Abstract
One half million patients suffer from colorectal cancer in industrialized nations, yet this disease exhibits a low incidence in under-developed countries. This geographic imbalance suggests an environmental contribution to the resistance of endemic populations to intestinal neoplasia. A common epidemiological characteristic of these colon cancer-spared regions is the prevalence of enterotoxigenic bacteria associated with diarrheal disease. Here, a bacterial heat-stable enterotoxin was demonstrated to suppress colon cancer cell proliferation by a guanylyl cyclase C-mediated signaling cascade. The heat-stable enterotoxin suppressed proliferation by increasing intracellular cGMP, an effect mimicked by the cell-permeant analog 8-br-cGMP. The antiproliferative effects of the enterotoxin and 8-br-cGMP were reversed by L-cis-diltiazem, a cyclic nucleotide-gated channel inhibitor, as well as by removal of extracellular Ca2+, or chelation of intracellular Ca2+. In fact, both the enterotoxin and 8-br-cGMP induced an L-cis-diltiazem-sensitive conductance, promoting Ca2+ influx and inhibition of DNA synthesis in colon cancer cells. Induction of this previously unrecognized antiproliferative signaling pathway by bacterial enterotoxin could contribute to the resistance of endemic populations to intestinal neoplasia, and offers a paradigm for targeted prevention and therapy of primary and metastatic colorectal cancer.
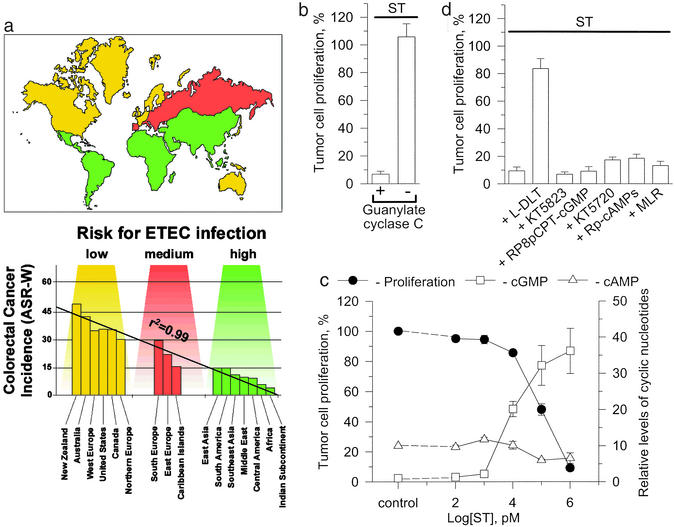
Colorectal cancer is the fourth leading cause of cancer and cancer-related mortality in the world, with a geographic distribution primarily affecting patients in developed countries (Fig. 1a; ref. 1). Although the epidemiology of this disease remains poorly understood, there is an unexplained inverse relationship between the incidence of colorectal cancer and enterotoxigenic Escherichia coli (ETEC) infections (Fig. 1a). Indeed, the age-adjusted incidence of colorectal cancer is lowest in under-developed countries (1), where ETEC infections are the highest (Fig. 1a; refs. 2 and 3).
Figure 1.
The geographic imbalance between colorectal cancer and infections with ETEC which produce enterotoxins that suppress human colon carcinoma cell proliferation. (a) Worldwide geographic distribution of ST-producing ETEC infections and the incidence of colorectal cancer. The risk of infection with ST-producing ETEC was estimated from the risk of travelers' diarrhea. The incidence of colorectal cancer is represented as the age-adjusted rate using the World Standard Population (ASR-W) and is expressed per 100,000. Linear regression analysis was generated to fit the mean values for each risk category. (b) ST (1 μM) inhibits DNA synthesis in colon cancer cells expressing GC-C [T84 cells; P < 0.01 for control vs. Student's t test (ST)] but not in those cells that do not express GC-C (SW480 cells). Tumor cell proliferation, % (y axis) is defined as the amount of DNA synthesis in treated cells as a percentage of the amount of DNA synthesis in parallel control cultures. (c) In T84 cells, the antiproliferative effects of ST correlate with [cGMP]i but not intracellular cAMP accumulation. (d) Inhibitors of downstream effectors of cGMP, including PKG (1 μM KT5823, 50 μM RP8pCPT-cGMP), cAMP-dependent protein kinase (0.5 μM KT5720, 50 μM RP-cAMPs), and cGMP-regulated phosphodiesterase 3 [10 μM milrinone (MRL)], did not alter the inhibition of proliferation induced by ST. In contrast, an inhibitor of CNG channels, L-DLT (200 μM), blocked the effect of ST on proliferation (P > 0.1 for control vs. L-DLT, Student's t test). The concentrations of inhibitors used are those that selectively and completely inhibit their target enzymes: PKG-I and –II (KT5823: Ki = 234 nM; RP8pCPT-cGMP: Ki = 500 nM; refs. 20, 48, and 49), cAMP-dependent protein kinase I and II (KT5720: Ki = 56 nM; RP-cAMPs: Ki = 10 μM; refs. 50 and 51), and phosphodiesterase 3 (milrinone: Ki = 300 nM; ref. 52). The concentration of L-DLT used abolished ST-induced 45Ca2+ influx in the rat colon (40). Data are the mean ± SEM of a representative experiment performed in triplicate.
ETEC produce heat-stable enterotoxins (STs; refs. 4 and 5), a principle cause of secretory diarrhea in endemic populations, travelers, and agriculturally important animal herds (6, 7). These enterotoxins are plasmid-encoded small (≤19 amino acids) peptides that bind to guanylyl cyclase C (GC-C), specifically expressed in intestinal epithelial cells (4, 8). Association of STs with the extracellular domain of GC-C activates the intracellular catalytic domain that converts GTP into cGMP (9, 10). The second messenger cGMP, in turn, activates cGMP-dependent protein kinase (PKG) II, the conventional downstream effector for this cyclic nucleotide, resulting in secretory diarrhea (9–11). In this way, STs represent molecular mimicry wherein enterotoxigenic bacteria have evolved a strategy for dissemination and propagation that exploits normal intestinal physiology. Indeed, STs are structurally and functionally homologous to the endogenous peptides guanylin and uroguanylin (12, 13), which mediate autocrine/paracrine control of intestinal fluid and electrolyte homeostasis (14).
Beyond volume homeostasis, GC-C and its ligands have been implicated in the regulation of the balance of proliferation and differentiation along the crypt-to-villus axis in the intestine (15). Expression of endogenous GC-C ligands is frequently lost during tumorigenesis, and subsequent loss of signaling may represent one key mutational event underlying neoplastic transformation in the colon (16–18). In principle, this putative role for GC-C as a tumor suppressor may contribute to the inverse epidemiological association between colorectal cancer (1) and ETEC infections (2, 3) reflecting, in part, longitudinal exposure in under-developed countries to ST-producing bacteria. However, the mechanisms by which STs repress colorectal tumorigenesis are unknown. The present study revealed a previously unrecognized ST-induced cGMP-dependent signaling pathway, through cyclic nucleotide-gated (CNG) channels and calcium, responsible for the antiproliferative action of bacterial enterotoxins on human colon carcinoma cells.
Materials and Methods
Cell Culture.
T84 (passages 50–70) and SW480 (passage 100–120) human colon carcinoma cells, obtained from the American Type Culture Collection, were maintained at 37°C (5% CO2) in DMEM/F12 containing 2.5 mM l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 10% (vol/vol) FBS.
DNA Synthesis.
Exponentially growing cancer cells (≈60% confluent in 96-well plates) were synchronized by serum starvation in Eagle's minimal essential medium (EMEM) for 48 h, followed by proliferative induction with 10 mM l-glutamine (in EMEM) for 24 h. ST and other agents were added to cells 15 min before 0.2 μCi per well (1 Ci = 37 GBq) of [methyl-3H]thymidine, which was added for 3 h, followed by quantification of [3H]thymidine incorporation into DNA (15).
Current Measurements.
The perforated mode of the whole-cell patch-clamp recording was applied to human T84 colon cancer cells. Membrane potential was controlled through the electrical access obtained by membrane perforation induced by amphotericin B (9, 10). The pipette solution supplemented by amphotericin B (200–240 mg/ml) contained: 140 mM KCl, 1 mM MgCl2, 5 mM EGTA, and 20 mM Hepes-KOH (pH 7.3). The bath solution contained (in mM): 136.5 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.5 mM MgCl2, 5.5 mM glucose, and 5 mM Hepes-NaOH (pH 7.4). Voltage-clamp recordings were performed with an Axopatch 1-C patch-clamp amplifier (Axon Instruments, Foster City, CA) at 31 ± 1°C using an HCC-100 temperature controller (Dagan Instruments, Minneapolis; ref. 19).
Cyclic Nucleotides.
cGMP and cAMP were quantified in exponentially growing T84 cells (≈60% confluent in 96-well plates) employing ELISA (Amersham Pharmacia) or RIA. In experiments employing ELISA, media was aspirated, reactions were terminated by the addition of a lysis solution (200 μl per well) containing 0.5% dodecyltrimethylammonium bromide, and aliquots (100 μl) from each well were processed for cyclic nucleotide determinations. In experiments employing RIA, reactions were terminated with ice-cold 100% ethanol, and supernatants were separated from pellets by centrifugation and processed for cGMP determinations (8).
Calcium Transport.
Exponentially growing T84 cells (40–80% confluent in 24-well plates) were incubated in media (S-MEM, Life Technologies, Rockville, MD) containing low (300 μM) CaCl2 to maximize the signal-to-noise ratio for L-cis-diltiazem (L-DLT)-sensitive 45Ca2+ transport determinations. Cells were treated with ST, 8-br-cGMP, and/or L-DLT for the indicated times, followed by the addition of 1 μCi per well 45Ca2+ for the last 15 min. Incubations were terminated by washing four times in cold PBS buffer (145 mM NaCl/5 mM KCl/1 mM MgCl2/10 mM glucose/5 mM Hepes, pH 7.4) containing 0.1 mM EGTA, cells were solubilized with cold NaOH (0.1 M), and radioactivity was quantified in 100-μl aliquots.
Results and Discussion
ST inhibited DNA synthesis in human colon cancer cells that express GC-C, but not in tumor cells deficient in GC-C (Fig. 1b). The concentration-dependence of ST inhibition of proliferation corresponded to the accumulation of intracellular cGMP [cGMP]i, but not other cyclic nucleotides (Fig. 1c). Yet, selective inhibitors of PKG, which disrupt ST induction of intestinal secretion (20), did not prevent the antiproliferative action of the enterotoxin (Fig. 1d). Moreover, inhibitors of cAMP-dependent protein kinase or cGMP-regulated phosphodiesterase 3 did not influence inhibition of proliferation by ST (Fig. 1d). Thus, the antiproliferative actions of ST on human colon carcinoma cells were not mediated by conventional downstream effectors of cGMP.
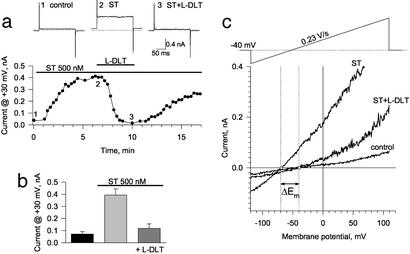
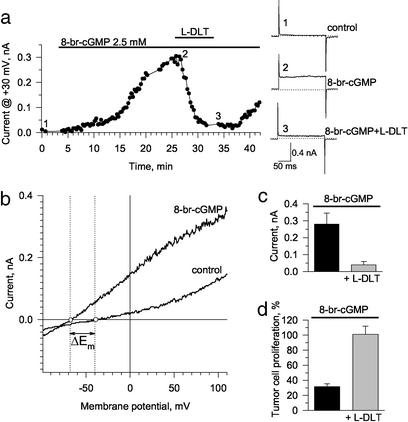
Rather, a specific reversible inhibitor of CNG channels, L-DLT (21), prevented ST inhibition of proliferation (Fig. 1d). In fact, ST induced membrane current in voltage-clamped human colon cancer cells that was reversibly blocked by L-DLT, indicating that the enterotoxin activates CNG channels (Fig. 2 a and b). Throughout the range of the imposed membrane potential ramp, ST significantly increased current, an effect largely reversed by L-DLT (Fig. 2c). Although the reversal potential (Em) of colon cancer cells was −39.3 ± 3.9 mV (n = 6), ST shifted Em to −66.3 ± 3.6 mV (n = 6; Fig. 2c), an effect abolished by L-DLT that restored Em to −39.5 ± 5.2 mV (n = 4; Fig. 2c). The action of ST on membrane current in colon cancer cells was mimicked by the membrane-permeant cGMP analog, 8-br-cGMP, which also induced an L-DLT-sensitive current and produced a significant shift in Em (ΔEm = −25.8 ± 4.6 mV, n = 3; Fig. 3 a–c). Moreover, 8-br-cGMP inhibited tumor cell DNA synthesis in an L-DLT sensitive manner (Fig. 3d). Identical actions of ST and 8-br-cGMP in colon cancer cells demonstrate that a [cGMP]i-signaling pathway mediates the action of the enterotoxin on activation of CNG channels and suppression of tumor cell proliferation.
Figure 2.
ST induces an L-DLT-sensitive current in human colon carcinoma cells. (a) Time-course of steady-state outward current recorded at the end of 200- ms-long depolarizing rectangular pulses from a holding potential of −40 mV to +30 mV. The period of drug application is indicated by corresponding horizontal bars above the data plot. (Top) Original current traces corresponding to specific points along the time course under control conditions (1), following application of 500 nM ST (2), and in the combined presence of ST plus 200 μM L-DLT (3). (b) Average current at +30 mV under control conditions, in the presence of ST, and in the presence of ST plus L-DLT (n = 6, mean ± SEM). (c) Voltage–current relationships obtained at the holding potential of −40 mV in response to 1-s-long ramp pulses from −120 mV to +110 mV under control conditions, in the presence of ST, and in the presence of ST plus L-DLT. ST induced a significant shift of the reversal potential (ΔEm = −27.5 ± 2.6 mV, n = 6, mean ± SEM) that was reversed by L-DLT.
Figure 3.
8-Br-cGMP induces an L-DLT-sensitive current in, and inhibits proliferation of, human colon carcinoma cells. (a) [cGMP]i was increased by application of the membrane-permeable analog, 8-br-cGMP (2.5. mM), which, in a time-dependent manner, activated a membrane current sensitive to 200 μM L-DLT. (Right) Original current traces recorded in response to 200-ms depolarizing rectangular pulses from a holding potential of −40 mV to +30 mV correspond to points along the time course under control conditions (1), in the presence of 8-br-cGMP (2), and in the combined presence of 8-br-cGMP plus L-DLT (3). (b) Voltage–current relationships obtained at the holding potential of −40 mV in response to 1-s-long ramp pulses from −100 mV to +110 mV under control conditions and in the presence of 8-br-cGMP. (c) Average current at +30 mV in the presence of 8-br-cGMP and in the presence of 8-br-cGMP plus L-DLT (n = 4, mean ± SEM). (d) Inhibition of cell proliferation by 8-br-cGMP (5 mM) was blocked by 200 μM L-DLT. Data are the mean ± SEM of a representative experiment performed in triplicate.
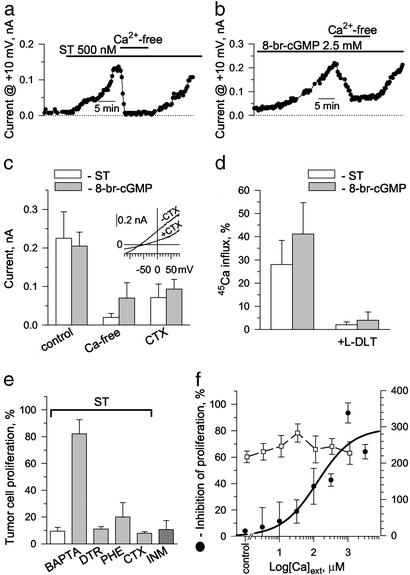
The nonselective CNG channel preferentially conducts Ca2+ ions (22). Accordingly, removal of extracellular Ca2+ ([Ca2+]ext) reduced both ST and 8-br-cGMP currents, from 0.23 ± 0.07 to 0.02 ± 0.01 nA and from 0.21 ± 0.04 to 0.07 ± 0.04 nA, respectively (n = 5; Fig. 4 a–c). As a further indicator of Ca2+ entry, Ca2+-dependent K+ current (KCa), previously reported in this cancer cell line (23) and identified here by sensitivity to the specific blocker charybdotoxin, was found to be induced by both ST and 8-br-cGMP (Fig. 4c). In accordance, reversal potentials derived from the ST- and 8-br-cGMP-induced current–voltage relationships approached −70 mV (Figs. 2c, 3b, and 4c Inset), which closely approximates the theoretical equilibrium potential for K+ ions (EK≈ −75 mV) calculated from the Nernst equation (24). Such shift in Em away from Ca2+ equilibrium would drive Ca2+ influx. However, failure to exactly match the theoretical K+ equilibrium reflects current contribution by non-K+ conductances, such as the chloride-carrying CFTR and/or nonselective current through CNG channels. Finally, ST and 8-br-cGMP-mediated Ca2+ entry through CNG channels was demonstrated by direct measurement of radioactive calcium flux into colon cancer cells that was inhibited by L-DLT (Fig. 4d).
Figure 4.
ST and 8-br-cGMP alter the membrane conductance and proliferation of human colon carcinoma cells by inducing CNG channel-mediated calcium influx. (a and b) Currents induced by ST (a) and 8-br-cGMP (b) are reversed in calcium-free solution. Steady-state outward current recorded at the end of 200-ms-long depolarizing rectangular pulses from a holding potential of −40 mV to +10 mV. (c) Average effect of ST (white bars) and 8-br-cGMP (gray bars) in the presence (control; 1.8 mM CaCl2) and absence (Ca-free) of [Ca2+]ext, as well as in the presence of 100 nM charybdotoxin, a specific inhibitor of Kca channels (CTX in the presence of 1.8 mM CaCl2). Data are the mean ± SEM of five separate experiments. (Inset) A representative current–voltage relationship defining the effect of charybdotoxin on ST-induced current obtained in response to 0.25 V/s ramped membrane depolarization. (d) ST (1 μM, 20 min) or 8-br-cGMP (5 mM, 40 min) induced influx of 45Ca2+ into colon cancer cells, which was abolished by pretreatment (30 min) with 250 μM L-DLT. Results are expressed as percent increase over respective controls and are the mean ± SEM of six (ST) or five (8-br-cGMP) experiments performed in duplicate. (e) ST inhibits cell proliferation, an effect abolished by the cytosolic calcium chelator BAPTA-AM (BAPTA, 20 μM). Dantrolene (DTR, 50 μM), which blocks Ca2+ mobilization from the endoplasmic reticulum, and phenamil (PHE, 1 μM), which blocks Na+/Ca2+ exchange as well as CTX (100 nM), did not reverse ST-mediated inhibition of proliferation. Ionomycin (INM, 1 μM), a calcium ionophore, mimicked on its own the effects of ST (1 μM). Results are expressed as percentage of respective controls and are the mean ± SEM of a representative experiment performed in triplicate. (f) ST inhibition of cell proliferation (left scale) depended on [Ca2+]ext, with an EC50 estimated at 127 μM. The inability of ST to inhibit proliferation in the absence of [Ca2+]ext did not reflect failure of ST to bind to GC-C, because induction of [cGMP]i accumulation (right scale) by ST was independent of [Ca2+]ext. Data are the mean ± SEM of a representative experiment performed in triplicate.
The essential role of Ca2+ influx in the ST-mediated regulation of cancer cell proliferation is underscored by reversal of ST antiproliferative action through cytosolic Ca2+ chelation with BAPTA-AM (Fig. 4e). Furthermore, without influencing [cGMP]i, depletion of [Ca2+]ext abolished the ability of ST to inhibit cancer cell proliferation, whereas increases in [Ca2+]ext restored in a concentration-dependent manner the antiproliferative effect of ST (Fig. 4f). In fact, ionomycin, a Ca2+ ionophore, mimicked, whereas dantrolene, which blocks Ca2+ release from intracellular pools, or phenamil, an inhibitor of electrogenic Na+ influx, did not affect the ability of ST to inhibit cancer cell proliferation (Fig. 4e). The absence of charybdotoxin effect on ST inhibition of cancer cell proliferation further indicates that Ca2+ entry rather than the consequent Ca2+-sensitive K+ current is required for enterotoxin-mediated antiproliferation (Fig. 4e). Thus, ST inhibits DNA synthesis in colon carcinoma cells by a signaling mechanism initiated by activation of GC-C, accumulation of [cGMP]i, and Ca2+ influx through CNG channels.
cGMP is emerging as an important regulator of cell proliferation, although the molecular mechanisms mediating that activity appear to be varied and cell-specific. Thus, in human vascular smooth muscle cells, cGMP delays the G1/S transition by down-regulation of cyclin D1 and cyclin-dependent kinase 4 activities after platelet-derived growth factor stimulation (25). Also, cGMP suppresses human T cell proliferation by inhibiting IL-2 release (26). In addition, proliferation of glomerular mesangial cells by phorbol 12,13-dibutyrate-mediated activation of mitogen-activated protein kinase is antagonized by cGMP-induced expression of the specific phosphatase MKP-1 (27). Further, in colorectal cancer cells, the antiproliferative effects of cGMP may reflect activation of apoptotic pathways (28, 29) or regulation of cell cycle (15). Conversely, cGMP-dependent activation of PKG promotes human umbilical vein endothelial cell proliferation by stimulating Raf-1 kinase activity (30).
In the present study, proliferation of intestinal epithelial cells was suppressed by a signaling mechanism initiated by ST interaction with GC-C. Indeed, cells that lack this receptor/enzyme molecule and do not exhibit ST-induced accumulation of [cGMP]i (31) were unresponsive to the antiproliferative effects of ST. ST/GC-C interaction induced the accumulation of [cGMP]i, which mediated suppression of proliferation by activating CNG channels. It is notable that cGMP inhibition of proliferation in intestinal cells is mediated through the L-DLT-sensitive CNG pathway, because specific inhibitors of cAMP-dependent protein kinase (32), phosphodiesterase 3 (11), and PKG (20), which mediate GC-C-dependent secretion, did not alter the effect of ST on DNA synthesis. Thus, ST-mediated [cGMP]i accumulation does not cause functional transactivation of cAMP-dependent pathways because ST did not induce [cAMP]i accumulation (Fig. 1c), activation of cAMP-dependent protein kinase, or inhibition of phosphodiesterase 3 (Fig. 1d). This report details the regulation of cell proliferation by a cGMP-dependent mechanism mediated by CNG channels.
Ca2+ serves as the third messenger in the signaling cascade linking GC-C at the cell surface to regulation of proliferation in the nucleus. Indeed, the ability of the second messenger cGMP to inhibit DNA synthesis was mediated by [Ca2+]ext influx through CNG channels (see Fig. 4 d–f). cGMP-dependent CNG channel-mediated Ca2+ influx has been described in excitable cells, although it mediates different functions. In this way, in cone and rod photoreceptors, high [cGMP]i sustains dark-state functions, in part, by inducing Ca2+ influx through CNG channels (33). Similarly, in the brain, cGMP-dependent activation of CNG channels regulates neurotransmitter release and potentiates synaptic transmission through Ca2+ influx (34). Additionally, regulation of intracellular Ca2+ by cGMP has been described in several cell systems, including vascular smooth muscle (35), platelets (36), and neurons (37). In intestinal cells, ST activation of GC-C mobilizes intracellular Ca2+ by a cGMP-dependent mechanism (38, 39). Further, ST and 8-br-cGMP induce Ca2+ influx through CNG channels in colon (40). In close agreement, the present study demonstrates that ST and 8-br-cGMP induce a structurally and functionally compartmentalized influx of Ca2+ through CNG channels in human colon carcinoma cells. Indeed, cGMP does not evoke electrophysiologically detectable Ca2+ currents in these cells (41), although its influx could be detected with radioactive Ca2+. The precise mode of Ca2+ delivery to its intracellular site of action to induce intestinal cell cytostasis remains undefined. Yet, this antiproliferative process must be tightly regulated because Ca2+ can, upon intracellular accumulation, promote apoptosis (42), which is not induced by ST (15).
In summary, these data demonstrate that bacterial enterotoxins suppress colon carcinoma cells through a GC-C-based calcium-dependent signaling pathway with a newly identified role in regulating cell proliferation. Endogenous GC-C ligands, guanylin and uroguanylin, may activate this pathway and promote the transition from proliferation to differentiation of enterocytes along the crypt-villus axis in normal intestine (15). In addition, these observations offer a possible mechanistic insight into the resistance to colorectal cancer observed in geographic areas in which ETEC is endemic. Although additional factors contribute to the epidemiology of colorectal cancer (43, 44), the significance of this antiproliferative pathway is highlighted by the neoplastic transformation of epithelial cells that follows loss of expression of endogenous GC-C ligands in the intestine (16–18). In turn, the conservation of GC-C itself and its downstream effectors by colorectal tumors provides a therapeutic target for restoration of this signaling cascade and maintenance of the tumor-suppressor phenotype. Indeed, oral administration of GC-C ligands or downstream effectors of that pathway, such as calcium, offer a hitherto unknown approach to the primary prevention of intestinal neoplasia and/or therapy of colorectal cancer metastases (29, 45–47).
Acknowledgments
This work was supported by National Institutes of Health Grants HL65921, CA7512, CA7966, HL59214, HL07111, and HL64822, Targeted Diagnostic and Therapeutics, Inc., the Landenberger Foundation, the American Heart Association, the Marriott Foundation, the Miami Heart Research Institute, the Bruce and Ruth Rappaport Program, and American Physicians Fellowship for Medicine in Israel. S.A.W. is the Samuel M. V. Hamilton Professor. A.T. is an Established Investigator of the American Heart Association.
Abbreviations
- ETEC
enterotoxigenic Escherichia coli
- STs
heat-stable enterotoxins
- GC-C
guanylyl cyclase C
- PKG
cyclic GMP-dependent protein kinase
- CNG channels
cyclic nucleotide-gated channels
- L-DLT
L-cis-diltiazem
- [cGMP]i
intracellular cGMP
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ferlay J, Bray F, Pisani P, Parkin D M. GLOBOCAN 2000: Cancer Incidence, Mortality and Prevalence Worldwide, Version 1.0. Lyon: International Agency for Research on Cancer; 2001. [Google Scholar]
- 2.Hill D R, Pearson R D. Ann Intern Med. 1988;108:839–852. doi: 10.7326/0003-4819-108-6-839. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Health Information for International Travel 1999–2000. Atlanta: Department of Health and Human Services; 2001. [Google Scholar]
- 4.Schulz S, Green C K, Yuen P S, Garbers D L. Cell. 1990;63:941–948. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- 5.Hughes J M, Murad F, Chang B, Guerrant R L. Nature. 1978;271:755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- 6.Guarino A, Cohen M B, Giannella R A. Pediatr Res. 1987;21:551–555. doi: 10.1203/00006450-198706000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Giannella R A. J Lab Clin Med. 1995;125:173–181. [PubMed] [Google Scholar]
- 8.Carrithers S L, Barber M T, Biswas S, Parkinson S J, Park P K, Goldstein S D, Waldman S A. Proc Natl Acad Sci USA. 1996;93:14827–14832. doi: 10.1073/pnas.93.25.14827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkinson S J, Alekseev A E, Gomez L A, Wagner F, Terzic A, Waldman S A. J Biol Chem. 1997;272:754–758. doi: 10.1074/jbc.272.2.754. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Mannan I, Schulz S, Parkinson S J, Alekseev A E, Gomez L A, Terzic A, Waldman S A. FASEB J. 1999;13:913–922. doi: 10.1096/fasebj.13.8.913. [DOI] [PubMed] [Google Scholar]
- 11.Vaandrager A B, Bot A G, Ruth P, Pfeifer A, Hofmann F, De Jonge H R. Gastroenterology. 2000;118:108–114. doi: 10.1016/s0016-5085(00)70419-7. [DOI] [PubMed] [Google Scholar]
- 12.Currie M G, Fok K F, Kato J, Moore R J, Hamra F K, Duffin K L, Smith C E. Proc Natl Acad Sci USA. 1992;89:947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamra F K, Forte L R, Eber S L, Pidhorodeckyj N V, Krause W J, Freeman R H, Chin D T, Tompkins J A, Fok K F, Smith C E, et al. Proc Natl Acad Sci USA. 1993;90:10464–10468. doi: 10.1073/pnas.90.22.10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forte L R. Regul Pept. 1999;81:25–39. doi: 10.1016/s0167-0115(99)00033-6. [DOI] [PubMed] [Google Scholar]
- 15.Pitari G M, Di Guglielmo M D, Park J, Schulz S, Waldman S A. Proc Natl Acad Sci USA. 2001;98:7846–7851. doi: 10.1073/pnas.141124698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen M B, Hawkins J A, Witte D P. Lab Invest. 1998;78:101–108. [PubMed] [Google Scholar]
- 17.Notterman D A, Alon U, Sierk A J, Levine A J. Cancer Res. 2001;61:3124–3130. [PubMed] [Google Scholar]
- 18.Birkenkamp-Demtroder K, Lotte Christensen L, Harder Olesen S, Frederiksen C M, Laiho P, Aaltonen L A, Laurberg S, Sorensen F B, Hagemann R, Orntoft T F. Cancer Res. 2002;62:4352–4363. [PubMed] [Google Scholar]
- 19.Zingman L V, Alekseev A E, Bienengraeber M, Hodgson D, Karger A B, Dzeja P P, Terzic A. Neuron. 2001;31:233–245. doi: 10.1016/s0896-6273(01)00356-7. [DOI] [PubMed] [Google Scholar]
- 20.Vaandrager A B, Bot A G, De Jonge H R. Gastroenterology. 1997;112:437–443. doi: 10.1053/gast.1997.v112.pm9024297. [DOI] [PubMed] [Google Scholar]
- 21.Stern J H, Kaupp U B, MacLeish P R. Proc Natl Acad Sci USA. 1986;83:1163–1167. doi: 10.1073/pnas.83.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dzeja C, Hagen V, Kaupp U B, Frings S. EMBO J. 1999;18:131–144. doi: 10.1093/emboj/18.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devor D C, Frizzell R A. Am J Physiol. 1998;274:C138–C148. doi: 10.1152/ajpcell.1998.274.1.C138. [DOI] [PubMed] [Google Scholar]
- 24.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates; 1984. [Google Scholar]
- 25.Fukumoto S, Koyama H, Hosoi M, Yamakawa K, Tanaka S, Morii H, Nishizawa Y. Circ Res. 1999;85:985–991. doi: 10.1161/01.res.85.11.985. [DOI] [PubMed] [Google Scholar]
- 26.Fischer T A, Palmetshofer A, Gambaryan S, Butt E, Jassoy C, Walter U, Sopper S, Lohmann S M. J Biol Chem. 2001;276:5967–5974. doi: 10.1074/jbc.M009781200. [DOI] [PubMed] [Google Scholar]
- 27.Sugimoto T, Haneda M, Togawa M, Isono M, Shikano T, Araki S, Nakagawa T, Kashiwagi A, Guan K L, Kikkawa R. J Biol Chem. 1996;271:544–547. doi: 10.1074/jbc.271.1.544. [DOI] [PubMed] [Google Scholar]
- 28.Thompson W J, Piazza G A, Li H, Liu L, Fetter J, Zhu B, Sperl G, Ahnen D, Pamukcu R. Cancer Res. 2000;60:3338–3342. [PubMed] [Google Scholar]
- 29.Shailubhai K, Yu H H, Karunanandaa K, Wang J Y, Eber S L, Wang Y, Joo N S, Kim H D, Miedema B W, Abbas S Z, et al. Cancer Res. 2000;60:5151–5157. [PubMed] [Google Scholar]
- 30.Hood J, Granger H J. J Biol Chem. 1998;273:23504–23508. doi: 10.1074/jbc.273.36.23504. [DOI] [PubMed] [Google Scholar]
- 31.Waldman S A, Barber M, Pearlman J, Park J, George R, Parkinson S J. Cancer Epidemiol Biomarkers Prev. 1998;7:505–514. [PubMed] [Google Scholar]
- 32.Chao A C, de Sauvage F J, Dong Y J, Wagner J A, Goeddel D V, Gardner P. EMBO J. 1994;13:1065–1072. doi: 10.1002/j.1460-2075.1994.tb06355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ames J B, Dizhoor A M, Ikura M, Palczewski K, Stryer L. J Biol Chem. 1999;274:19329–19337. doi: 10.1074/jbc.274.27.19329. [DOI] [PubMed] [Google Scholar]
- 34.Zufall F, Shepherd G M, Barnstable C J. Curr Opin Neurobiol. 1997;7:404–412. doi: 10.1016/s0959-4388(97)80070-0. [DOI] [PubMed] [Google Scholar]
- 35.Lucas K A, Pitari G M, Kazerounian S, Ruiz Stewart I, Park J, Schulz S, Chepenik K P, Waldman S A. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- 36.Rosado J A, Porras T, Conde M, Sage S O. J Biol Chem. 2001;276:15666–15675. doi: 10.1074/jbc.M009217200. [DOI] [PubMed] [Google Scholar]
- 37.Andric S A, Kostic T S, Tomic M, Koshimizu T, Stojilkovic S S. J Biol Chem. 2001;276:844–849. doi: 10.1074/jbc.M004406200. [DOI] [PubMed] [Google Scholar]
- 38.Knoop F C, Owens M. J Pharmacol Toxicol Methods. 1992;28:67–72. doi: 10.1016/1056-8719(92)90049-7. [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharya J, Chakrabarti M K. Biochim Biophys Acta. 1998;1403:1–4. doi: 10.1016/s0167-4889(98)00026-3. [DOI] [PubMed] [Google Scholar]
- 40.Qiu W, Lee B, Lancaster M, Xu W, Leung S, Guggino S E. Am J Physiol. 2000;278:C336–C343. doi: 10.1152/ajpcell.2000.278.2.C336. [DOI] [PubMed] [Google Scholar]
- 41.Biel M, Sautter A, Ludwig A, Hofmann F, Zong X. Naunyn-Schmiedeberg's Arch Pharmacol. 1998;358:140–144. doi: 10.1007/pl00005235. [DOI] [PubMed] [Google Scholar]
- 42.Berridge M J, Bootman M D, Lipp P. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 43.Briskey E N, Pamies R J. J Natl Med Assoc. 2000;92:222–230. [PMC free article] [PubMed] [Google Scholar]
- 44.Wilmink A B. Dis Colon Rectum. 1997;40:483–493. doi: 10.1007/BF02258397. [DOI] [PubMed] [Google Scholar]
- 45.Buset M, Lipkin M, Winawer S, Swaroop S, Friedman E. Cancer Res. 1986;46:5426–5430. [PubMed] [Google Scholar]
- 46.Penman I D, Liang Q L, Bode J, Eastwood M A, Arends M J. J Clin Pathol. 2000;53:302–307. doi: 10.1136/jcp.53.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sesink A L, Termont D S, Kleibeuker J H, Van der Meer R. Carcinogenesis. 2001;22:1653–1659. doi: 10.1093/carcin/22.10.1653. [DOI] [PubMed] [Google Scholar]
- 48.Grider J R. Am J Physiol. 1993;264:G334–G340. doi: 10.1152/ajpgi.1993.264.2.G334. [DOI] [PubMed] [Google Scholar]
- 49.Butt E, Eigenthaler M, Genieser H G. Eur J Pharmacol. 1994;269:265–268. doi: 10.1016/0922-4106(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 50.Gadbois D M, Crissman H A, Tobey R A, Bradbury E M. Proc Natl Acad Sci USA. 1992;89:8626–8630. doi: 10.1073/pnas.89.18.8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dostmann W R. FEBS Lett. 1995;375:231–234. doi: 10.1016/0014-5793(95)01201-o. [DOI] [PubMed] [Google Scholar]
- 52.Harrison S A, Reifsnyder D H, Gallis B, Cadd G G, Beavo J A. Mol Pharmacol. 1986;29:506–514. [PubMed] [Google Scholar]