 min in the pFN-deficient animal, and at termination of the experiment (40 min) the vessel was still patent. The lack of stability of the thrombus formed in the pFN-deficient mouse is better observed in Movie 1, where the two arterial segments are shown in real time.
Early Platelet Adhesion.
Shortly after the endothelial injury, platelets began to adhere to the vessel wall and these interactions were mostly transient (Fig. 2). The numbers of single adherent platelets per min, determined in the interval 3–5 min after the injury, were not significantly affected by the absence of pFN (Fig. 3). Tissue FN is not expected to be reduced by the polyI-polyC treatment (11), consistent with our observations. Thus, pFN is not necessary for the early platelet vessel wall adhesion at high shear rate, which is a function primarily attributed to vWF (14). In the absence of vWF a much delayed platelet adhesion still occurs (2), and we cannot exclude that FN along with other basement membrane components might contribute to this process.
Figure 3.
Quantitative analysis of formation of thrombi in control (black bar) and pFN-deficient (striated bar) arterioles. (A) Initial platelet deposition. The number of fluorescent platelets deposited per min was determined in the interval of 3–5 min after injury. pFN appeared not to influence significantly the early platelet interactions with the subendothelium. (B) Time required for the formation of the first thrombus >20 μm in diameter. The absence of pFN significantly delayed the formation of thrombi. (C) Embolization. The number of large emboli >30 μm generated in the period before occlusion was determined. Although in the absence of pFN the thrombi were dissolving by shedding single or small groups of platelets, formation of large emboli was just as infrequent as in animals with WT levels of pFN.
Thrombus Formation.
Several minutes after the injury, platelets started to adhere more stably to the vessel wall and the first aggregates formed (Fig. 2). We noted that the aggregate formation was significantly delayed in pFN-deficient mice and that the thrombi increased in size slowly as they grew and dissolved simultaneously (see Movie 1, which is published as supporting information on the PNAS web site, www.pnas.org). For each animal we measured the time from injury to formation of a thrombus of >20 μm in diameter (Fig. 3) and found that for the pFN-deficient mice this period was 8 min longer than for the mice with normal pFN content (pFN-positive 10.7 ± 2.9, pFN-deficient 18.6 ± 1.9 min). There was little embolization of large thrombi (>30 μm in diameter, Fig. 3) in either group (<1 embolus per mouse per observation period). Thus the pFN-deficient thrombi, although in a constant state of flux, were well anchored to the vessel wall. This finding was in sharp contrast with fibrinogen-deficient arterioles where we observed close to 20 large emboli before the vessel occluded (2). Most frequently these emboli formed by dislodging from the vessel wall. As a consequence of this embolization, all fibrinogen-deficient arterioles occlude downstream from the injured area where the emboli accumulated (2). All pFN-deficient arterioles that occluded (see below) in our study did so at the site of injury. Thus, pFN is not necessary for anchoring the thrombi to the vessel wall and rather appears to be implicated in fortifying the platelet/platelet interactions within the thrombus.
Vessel Occlusion.
The polyI-polyC treatment inducing interferons had an apparent antithrombotic effect on the mice. The mean occlusion time of the polyI-polyC-treated mice was a few minutes longer than we generally observe in WT mice. This finding could also be caused by the mixed genetic background of these mice. Thus we made sure to use pFN-positive and pFN-negative littermates, and all of the mice were treated in identical fashion. The lower efficiency of thrombus growth in pFN-deficient arterioles translated into a significant delay in vessel occlusion as compared with the control mice (Fig. 4). In some pFN-deficient animals, channels opened up through large thrombi, leading to partial or complete dissolution of the thrombus. Other thrombi were continuously eroded from their surfaces (see Movie 1). The majority of the pFN-deficient arterioles did not occlude by 40 min after the injury, the time when the experiment was terminated (Fig. 4). The occlusion time of these animals was scored as 40 min and the difference between the pFN-deficient mice and the control mice was highly statistically significant (P < 0.009) despite the underestimate of the actual occlusion time in the mice lacking pFN. It is most likely that the observed defect in platelet cohesion was caused by a deficit of pFN rather than lack of platelet α-granule FN function, as the platelet FN was not completely eliminated by the polyI-polyC treatment, as discussed above. Nevertheless, the contribution of α-granule FN or its complex, e.g., fibrinogen–FN, cannot be excluded.
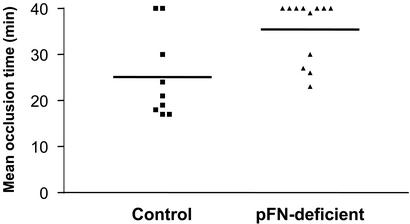
Figure 4.
The role of pFN in occlusion of injured arterioles by platelet thrombi. The time needed to form an occlusive thrombus in an injured arteriole was determined in 9 control and 12 pFN-deficient animals. Occlusion was noted when blood flow completely stopped for at least 10 s. If this did not occur during the observation period of 40 min, 40 min was taken as the occlusion time. The majority of vessels did not occlude in pFN-deficient mice, whereas this happened in only two control animals. Thus there was a significant defect in generation of occlusive thrombi in the absence of pFN. P < 0.009.
The thrombotic phenotype of the pFN-deficient mice was different from those of all genetically modified mice we have observed in this assay in our laboratory to date. Unlike the situation in all of the other mutant mice, thrombus progression in pFN-deficient mice was abnormal from beginning to end. The platelets appeared less sticky to one another and, even in aggregates, they dissociated more easily than did control platelets (Movie 1). This defect in aggregation was shear-dependent as it was not seen in standard in vitro aggregation assays nor was it reflected in the mouse tail bleeding time (11). This finding is reminiscent of CD40L−/− mice that also had normal platelet aggregation in an aggregometer and normal bleeding time but presented thrombus instability specifically at arterial shear rates (15). In the same intravital arterial thrombosis model, the pFN deficiency produced a more severe defect than that of CD40L. Absence of pFN affected the growth of both small and large thrombi (Fig. 3), whereas CD40L affected the stability only of large thrombi. It was hypothesized that CD40L promotes platelet activation as it induces platelet spreading (15). pFN does the same (16) and, in addition, its large dimeric size may crosslink the platelets. vWF function is enhanced at high-shear conditions possibly by conformational changes occurring in the vWF binding site for its platelet receptor GPIb (17–19). FN is also known to undergo conformational changes under mechanical stress (20) and thus its function may also be modulated by flow. Sixma and colleagues (21–24) have reported a contribution of FN to platelet adhesion especially under high shear conditions.
The effect of pFN on platelet aggregation is likely through the major platelet integrin αIIbβ3, although it could also function through other receptors. Because FN can bind both fibrin(ogen) and thrombospondin, it could also contribute to crosslinking among these proteins and with platelets (8, 9). Thrombasthenic platelets (lacking integrin αIIbβ3) stimulated with thrombin were shown to bind FN poorly in comparison with stimulated WT platelets (25). In addition, the ligand mediating the residual platelet aggregation in the vWF/fibrinogen doubly deficient mice likely interacts with the αIIbβ3 integrin as we observed that platelets deficient in this integrin do not form any thrombi in this thrombosis model (unpublished data). αIIbβ3 integrin also probably internalizes pFN into α-granules as it does fibrinogen. Platelet fibrinogen is greatly reduced in Glanzmann thrombasthenic platelets (26, 27) and β3-deficient mice (28). Furthermore, in the absence of fibrinogen (2) or in mice with a mutation of the αIIbβ3- binding sequence of fibrinogen (29), platelets have elevated FN levels (unpublished work), suggesting enhanced uptake of pFN in the absence of competing fibrinogen. However, this cannot be the only source of platelet FN because β3-null mouse platelets still contain some FN (unpublished data). Other potential sources of platelet FN include uptake by other pathways or synthesis in the megakaryocyte lineage. It is possible that this residual platelet FN contributes to platelet thrombus formation and that complete absence of FN might yield even more radical defects.
In conclusion, it appears that, in addition to vWF and fibrinogen, FN contributes significantly to thrombus development in arteries. Each of these adhesion molecules may have its own special function: vWF in initiating single platelet adhesion and supporting platelet/platelet interactions at extreme shear rates, fibrinogen/fibrin in providing thrombus stability by anchoring it to the vessel wall, and finally FN in gluing the platelets to each other at all stages of thrombus growth. It is likely that this fundamental role of FN remained unnoticed because of the severe consequences of FN deficiency to embryonic development and also because most in vitro studies in the past were performed under static or low shear rate conditions.
Supplementary Material
Acknowledgments
We thank Lesley Cowan for help with preparation of the manuscript and Mary Connolly for technical assistance. This work was supported by National Heart, Lung, and Blood Institute Grants R37HL41002 and P01HL56949 (to D.D.W.) and P01HL66105 (to R.O.H.), the Howard Hughes Medical Institute, of which R.O.H. is an Investigator, and the Heart and Stroke Foundation of Canada, of which H.N. was a Fellow. R.F. was supported by the Fonds der Chemischen Industrie.
Abbreviations
- FN
fibronectin
- pFN
plasma FN
- vWF
von Willebrand factor
- polyI-polyC
poly inosine-poly cytosine
References
- 1.Denis C, Methia N, Frenette P S, Rayburn H, Ullman-Cullere M, Hynes R O, Wagner D D. Proc Natl Acad Sci USA. 1998;95:9524–9529. doi: 10.1073/pnas.95.16.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ni H, Denis C V, Subbarao S, Degen J L, Sato T N, Hynes R O, Wagner D D. J Clin Invest. 2000;106:385–392. doi: 10.1172/JCI9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss H J, Turitto V T, Vicic W J, Baumgartner H R. Blood. 1984;63:1004–1014. [PubMed] [Google Scholar]
- 4.Tsuji S, Sugimoto M, Miyata S, Kuwahara M, Kinoshita S, Yoshioka A. Blood. 1999;94:968–975. [PubMed] [Google Scholar]
- 5.Lawler J, Sunday M, Thibert V, Duquette M, George E L, Rayburn H, Hynes R O. J Clin Invest. 1998;101:982–992. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fay W P, Parker A C, Ansari M N, Zheng X, Ginsburg D. Blood. 1999;93:1825–1830. [PubMed] [Google Scholar]
- 7.Konstantinides S, Schafer K, Thinnes T, Loskutoff D J. Circulation. 2001;103:576–583. doi: 10.1161/01.cir.103.4.576. [DOI] [PubMed] [Google Scholar]
- 8.Ginsberg M H, Plow E F. In: Fibronectin. Mosher D F, editor. New York: Academic; 1989. pp. 273–293. [Google Scholar]
- 9.Hynes R O. Fibronectin. New York: Springer; 1990. [Google Scholar]
- 10.George E L, Georges-Labouesse E N, Patel-King R S, Rayburn H, Hynes R O. Development (Cambridge, UK) 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 11.Sakai T, Johnson K J, Murozono M, Sakai K, Magnuson M A, Wieloch T, Cronberg T, Isshiki A, Erickson H P, Fassler R. Nat Med. 2001;7:324–330. doi: 10.1038/85471. [DOI] [PubMed] [Google Scholar]
- 12.Ni H, Ramakrishnan V, Ruggeri Z M, Papalia J M, Phillips D R, Wagner D D. Blood. 2001;96:368–373. doi: 10.1182/blood.v98.2.368. [DOI] [PubMed] [Google Scholar]
- 13.Kurz K D, Main B W, Sandusky G E. Thromb Res. 1990;60:269–280. doi: 10.1016/0049-3848(90)90106-m. [DOI] [PubMed] [Google Scholar]
- 14.Ruggeri Z M. Thromb Haemostasis. 1997;78:611–616. [PubMed] [Google Scholar]
- 15.Andre P, Prasad K S, Denis C V, He M, Papalia J M, Hynes R O, Phillips D R, Wagner D D. Nat Med. 2002;8:247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 16.Hynes R O, Ali I U, Destree A T, Mautner V, Perkins M E, Senger D R, Wagner D D, Smith K K. Ann NY Acad Sci. 1978;312:317–342. doi: 10.1111/j.1749-6632.1978.tb16811.x. [DOI] [PubMed] [Google Scholar]
- 17.Savage B, Saldivar E, Ruggeri Z M. Cell. 1996;84:289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 18.Siedlecki C A, Lestini B J, Kottke-Marchant K K, Eppell S J, Wilson D L, Marchant R E. Blood. 1996;88:2939–2950. [PubMed] [Google Scholar]
- 19.Sugimoto M, Tsuji S, Kuwahara M, Matsui H, Miyata S, Fujimura Y, Yoshioka A. Int J Hematol. 1999;69:48–53. [PubMed] [Google Scholar]
- 20.Hynes R O. Proc Natl Acad Sci USA. 1999;96:2588–2590. doi: 10.1073/pnas.96.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houdijk W P, Sixma J J. Blood. 1985;65:598–604. [PubMed] [Google Scholar]
- 22.Houdijk W P, Sakariassen K S, Nievelstein P F, Sixma J J. J Clin Invest. 1985;75:531–540. doi: 10.1172/JCI111729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houdijk W P, de Groot P G, Nievelstein P F, Sakariassen K S, Sixma J J. Arteriosclerosis. 1986;6:24–33. doi: 10.1161/01.atv.6.1.24. [DOI] [PubMed] [Google Scholar]
- 24.Bastida E, Escolar G, Ordinas A, Sixma J J. Blood. 1987;70:1437–1442. [PubMed] [Google Scholar]
- 25.Ginsberg M H, Forsyth J, Lightsey A, Chediak J, Plow E F. J Clin Invest. 1983;71:619–624. doi: 10.1172/JCI110808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George J N, Caen J P, Nurden A T. Blood. 1990;75:1383–1395. [PubMed] [Google Scholar]
- 27.Kato A. Crit Rev Oncol Hematol. 1997;26:1–23. doi: 10.1016/s1040-8428(97)00011-5. [DOI] [PubMed] [Google Scholar]
- 28.Hodivala-Dilke K M, McHugh K P, Tsakiris D A, Rayburn H, Crowley D, Ullman-Cullere M, Ross F P, Coller B S, Teitelbaum S, Hynes R O. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmback K, Danton M J, Suh T T, Daugherty C C, Degen J L. EMBO J. 1996;15:5760–5771. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.