Abstract
The lack of genetic means has been a serious limitation in studying mosquito immunity. We generated Relish-mediated immune deficiency (RMID) by transforming Aedes aegypti with the ΔRel transgene driven by the vitellogenin (Vg) promoter using the pBac[3xP3-EGFP, afm] vector. A stable transformed line had a single copy of the Vg-ΔRel transgene. The Vg-ΔRel transgene expression was highly activated by blood feeding, and transgenic mosquitoes were extremely susceptible to the infection by Gram-negative bacteria. This RMID phenotype was characterized by severely reduced postinfection levels of antimicrobial peptides genes, defensin and cecropin. Crossing the RMID line with the wild-type strain produced the same RMID phenotype, indicating its dominant nature, whereas crossing with the Vg-def transgenic line, in which Defensin A was activated by blood feeding, restored the immunity to Enterobacter cloacae.
Mosquitoes transmit numerous human diseases. Malaria is particularly devastating, taking a heavy toll on the human population in many parts of the world by infecting >300 million and killing >2 million people each year (1, 2). Diseases caused by mosquito-borne viruses, most importantly Dengue fever, are reaching disastrous levels (3). In the U.S., the West Nile encephalitis virus is rapidly spreading westward. Lymphatic filariasis, a nematode-based disease transmitted by mosquitoes, affects millions of people in tropical regions of the world (4). The major reasons for this tragic situation are the unavailability of effective vaccines for malaria and other mosquito-borne diseases and the development of insecticide and drug resistance by the vectors and pathogens, respectively (5, 6). Therefore, there is an urgent need to explore every possible avenue for developing novel control strategies against these mosquito-borne menacing diseases.
Activation of innate immune proteins in both mammals and insects shares conserved pathways in which Rel/nuclear factor (NF)-κB transcription factors are principal regulators (7). Tremendous progress has been made in the understanding of innate immunity using Drosophila as a model. The ability to manipulate Drosophila genetically has been one of the major reasons for this progress. In Drosophila, three Rel/NF-κB molecules are involved in the two distinct pathways of the innate immune response: (i) the Toll pathway, which activates primarily antifungal responses and is mediated by Dorsal and Dif factors, and (ii) the IMD/Relish pathway, which is antibacterial and requires the Rel/NF-κB factor Relish (8, 9).
Little is known about the pathways regulating the immune responses in mosquitoes, despite the enormous importance of such knowledge for our understanding of the immune system of these disease vectors. Thus, we have isolated and characterized the gene homologous to Drosophila Relish from the mosquito Aedes aegypti (10). However, the primary structure of the Aedes Relish gene exhibits three unique features. Compared with Drosophila Relish, the mosquito Relish gene encodes three alternatively spliced transcripts that give rise to different proteins: there is Death Domain at the extreme C terminus and a short His/Gln-rich stretch followed by a long S-rich region at the putative N-terminal transactivation domain. Interestingly, the mosquito Relish is more similar to the mammalian p105 and p100; the same NF-κB1 gene that encodes p105 generates the NF-κB inhibitor IκBγ by alternative splicing (11).
The lack of genetic means has been a serious limitation to further progress in studying immune pathways in mosquitoes. Here, we have taken a transgenic approach to elucidate the regulatory role of Aedes Relish in the mosquito innate immunity. The Vg-ΔRel transgenic mosquitoes showed a marked susceptibility to Gram-negative bacteria infections and a severely compromised induction in the levels of expression of both Defensin and Cecropin genes, very similar phenotypes shown at the mutant studies of Drosophila IMD/Relish pathways. Our results clearly show that Aedes Relish is a key transcriptional regulator of mosquito immune response against Gram-negative bacteria. Generation of transgenic dominant negative Relish mosquito mutant opens the door of utilization of reverse genetic approach to the elucidating immune responses of the mosquito to various pathogenic agents of mosquito-borne human diseases.
Materials and Methods
Transformation Vector.
The pBac[3xP3-EGFP, afm] transformation vector containing an eye-specific promoter (3xP3), in front of a TATA box and enhanced GFP (EGFP), was described by Horn and Wimmer (12). The 2.4-kb C8 mosquito Relish clone, which encodes the Rel-type protein without the putative transactivation domain (ΔRel), was used for these transgenic experiments. The Vg-ΔRel construct was made by linking the vitellogenin (Vg) promoter, ΔRel, and SV40 polyadenylation element. This 4.5-kb Vg-ΔRel was introduced into the pBac[3xP3-EGFP, afm] plasmid at the AscI–FseI unique cloning site. The resulting plasmid pBac[3xP3-EGFP, afm, Vg-ΔRel] was used for injection of A. aegypti embryos.
Generation of Transgenic Mosquitoes.
A. aegypti wild-type Rockefeller/UGAL strain was reared according to the method described by Hays and Raikhel (13). Three-day-old previtellogenic females were fed on anesthetized white rats. Eggs were then collected for microinjection 72 h postblood meal (PBM). Embryo injections and development of transgenics were performed as described before by Kokoza et al. (14). Embryos were injected with a mixture of pBac[3xP3-EGFP, afm, Vg-ΔRel] and the phsp-pBac helper plasmid at a final concentration of 0.3 μg/ml and 0.5 μg/ml, respectively, in 5 mM KCl and 0.1 mM NaH2PO4 (pH 6.8). After DNA microinjection, 16- to 20-h-old injected embryos were heat-shocked at 39°C for 60 min and then placed at 27°C, 85% humidity. G0 adults were crossed with virgin wild-type females or males, and the resulting G1 progeny was screened for eye EGFP fluorescent marker using a fluorescence microscope (Nikon SMZ 800) equipped with a GFP-B filter (GFP band pass, Ex 470/40 DM 495 BA 525/50) and a GFP-L filter [GFP long pass, Ex 470/40 DM 495 BA 500 (LP)], or using the Illumatool Tunable Lighting system adapter for a Nikon dissecting microscope equipped with an EGFP long-pass filter set.
Southern and Northern Hybridizations.
Genomic DNA from transgenic and control mosquitoes was purified using the method of Bender et al. (15). Aliquots of DNA (5 μg) were digested with the corresponding endonucleases; the DNA fragments were separated by means of electrophoresis in a 0.8% agarose gel, transferred to nitrocellulose filters, and hybridized with a 1.4-kb DNA fragment of Aedes Relish. Total RNA from the previtellogenic and vitellogenic mosquitoes (at 5 and 24 h PBM) was used for the expression study of transgenic Vg-ΔRel. For Northern hybridization of defensin and cecropin genes, the vitellogenic mosquitoes at 24 h PBM were injected with a stationary-phase culture of Enterobacter cloacae and collected 5 h after injection. Total RNA was prepared using the Trizol technique (GIBCO/BRL). Samples of 5 μg total RNA were separated on a formaldehyde gel, blotted, and hybridized with corresponding DNA probes.
Immunoblot Analysis.
Protein extracts from whole mosquitoes were prepared by homogenization in the lysis buffer [10 mM Hepes, pH 7.9/10 mM KCl/1 mM DTT/0.6% (vol/vol) Nonidet P-40] with protease inhibitor mixture (Roche Molecular Biochemicals). Samples were loaded directly onto an SDS/PAGE set-up. Anti-DefA IgG was prepared by Cho et al. (16). Immunoblots were developed using the ECL detection system (Amersham Pharmacia).
Microorganisms and Survival Experiments.
Microbial challenge was performed by pricking the mosquitoes with a Hamilton 33S needle dipped into a bacterial suspension. Bacteria were precultured in LB medium. The following are the bacterial strains used in this study: Escherichia coli (ATCC 8739), Pseudomonas aeruginosa (ATCC 9027), Staphylococcus aureus (ATCC 6538), Bacillus subtilis (ATCC 6633), and Micrococcus luteus (ATCC 9341). Inoculations were done by pricking mosquitoes in the rear part of the abdomen. Abdominal injections have been routinely performed in physiological experiments with mosquitoes yielding high level of survival, particularly in blood-fed females (17).
Results and Discussion
We have taken the recently developed mosquito transgenic technique to elucidate the in vivo regulatory role of Aedes Relish in mosquito innate immunity. We used a well characterized 5′ regulatory region of the Vg gene, which is fat-body specific and strongly activated by blood feeding (14). The C8 cDNA clone, which represents the Aedes Relish splice variant that has the DNA binding domain but lacks the putative N-terminal transactivation domain and IκB-region (Fig. 1A), was chosen for expression. The translated protein of this cDNA clone (ΔRel) specifically binds to κB motifs in Drosophila cecropin A1 and Aedes defensin promoters (10).
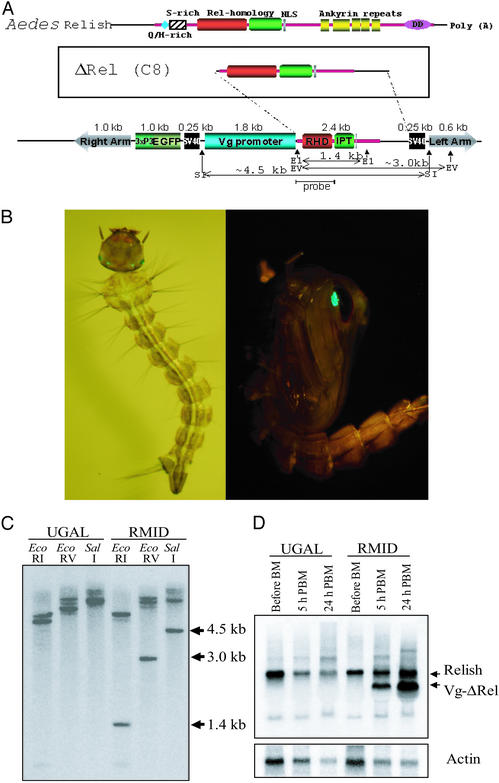
Figure 1.
Structure of the pBac[3xP3-EGFP, afm, Vg-ΔRel] transformation vector and its expression in transgenic mosquitoes. (A) Schematic diagram of the pBac[3xP3-EGFP, afm, Vg-ΔRel] transformation vector that was transformed into the A. aegypti germ line. (B) Detection of the transgenic Vg-ΔRel mosquitoes by transformation marker-mediated fluorescence. (C) Southern blot analysis of genomic DNA extracted from the Vg-ΔRel transgenic mosquitoes and the parental UGAL strain, digested with EcoRI, EcoRV, and SalI. The probe region and the restriction sites of the transformation vector are indicated in A. (D) The expression profile of Relish and transgenic Vg-ΔRel transcripts after blood feeding. Endogenous ≈3.9-kb Relish transcript was detected in both transgenic and parental mosquitoes at any stage. An additional band of ≈3.0 kb was detected in the transgenic mosquitoes; it appeared after bloodfeeding and reached its peak at 24 h PBM.
A Vg-ΔRel construct, obtained by aligning the Vg promoter, ΔRel, and SV40 polyadenylation signal, was inserted into the pBac[3xP3-EGFP, afm] vector (Fig. 1A) and transformed into the germ line of mosquito A. aegypti embryos. From 500 injected embryos, 50 survived into adulthood and 1 (no. 80) yielded green fluorescence protein (EGFP)-positive progeny (Fig. 1B). Genomic Southern analysis of this progeny showed correct incorporation of the Vg-ΔRel construct into the genome (Fig. 1C). The band density comparison between the endogenous and transgenic Relish indicated a single copy insertion. Additionally, we confirmed a single copy insertion using Southern blotting with probes from the left and right arms of the vector (data not shown). Northern blot analysis indicated that the Vg-ΔRel transcript was significantly induced by blood feeding in transgenic mosquitoes 5 h PBM and greatly accumulated by 24 h PBM (Fig. 1D). This pattern is consistent with that previously observed for the defensin gene driven by the Vg promoter (Vg-DefA). Transcription of the Vg-DefA transgene was activated to a high level after a blood meal, and its maximal expression was observed at 24 h PBM, corresponding to the peak expression of the endogenous Vg gene (18, 19). The 3.9-kb Relish transcript was expressed constitutively in both the wild-type UGAL and transgenic mosquitoes. Surprisingly, it was elevated in transgenic mosquitoes at 24 h PBM. It is possible that ΔRel interfered with regulation of 3.9-kb Relish transcript expression.
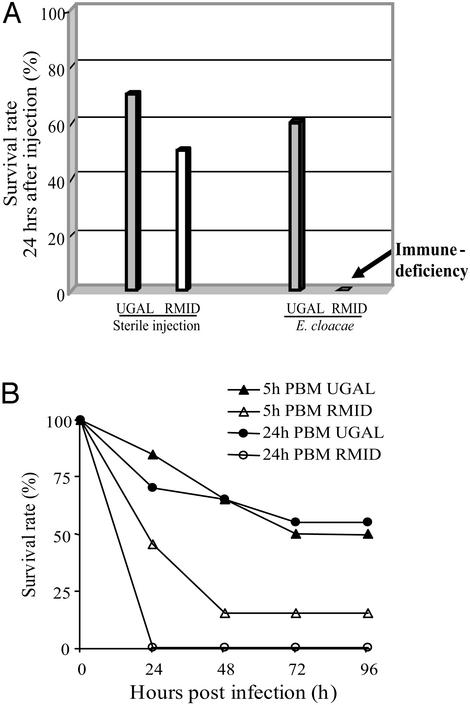
We applied the survival test to examine the effect of the Vg-ΔRel transgene expression on mosquito immunity. All Vg-ΔRel transgenic mosquitoes challenged with E. cloacae died within 24 h after the infection, whereas only 50% died when just pricked with the needle without any bacteria (Fig. 2A). The control experiment with the parental UGAL strain showed 60% and 70% survival rates when challenged with or without bacteria, respectively (Fig. 2A). We concluded that the high expression level of ΔRel protein is likely the cause of the immune-deficient phenotype in the transgenic mosquitoes.
Figure 2.
Susceptibility of the Vg-ΔRel transgenic mosquitoes to bacterial infection. (A) Survival rate of the parental wild-type and transgenic mosquitoes 24 h after the injection of E. cloacae are present. All of the transgenic mosquitoes at 24 h PBM could not survive >24 h, presenting the immune-deficient phenotype. (B) Dependence of the survival rate to the expression level of Vg-ΔRel transcript. The Vg-ΔRel transcript was induced by a blood meal, and then mosquitoes were challenged by E. cloacae at 5 or 24 h PBM. The mortality of the challenged mosquitoes was assayed at the different time points postinfection indicated in the graphs. In both experiments, 20 mosquitoes were injected for each experimental point. The results shown are representative of at least two independent experiments.
If this is the case, the sensitivity of mosquitoes should depend on the amount of ΔRel protein produced after blood feeding. To examine this suggestion, we immune-challenged transgenic mosquitoes at 5 and 24 h PBM that exhibited profoundly different levels of the ΔRel transcript accumulation (Fig. 1D). Indeed, the susceptibility of the Vg-ΔRel transgenic mosquitoes to E. cloacae was dependent on the expression level of the Vg-ΔRel transcript. The transgenic mosquitoes at 5 h PBM, which expressed a lower level of Vg-ΔRel transcript than that at 24 h PBM (Fig. 1D), were less susceptible to the infection by E. cloacae (Fig. 2B). Whereas all of the transgenic mosquitoes challenged at 24 h PBM died within 24 h, almost half (45%) of the transgenic mosquitoes challenged at 5 h PBM survived at 24 h after the challenge with E. cloacae (Fig. 2B). The wild-type UGAL strain, injected at 5 h and 24 h PBM, showed a >50% survival rate when challenged with Gram-negative bacteria, E. cloacae (Fig. 2B).
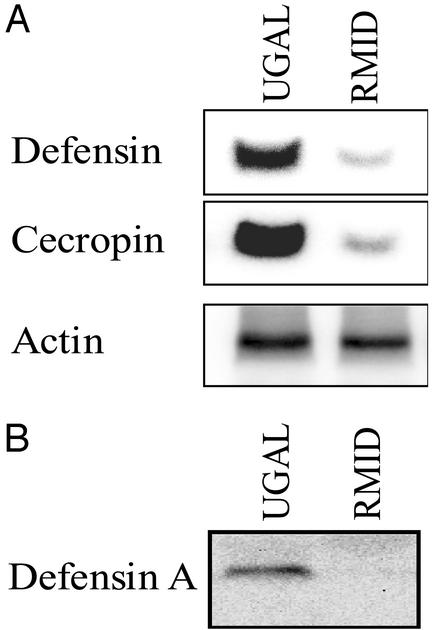
When challenged with E. cloacae at 24 h PBM, the Relish-mediated immune deficiency (RMID) transgenic mosquitoes (Vg-ΔRel transgenic mosquitoes) exhibited severely compromised expression levels of the two antimicrobial peptide (AMP) genes defensin and cecropin (Fig. 3A). Western blot analysis demonstrated the absence of Defensin peptide at 5 h after bacterial challenge in the whole body extracts of the RMID transgenic mosquitoes (Fig. 3B).
Figure 3.
The effect of the transgenic Vg-ΔRel on the AMP response. (A) The Northern blot demonstrating the severely compromised induction in the levels of expression of two known antibacterial peptide genes, defensin and cecropin, in the transgenic mosquitoes. (B) The Western blot showing the severely reduced level of Defensin peptide in the transgenic mosquitoes after blood feeding.
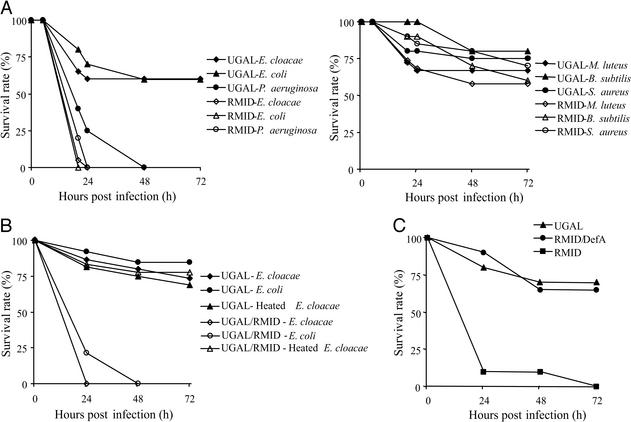
Survival experiments with the transgenic mosquitoes showed a marked susceptibility to Gram-negative infections (E. cloacae, E. coli, and P. aeruginosa) (Fig. 4A). All RMID transgenic mosquitoes injected by Gram-negative bacteria did not survive beyond 24 h postinfection. The parental UGAL strain showed <40% lethality to the injection of E. cloacae and E. coli and less susceptibility than the transgenic mosquitoes to the injection of P. aerusinosa. This human opportunistic pathogen is highly pathogenic to Drosophila (20) and even killed all of the wild-type mosquitoes in <48 h. The susceptibility to Gram-positive infection was not markedly different between the transgenic and wild-type mosquitoes in the present experiment (Fig. 4A). All three Gram-positive bacterial infections could not kill >50% of both wild-type and transgenic mosquitoes. The different susceptibility between Gram-negative and Gram-positive bacteria in the RMID transgenic mosquitoes indicates the conservation of the immune response between mosquitoes and flies. In Drosophila, the immune system discriminates Gram-negative and Gram-positive bacteria by different peptidoglycan recognition proteins, PGRP-LC and PGRP-SA, and then the recognition triggers several immune responses, in addition to the AMP response, by the IMD/Relish and Toll pathways, respectively (21). It has been shown that mutants of the IMD/Relish pathway are more susceptible than wild-type flies to Gram-negative bacterial infection, whereas mutants of the Toll pathway are more susceptible to fungal and Gram-positive bacterial infections (22, 23). Interestingly, double-stranded RNA knockout of Defensin gene in Anopheles gambiae resulted in an increased susceptibility to Gram-positive bacteria (24). Further studies are required to understand whether or not the detected differences reflect properties of Defensins in these insects.
Figure 4.
(A) High susceptibility of the Vg-ΔRel transgenic mosquitoes to infection by Gram-negative bacteria. The survival rates of the transgenic and parental mosquitoes infected with the indicated microorganisms are presented. Each experiment was performed using 18–20 mosquitoes for each case, and the results shown are representative of at least two independent experiments. (Left) Result challenged by three Gram-negative bacteria: E. cloacae, E. coli, and P. aeruginosa. (Right) Result challenged by three Gram-positive bacteria: S. aureus, B. subtilis, and M. luteus. (B) Genetically dominant phenotype of the transgene Vg-ΔRel. Female transgenic mosquitoes were mated to male wild-type UGAL mosquitoes, and their progeny were challenged. These heterozygous mosquitoes showed a marked susceptibility to alive bacteria, E. cloacae and E. coli. Heat inactivation of E. cloacae was performed by incubating the bacterial suspension at 95°C for 30 min. (C) Wild-type resistance recovery of immune-compromised mosquitoes overexpressing the defensin gene. Female Vg-ΔRel transgenic mosquitoes were mated to male Vg-DefA transgenic mosquitoes, and their progeny were applied to survival test with E. cloacae. These Vg-DefA transgenic mosquitoes were constructed using the same transformation vector pBac[3xP3-EGFP, afm] and Vg promoter as pBac[3xP3-EGFP, afm, Vg-ΔRel].
Next, we asked the question whether or not crossing of the wild-type UGAL strain with the RMID transgenic mosquitoes would restore resistance to E. cloacae. However, the heterozygous RMID/UGAL mosquitoes exhibited the same susceptibility to the E. cloacae infection as the RMID transgenic mosquitoes (Fig. 4B). All heterozygous mosquitoes challenged with two Gram-negative bacteria, E. cloacae or E. coli, at 24 h PBM died within 24 or 48 h after infection, respectively. The challenge with heat-inactivated bacteria did not show any significant difference in the survival rate between the parental UGAL and the heterozygous mosquitoes. These crossing experiments clearly showed that the RMID phenotype originated from the Vg-ΔRel transgene and that it was genetically dominant.
Tzou et al. (25) have reported that the overexpression of a single AMP was sufficient to rescue mutant immune-deficient flies from susceptibility to microbial infection. We mated transgenic RMID (Vg-ΔRel) with Vg-DefA mosquitoes, and the progeny was challenged with E. cloacae to monitor the contribution of Defensin peptide in resistance to infection by E. cloacae. The growth of E. cloacae was inhibited in these mosquitoes at a relatively low minimal concentration (<2 μM) (26). Thus, in these double-transgenic mosquitoes, Defensin A, which is stimulated by the Vg promoter after a blood meal independently of Relish-mediated activation, restored the resistance to E. cloacae (Fig. 4C). These results demonstrated that the RMID phenotype of the transgenic mosquitoes could be directly linked to the lack of AMP expression.
The data presented here clearly point toward an involvement of the Aedes Relish gene in the regulation of the immune response to bacterial challenge. Defensin A expression levels, and the survival studies both indicate that the transgenic mosquitoes have an immune response similar to that described for loss-of-function mutants of the Drosophila IMD/Relish pathway (27–29), where the transcription factor Relish directly activates antibacterial target genes. In that system, the immune challenge has been reported to induce cleavage of the latent cytoplasmic Relish, allowing for nuclear translocation of the amino-terminal Rel-homology domain fragment and activation of AMP genes (30). In mosquitoes, the overexpression of the Vg-ΔRel transgene showed an immune-deficient phenotype similar to that of Drosophila IMD/Relish pathway mutants, indicating the conserved regulatory role of Relish in the innate immune response and the presence of a similar Relish pathway in both dipteran insects.
Based on the previous study on the expression pattern and binding to κB motifs, Aedes Relish was predicted to be a transcriptional activator of mosquito immune genes including AMP genes (10). The transgenic ΔRel interfered with the activation of AMP genes by the functional Rel protein, resulting in the immune-deficient phenotype. The most likely explanation for this interference is the competitive binding to the κB motif between the transgenic ΔRel and the functional Rel protein. The transgenic ΔRel, which can bind to the κB motif but lacks the putative transactivation domain, may function as a repressor against endogenous Relish such as p52 or p50 against mammalian Rel proteins.
The elucidation of the mosquito immune response itself may be a crucial step in the understanding of the vector–parasite relationship that underlies several mosquito-borne diseases including malaria. Advances in the studies of the immune response of the model organism Drosophila and the development of molecular approaches have made it possible to isolate genes associated with mosquito immunity, including immune-regulatory genes (31). However, the lack of genetic approaches, such as mutagenesis and transgenic techniques, which are standard in Drosophila studies, has blocked the further advance of these studies in the mosquito. Recently, Blandin et al. (24) have reported successful double-stranded RNA knockout of Defensin gene in Anopheles gambiae. The double-stranded RNA knockout approach provides a valuable tool in mosquito research for elucidating in vivo functions of individual genes. However, it cannot lead to establishment of genetically stable mutant lines with a desired phenotype. In this report, we generated genetically immune-deficient transgenic mosquitoes by overexpression of a dominantly negative construct of Aedes Relish. Utilization of transgenesis has many advantages, principally the establishment of genetically stable transgenic mosquito lines that can be crossed with other transgenic and wild-type mosquitoes to produce new genetic variations, as demonstrated in this report. The development of such a reverse-genetics approach has opened a door for further exploration of the mosquito immune system and its interaction with pathogens transmitted by these vectors.
Acknowledgments
We thank Aileen McAinsh for editing the manuscript. This work was supported by National Institutes of Health Grants R37 AI2416 and AI45123.
Abbreviations
- AMP
antimicrobial peptide
- PBM
postblood meal
- RMID
Relish-mediated immune deficiency
- Vg
vitellogenin
- EGFP
enhanced GFP
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Beier J C. Annu Rev Entomol. 1998;43:519–543. doi: 10.1146/annurev.ento.43.1.519. [DOI] [PubMed] [Google Scholar]
- 2.Bremen J. Am J Trop Med Hyg. 2001;64,Suppl.:1–11. doi: 10.4269/ajtmh.2001.64.1. [DOI] [PubMed] [Google Scholar]
- 3.Beaty B. Proc Natl Acad Sci USA. 2000;97:10295–10297. doi: 10.1073/pnas.97.19.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wattam A R, Christensen B M. Proc Natl Acad Sci USA. 1992;89:6502–6505. doi: 10.1073/pnas.89.14.6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtis C F, Lines J D. Parasitol Today. 2000;16:119–121. doi: 10.1016/s0169-4758(99)01605-1. [DOI] [PubMed] [Google Scholar]
- 6. Lycett, G. J. & Kafatos, F. C. (2002) Nature 387–388. [DOI] [PubMed]
- 7.Ghosh S, May M J, Kopp E B. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 8.Dushay M S, Åsling B, Hultmark D. Proc Natl Acad Sci USA. 1996;93:10343–10347. doi: 10.1073/pnas.93.19.10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Gregorio E, Spellman P T, Tzou P, Rubin G M, Lemaitre B. EMBO J. 2002;21:2568–2579. doi: 10.1093/emboj/21.11.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin S W, Kokoza V, Ahmed A, Raikhel A S. Proc Natl Acad Sci USA. 2002;99:9978–9983. doi: 10.1073/pnas.162345999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grumont R J, Gerondakis S. Proc Natl Acad Sci USA. 1994;91:4367–4371. doi: 10.1073/pnas.91.10.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horn C, Wimmer E A. Dev Genes Evol. 2000;210:630–637. doi: 10.1007/s004270000110. [DOI] [PubMed] [Google Scholar]
- 13.Hays A R, Raikhel A S. Roux's Arch Dev Biol. 1990;199:114–121. doi: 10.1007/BF02029559. [DOI] [PubMed] [Google Scholar]
- 14.Kokoza V, Martin D, Mienaltowski M J, Ahmed A, Morton C M, Raikhel A S. Gene. 2001;274:47–65. doi: 10.1016/s0378-1119(01)00602-3. [DOI] [PubMed] [Google Scholar]
- 15.Bender W, Spierer P, Hogness D. J Mol Biol. 1983;168:17–33. doi: 10.1016/s0022-2836(83)80320-9. [DOI] [PubMed] [Google Scholar]
- 16.Cho W-L, Fu Y C, Chen C C, Ho C M. Insect Biochem Mol Biol. 1996;26:395–402. doi: 10.1016/0965-1748(95)00108-5. [DOI] [PubMed] [Google Scholar]
- 17.Raikhel A S, Lea A O. Physiol Entomol. 1982;7:55–64. [Google Scholar]
- 18.Kokoza V, Ahmed A, Cho W-L, Jasinskiene N, James A A, Raikhel A S. Proc Natl Acad Sci USA. 2000;97:9144–9149. doi: 10.1073/pnas.160258197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokoza V, Ahmed A, Wimmer E A, Raikhel A S. Insect Biochem Mol Biol. 2001;31:1137–1143. doi: 10.1016/s0965-1748(01)00120-5. [DOI] [PubMed] [Google Scholar]
- 20.Boman H G, Nilsson I, Rasmuson B. Nature. 1972;237:232–235. doi: 10.1038/237232a0. [DOI] [PubMed] [Google Scholar]
- 21.Khush R S, Leulier F, Lemaitre B. Science. 2002;296:273–275. doi: 10.1126/science.1071208. [DOI] [PubMed] [Google Scholar]
- 22.Lemaitre B, Nicolas E, Michaut L, Reichhart J M, Hoffmann J A. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 23.Rutschmann S, Kilinc A, Ferrandon D. J Immunol. 2002;168:1542–1546. doi: 10.4049/jimmunol.168.4.1542. [DOI] [PubMed] [Google Scholar]
- 24.Baldin S, Moita L F, Kocher T, Wilm M, Kafatos F C, Levashina E A. EMBO Rep. 2002;3:852–856. doi: 10.1093/embo-reports/kvf180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzou P, Reichhart J M, Lemaitre B. Proc Natl Acad Sci USA. 2002;99:2152–2157. doi: 10.1073/pnas.042411999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowenberger C, Bulet P, Charlet M, Hetru C, Hodgeman B, Christensen B M, Hoffmann J A. Insect Biochem Mol Biol. 1995;25:867–873. doi: 10.1016/0965-1748(95)00043-u. [DOI] [PubMed] [Google Scholar]
- 27.Hedengren M, Asling B, Dushay M S, Ando I, Ekengren S, Wihlborg M, Hultmark D. Mol Cell. 1999;4:827–837. doi: 10.1016/s1097-2765(00)80392-5. [DOI] [PubMed] [Google Scholar]
- 28.Choe K-M, Werner T, Stoven S, Hultmark D, Anderson K V. Science. 2002;296:359–362. doi: 10.1126/science.1070216. [DOI] [PubMed] [Google Scholar]
- 29.Gottar M, Gobert V, Michel T, Belvin M, Duyk G, Hoffmann J A, Ferrandon D, Royet J. Nature. 2002;416:640–644. doi: 10.1038/nature734. [DOI] [PubMed] [Google Scholar]
- 30.Stöven S, Ando I, Kadalayil L, Engström Y, Hultmark D. EMBO Rep. 2000;1:347–352. doi: 10.1093/embo-reports/kvd072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimopoulos G, Casavant T L, Chang S, Scheetz T, Roberts C, Donohue M, Schultz J, Benes V, Bork P, Ansorge W, et al. Proc Natl Acad Sci USA. 2000;97:6619–6624. doi: 10.1073/pnas.97.12.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]