Abstract
The Xenopus oocyte is used as a convenient cell expression system to study the structure and function of heterogenic transmitter receptors and ion channels. Recently, we introduced a method to microtransplant already assembled neurotransmitter receptors from the human brain to the plasma membrane of Xenopus oocytes. The same approach was used here to transplant neurotransmitter receptors expressed from cultured cells to the oocytes. Membrane vesicles prepared from a human embryonic kidney cell line (HEK293) stably expressing the rat glutamate receptor 1 were injected into oocytes, and, within a few hours, the oocyte plasma membrane acquired α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-type glutamate receptors, which had the same properties as those expressed in the original HEK cells. Analogously, oocytes injected with membranes prepared from rat pituitary GH(4)C1 cells, stably expressing homomeric human neuronal α7 nicotinic acetylcholine receptors (α7-AcChoRs), incorporated in their plasma membrane AcChoRs that behaved as those expressed in GH(4)C1 cells. Similar results were obtained with HEK cells stably expressing heteromeric human neuronal α4β2-AcChoRs. All this makes the Xenopus oocyte a powerful tool for detailed investigations of receptors and other proteins expressed in the membrane of cultured cells.
The original approach (1) of injecting heterogenic mRNAs, isolated from brains and other tissues, into Xenopus oocytes to express functional neurotransmitter receptors in the oocyte membrane (1) has been used extensively in the last 20 yr to study the molecular structure and function of many receptors and voltage-operated channels (2–4). A method was subsequently developed to incorporate in the oocyte membrane foreign receptors that had already been assembled in their native cells. Essentially, this method consists in injecting the oocytes with cell membranes prepared from foreign tissues. The oocyte plasma membrane incorporates the foreign membranes and efficiently acquires functional neurotransmitter receptors and voltage-operated channels (5–7). Those experiments showed that the incorporated receptors and channels behaved very much like the ones in the original cells. However, we deemed it important to determine in more detail whether the properties of the receptors incorporated in the oocyte membrane are the same as those of the receptors while still in the “donor” cells. For that purpose, we microtransplanted three types of neurotransmitter receptors assembled in the membranes of transfected cells to the oocytes and found that the properties of the receptors in their native membrane are retained after transplantation to the oocytes. Therefore, this method may be useful to study many of the proteins present in the membranes of cultured cells.
Materials and Methods
Cell Lines.
The cell lines used for electrophysiological recordings and membrane isolation were as follows: (i) HEK 293 cells stably transfected with the rat flip variant of glutamate receptor 1 (HEK-GluR1, a gift from Dr. P. Bregestovski, Centre National de la Recherche Scientifique, Marseille, France); (ii) HEK293 cells stably transfected with the human α4 and β2 nicotinic receptor subunits (HEK-α4β2 indicated elsewhere also as A4B2.2 cells; cf. ref. 20; a gift from from Merck, Whitehouse Station, NJ); and (iii) the rat pituitary GH(4)C1 cells stably transfected with the human α7 nicotinic receptor [GH(4)C1-α7; ref. 8; also a gift from Merck]. HEK cells were grown in DMEM and the GH(4)C1 in Ham's F10 media; and both were supplemented with 10% FBS, penicillin, streptomycin, and geneticin (G418 sulfate, 500 μg/ml). All culture media were purchased from Invitrogen.
Membrane Preparation.
Membranes were prepared as described (7). Briefly, about 107 cells were collected, rinsed in PBS and stored frozen at −80°C. Cells were then homogenized by using a Teflon glass homogenizer with 2 ml of glycine buffer of the following composition (in mM): 200 glycine, 150 NaCl, 50 EGTA, 50 EDTA, and 300 sucrose; plus 20 μl of protease inhibitors (P2714; Sigma); pH 9 was adjusted with NaOH. The homogenate was centrifuged for 15 min at 9,500 × g with a Beckman centrifuge (C1015 rotor). The supernatant was collected and centrifuged for 2 h at 105 × g with a TL-100 rotor at 4°C. The pellet was washed, resuspended in assay buffer (glycine 5 mM), and used directly, or aliquotted and stored at −80°C for later use.
Oocyte Injection of Membrane Vesicles.
Preparation of Xenopus laevis oocytes and injection procedures were detailed elsewhere (9). Oocytes were injected with membranes (≈100 nl; 9–14 mg of protein per ml) dissolved in 5 mM glycine, and maintained in modified Barth's solution plus antibiotics (kanamycin, 104 units⋅ml−1; penicillin, 104 units⋅ml−1; streptomycin, 10 mg⋅ml−1) at 16°C until electrophysiological recordings were performed. As controls, oocytes were injected with 100 nl of water or 5 mM glycine alone.
Patch-Clamp Recordings.
Whole-cell current recordings were performed from HEK-GluR1, HEK-α4β2 cells, or GH(4)C1-α7 cells 2–3 days after plating. Recordings and data analysis were performed as detailed (10) by using borosilicate glass patch pipette (3- to 6-MΩ tip resistance) connected to an Axopatch 200A amplifier (Axon Instruments, Foster City, CA). Data were stored on a PC computer by using pclamp8 software (Axon Instruments). Whole cell capacitance and patch series resistances (5–15 MΩ) were estimated from slow transient compensations, with a series resistance compensation of 75–90%. Unless otherwise indicated, the cells were voltage-clamped at a holding potential of −70 mV and continuously superfused by using a gravity-driven perfusion system consisting of independent tubes for standard and agonist-containing solutions (positioned 50–100 μm from the patched cell). To reduce the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) current desensitization, all measurements of AMPA currents in cultured cells were made in the presence of 50 μM cyclothiazide (stock was 50 mM in DMSO; ref. 11). Potential ramps (−120 mV to +60 mV, 1.5 s duration) were applied during the steady-state current induced by neurotrasmitters. Dose–response relationships were constructed as detailed in ref. 12 and below.
Oocyte Recordings.
A few hours (from 5 to 48) after cell membrane injection, membrane currents were recorded from voltage-clamped oocytes by using two microelectrodes filled with 3 M KCl (13). The oocytes were placed in a recording chamber (volume, 0.1 ml) and perfused continuously, 10–11 ml/min, with oocyte Ringer's solution at room temperature (20–22°C). To obtain agonist-dose/current response relations, the substances were applied to the oocytes at 4-min intervals, holding the oocyte membrane potential at −60 mV. The current-voltage relations were fitted with the Boltzmann equation as below. The half maximal concentration (EC50) was estimated by fitting the data to Hill equations, using least-square routines:
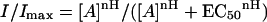 |
1 |
where [A] are the doses of neurotransmitter, nH is the Hill coefficient, and Imax is the maximum current response. Solution exchange was achieved by using electromagnetic valves and a computer controlled perfusion system (Biologique, Claix, France). Current-voltage relationships were constructed holding the cells at −60 mV and stepping the membrane potential for 2–4 min at the desired value before applying the neurotransmitter.
The current-voltage relations were fitted to the Boltzmann equation:
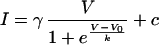 |
2 |
where I is the current, V is the membrane potential, γ is the conductance, V0 is the threshold potential, and c and k are constant values. To reduce AMPA current desensitization, all measurements of AMPA currents were again made in the presence of 50 μM cyclothiazide.
Chemicals and Solutions.
Oocyte Ringer's solution had the following composition (in mM): NaCl 82.5, KCl 2.5, CaCl2 2.5, MgCl2 1, and Hepes-NaOH 5, adjusted to pH 7.4 with NaOH. The HEK or GH(4)C1 cells were superfused with (in mM): NaCl 140, KCl 2.5, CaCl2 2, MgCl2 2, Hepes-NaOH 10, and glucose 10 (pH 7.3). Whole-cell patch-clamp recording pipettes were filled with (in mM): CsCl 140, MgCl2 2, Hepes 10, Na-ATP 4, and EGTA 0.25, equilibrated at pH 7.3 with CsOH. All drugs were purchased from Sigma, with the exception of AMPA, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and cyclothiazide from Tocris Neuramin (Bristol, U.K.); and of methyllycaconitine (MLA) from Research Biochemicals (Natick, MA).
Results
GluR1 Receptors.
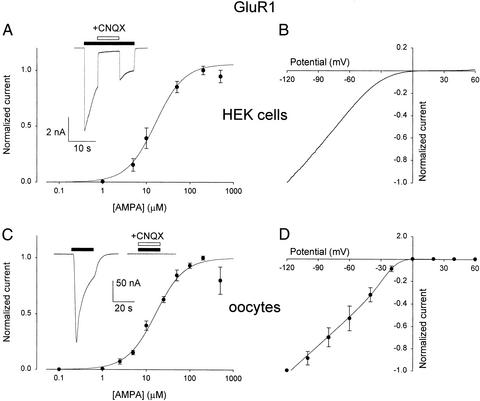
Application of AMPA (200 μM) to the HEK-GluR1 cells elicited inward currents, −5.3 ± 0.8 nA amplitude (mean ± SEM; n = 6), that decayed with a T0.1 value (the time for the current to decay by 10%) of 1.1 ± 0.2 s, and were rapidly and reversibly inhibited by the classical competitive blocker CNQX (Fig. 1A Inset). The amplitude of these currents increased with AMPA concentration, and the AMPA-dose/current-response curves, fitted to the Hill equation, gave nH and EC50 values of 1.3 and 16 μM (Fig. 1A). These values are similar to those reported for cerebellar granule cells (10). Moreover, the AMPA current was nonlinear, with membrane voltage showing a clear inward rectification beyond −40 mV (Fig. 1B), as described elsewhere using a variety of experimental approaches (14, 15).
Figure 1.
Properties of AMPA currents in HEK-GluR1 cells and in oocytes microtransplanted with HEK-GluR1 cell membranes. (A) AMPA-dose/current relation from six HEK-GluR1 cells. Peak currents normalized to Imax of each cell (mean Imax = −5.3 ± 0.8 nA; AMPA, 200 μM). Data represent mean ± SEM. (Inset) Sample current elicited by AMPA (100 μM) and blocked by CNQX (20 μM). In all figures, the solid bars show the timing of drug applications. (B) Current-voltage relation, from potential ramps, −120 mV to +60 mV, applied during the steady-state current induced by AMPA (50 μM). Data normalized to the current at −120 mV (−7.7 ± 1.6 nA; average of four HEK-GluR1 cells). (C) AMPA-dose/current response relation from 10 oocytes injected with HEK-GluR1 cell membranes. Peak currents normalized to Imax of each oocyte (mean Imax = −187 ± 92 nA; AMPA, 200 μM). (Inset) Drug concentrations and horizontal bars are as in A. (D) AMPA-current/voltage relation, from four oocytes injected as in C, fitted to a Boltzmann equation (see Materials and Methods). Data normalized to the currents, −149 ± 50 nA amplitude, elicited by AMPA (50 μM) at −120 mV.
Within 2 hr postinjection, the oocytes injected with HEK-GluR1 membranes acquired AMPA-type receptors that gated inward membrane currents, which varied in amplitude among different oocytes and also between donor frogs. For instance, 24 h after cell membrane injection, oocytes from one donor AMPA (200 μM) elicited currents of −179 ± 25 nA (n = 10; range, −62 to −307 nA). The AMPA currents in the oocytes desensitized fairly rapidly, showing a T0.1 value (0.49 ± 0.05 s; Fig. 1C Inset) significantly different compared with T0.1 value determined in HEK cells (Student's t test, P < 0.05). Furthermore, CNQX again blocked the currents elicited by AMPA in oocytes injected with HEK-GluR1 membranes. The AMPA-dose/current-response relation of the oocytes (Fig. 1C) yielded nH and EC50 values of 1.32 and 16.2 μM, respectively, matching the values reported here for HEK-GluR1 cells (see Fig. 1A), and elsewhere for Xenopus oocytes expressing GluR1 receptors, after the injection of GluR1 cRNA (16). Furthermore, the oocyte's AMPA currents increased fairly linearly, with membrane potential between −120 and −20 mV, and showed a considerable rectification beyond −20 mV (Fig. 1D). Fitting the I-V relationships to a Boltzmann equation (see Materials and Methods), we found similar slopes for both Xenopus oocytes and HEK cells (γHEK = 8.5 × 10−3 mV−1; γoocytes = 8.6 × 10−3 mV−1).
α7 Nicotinic Acetylcholine Receptors (AcChoR).
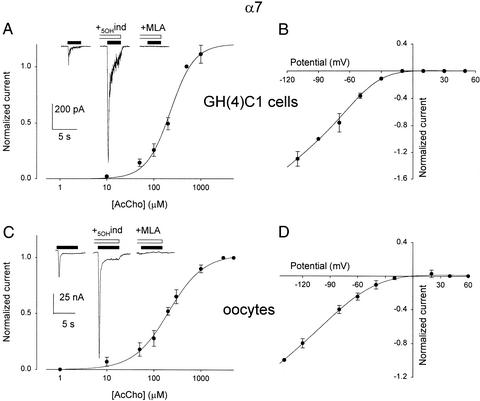
Patch-clamped GH(4)C1-α7 cells responded to AcCho application with an inward current that decayed very rapidly. Specifically, AcCho (200 μM) elicited a mean current of −167 ± 52 pA (n = 6), with a T0.1 value of 4.5 ± 1.6 ms, and the rate of decay became faster as the neurotransmitter concentration was raised (not shown). The AcCho currents were blocked by the α7 nicotinic antagonist MLA (e.g., Fig. 2A Inset), and were not affected by atropine (not shown). On the other hand, it is known that AcCho responses elicited by activation of α7 receptors, both in native nerve cells and in cell expression systems, are positively modulated by 5-hydroxyindole (5OHind; ref. 17). Accordingly, we found that the AcCho currents were greatly potentiated by 5OHind applied 4 s before the AcCho (e.g., Fig. 2A Inset). The AcCho concentration/current response relationship (Fig. 2A) gave EC50 and nH values of 239 μM and 1.9, similar to values reported elsewhere (18). Furthermore, the AcCho current was nonlinear, with membrane voltage showing marked rectification beyond −30 mV (Fig. 2B), as described (12).
Figure 2.
AcCho currents from GH(4)C1-α7 cells compared with those of oocytes injected with GH(4)C1-α7 cell membranes. (A) Dose-current response relation from six GH(4)C1-α7 cells. Peak currents normalized to Imax of each cell (mean Imax = −167 ± 52 pA ;n = 6; AcCho, 200 μM). (Inset Left) Current elicited by 100 μM AcCho; Center, plus 5OHind (1 mM; horizontal empty bar); Right, plus MLA (50 nM). (B) AcCho-current-voltage relation obtained with AcCho (100 μM) at different membrane potentials. Data normalized to the AcCho-current at −90 mV (−45 ± 14 pA), and averaged from three cells. (C) AcCho-dose/current response relation from six oocytes injected with GH(4)C1-α7 cell membranes. Peak currents normalized to normalized to Imax of each oocyte (mean Imax = −30.2 ± 5.3 nA; AcCho, 3 mM). (Inset) As in A. (D) AcCho-current/voltage relation, fitted as in Fig. 1, from four oocytes injected as in C. Data normalized to AcCho currents (−40 ± 7 nA; 1 mM AcCho), at −120 mV. MLA and 5OHind were preapplied for 4 s in A and 20 s in C.
As expected, injection of GH(4)C1-α7 cell membranes into the oocytes could lead to the incorporation of α7 receptors; and, 24 h postinjection, AcCho (200 μM) generated inward currents with a mean of 22 ± 3 nA (range, 15 to 35 nA, n = 6), and which decayed unlike those of the native cells (T0.1 = 220 ± 25 ms; P < 0.005). In addition, the AcCho currents were again potentiated by 5OHind, and blocked by MLA (Fig. 2C Inset); and the dose/current relation gave EC50 and nH values of 206 μM and 1.2 that matched those obtained for the GH(4)C1-α7 cells (Fig. 2C). Furthermore, the I-V relation was linear at hyperpolarized membrane potentials, while it was bent at −30 to +30 mV (e.g., Fig. 2D), with a pattern similar to that of native GH(4)C1-α7 cells. Furthermore, the I-V relationships fitted to a Boltzmann equation showed similar slopes (γHEK = 9.2 × 10−3 mV−1; γoocytes = 9.4 × 10−3 mV−1).
α4β2 Nicotinic AcChoRs.
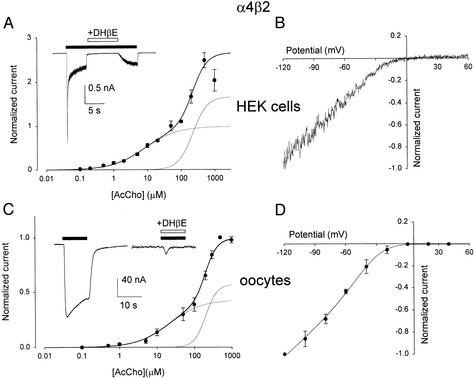
Application of AcCho (50 μM) to whole-cell patch-clamped HEK-α4β2 cells elicited inward currents, −536 ± 60 pA amplitude, that decayed with a T0.1 of 25 ± 3 ms (n = 18). The AcCho currents were inhibited by the competitive nicotinic blocker dihydro-β-erythoidine (DHβE; Fig. 3A Inset), and, as reported (19), the AcCho-dose/current-response curve was bimodal and was best fitted to the sum of two Hill equations (Fig. 3A). The AcCho current was nonlinear, rectifying strongly beyond −20 mV (Fig. 3B).
Figure 3.
AcCho currents of HEK-α4β2 cells compared with those of oocytes injected with HEK-α4β2 cell membranes. (A) Bimodal AcCho-dose/current relation (n = 6 for each point), best fitted to the sum of two Hill equations (superimposed lines) with the following best fitting parameters: nH = 0.90, EC50 = 8.3 μM; and nH = 2.0, EC50 = 221 μM. Data normalized to the Imax of each cell (mean peak current −536 ± 60 pA; n = 18; AcCho, 50 μM). (Inset) Current induced by AcCho (30 μM, horizontal filled bar) and reversibly blocked by DHβE (5 μM). (B) Current-voltage relation. Potential ramp, −120 mV to +60 mV, on the steady-state current induced by AcCho (50 μM). Data normalized to the current at −120 mV (−218 ± 52 pA; four cells). (C) AcCho-dose/current response relation from six oocytes injected with HEK-α4β2 cell membranes. Peak currents, normalized to Imax of each oocyte (mean Imax −405 ± 7 nA; AcCho, 500 μM) and best-fitted with a sum of two Hill equations (superimposed lines), gave: nH = 1 and EC50 = 21.4 μM; nH = 2.8 and EC50 = 200 μM, respectively. (Inset) As in A. (D) Current-voltage relation, fitted as in Fig. 2, from four oocytes injected as in C. Data normalized to currents (−331 ± 107 nA) elicited by AcCho (50 μM) at −120 mV.
Oocytes injected with HEK-α4β2 cell membranes rapidly acquired AcChoRs that gated inward membrane currents of variable amplitude. Specifically, 24 h after membrane injection, AcCho at 50 μM elicited currents that ranged from −42 and −220 nA (Fig. 3C Inset) and had a mean of −116 ± 27 nA (n = 9). The currents decayed with a T0.1 of 2.6 ± 0.65 s and, as illustrated in Fig. 3C Inset, DHβE again blocked the AcCho currents. The AcCho-dose/current relation of the oocytes was again bimodal (Fig. 3C), and the EC50 and nH values resembled those of the donor HEK-α4β2 cells (see also refs. 19 and 20). Furthermore, the AcCho currents increased with the membrane potential in the range −120 to −20 mV and showed a considerable rectification similarly to the currents elicited in the HEK-α4β2 (e.g., Fig. 3D). The I-V relationships fitted to the Boltzmann equation again showed similar slopes (γHEK = 7.6 × 10−3 mV−1; γoocytes = 8.4 × 10−3 mV−1).
Discussion
In this work, we report an efficient method to easily study transmitter receptors originally assembled in cultured cell lines and then microtransplanted to a sturdy and convenient host cell system, the Xenopus oocyte. This method has been recently used to transplant assembled transmitter receptors from human brain to Xenopus oocyte (7), following a method developed a few years ago to microtransplant AcChoRs and chloride channels from the electric organ of Torpedo to the Xenopus oocyte membrane (5, 6). Here, we injected membrane vesicles prepared from cultured cell lines, and this approach led to a rapid incorporation of neurotransmitter receptors in the oocyte plasma membrane. In this way, functional AMPA-type GluR1, α7-AcChoRs, and α4β2-AcChoRs from cultured cells were microtransplanted to the oocytes, and their respective transmitter-activated currents were analyzed.
We report here that the gross electrophysiological and pharmacological characteristics of the transmitter-gated receptors constitutively assembled in the cultured cells are, for the most part, retained after transplantation to the oocytes. Specifically, we found that (i) the receptor apparent transmitter affinity, (ii) the transmitter binding site cooperativity, (iii) the receptor sensitivity to the antagonists, and (iv) the I-V pattern observed in the native donor cells are substantially equivalent to those detected from membrane-injected oocytes, with the notable exception of the kinetic behavior of the current responses: α4β2- and α7-AcCho currents desensitized more rapidly when the receptors were in the native cells than when they were transplanted to the oocytes whereas GluR1-AMPA currents decayed faster in oocytes vs. HEK cells. The reasons for these differences are not known, and several possibilities need to be examined. To mention just two, it may be that the α4β2 receptors internalize after AcChoR activation due to internal signaling (21) and this process may be usually more marked in the cells than in the oocytes. Another possibility is raised by the fact that the intracellular medium and the cellular signaling machinery are different in the amphibian oocytes (22) vs. the mammalian cells (23). Whatever the correct explanation for the different rates of desensitization turns out to be, our results already show that these factors are not relevant to many other receptor properties and point to the molecular structure of the receptor as the main determining factor.
Finally, we also further report that the α7-AcChoRs are positively modulated by the allosteric modulator 5OHind, both in the donor cells and after receptor transplantation to the oocytes. Therefore, our approach may be useful to investigate also membrane receptor modulation due to the action of allosteric effectors and possible intracellular cascade signals, thus addressing many questions related to biochemical processes linking the cell membrane and its receptors to the whole cell.
A large body of evidence indicates that the functional properties of transmitter-gated receptors may be influenced by the host heterologous cell expression system (24, 25); but the roles played by the membrane, the intracellular ionic composition, the vast array of intracellular cell proteins, etc. are still not clear. One outcome of the present work is that our approach to microtransplant proteins, with their natural structure and still embedded in their natural lipid membrane, from donor cells to the host oocyte opens the way to many pathophysiological investigations.
Acknowledgments
We thank Drs. Francesca Grassi and Fabio Ruzzier for critical reading of the manuscript and Gregorio Torchia for help. This work had the financial support of Ministero Istruzione Università Ricerca (to F.E.) and the National Science Foundation (to R.M.).
Abbreviations
- T0.1
the time for the current to decay by 10%
- nH
Hill coefficient
- AcCho
acetylcholine
- AcChoR
AcCho receptor
- GluR1
glutamate receptor 1
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CNQX
cyano-7-nitroquinoxaline-2,3-dione
- MLA
methyllycaconitine
- 5OHind
5-hydroxyindole
- DHβE
dihydro-β-erythoidine
References
- 1.Barnard E A, Miledi R, Sumikawa K. Proc R Soc London Ser B Biol Sci. 1982;215:241–246. doi: 10.1098/rspb.1982.0040. [DOI] [PubMed] [Google Scholar]
- 2.Gundersen C B, Miledi R, Parker I. Nature. 1984;308:421–424. doi: 10.1038/308421a0. [DOI] [PubMed] [Google Scholar]
- 3.Miledi R, Parker I, Sumikawa K. In: Fidia Award Lecture Series. Smith J, editor. Vol. 3. New York: Raven; 1989. pp. 57–90. [Google Scholar]
- 4.Valera S, Hussy N, Evans R J, Adami N, North R A, Suprenant A, Buell G. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- 5.Marsal J, Tigyi G, Miledi R. Proc Natl Acad Sci USA. 1995;92:5224–5228. doi: 10.1073/pnas.92.11.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morales A, Aleu J, Ivorra I, Ferragut J A, Gonzalez-Ros J M, Miledi R. Proc Natl Acad Sci USA. 1995;92:8468–8472. doi: 10.1073/pnas.92.18.8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miledi R, Eusebi F, Martinez-Torres A, Palma E, Trettel F. Proc Natl Acad Sci USA. 2002;99:13238–13242. doi: 10.1073/pnas.192445299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virginio C, Giacometti A, Aldegheri L, Rimland J M, Terstappen G C. Eur J Pharmacol. 2002;445:153–161. doi: 10.1016/s0014-2999(02)01750-8. [DOI] [PubMed] [Google Scholar]
- 9.Palma E, Mileo A M, Eusebi F, Miledi R. Proc Natl Acad Sci USA. 1996;93:11231–11235. doi: 10.1073/pnas.93.20.11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lax P, Limatola C, Fucile S, Trettel F, Di Bartolomeo S, Renzi M, Ragozzino D, Eusebi F. J Neuroimmunol. 2002;129:66–73. doi: 10.1016/s0165-5728(02)00178-9. [DOI] [PubMed] [Google Scholar]
- 11.Patneau D K, Vyklicky L, Jr, Mayer M L. J Neurosci. 1993;13:3496–3509. doi: 10.1523/JNEUROSCI.13-08-03496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fucile S, Palma E, Mileo A M, Eusebi F, Miledi R. Proc Natl Acad Sci USA. 2000;97:3643–3648. doi: 10.1073/pnas.050582497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miledi R. Proc R Soc London Ser B Biol Sci. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- 14.Derkach V, Barria A, Soderling T R. Proc Natl Acad Sci USA. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palma E, Esposito V, Mileo A M, Di Gennaro G, Quarato P, Giangaspero F, Scoppetta C, Onorati P, Trettel F, Miledi R, Eusebi F. Proc Natl Acad Sci USA. 2002;99:15078–15083. doi: 10.1073/pnas.232574499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahl P, Madsen U, Banke T, Krogsgaard-Larsen P, Schousboe A. Eur J Pharmacol. 1996;308:211–218. doi: 10.1016/0014-2999(96)00292-0. [DOI] [PubMed] [Google Scholar]
- 17.Zwart R, De Filippi G, Broad L, McPhie G, Pearson K, Baldwinson T, Sher E. Neuropharmacology. 2002;43:374–384. doi: 10.1016/s0028-3908(02)00094-1. [DOI] [PubMed] [Google Scholar]
- 18.Gopalakrishnan M, Buisson B, Touma E, Giordano T, Campbell J E, Hu I C, Donnelly-Roberts D, Arneric S P, Bertrand D, Sullivan J P. Eur J Pharmacol. 1995;290:237–246. doi: 10.1016/0922-4106(95)00083-6. [DOI] [PubMed] [Google Scholar]
- 19.Buisson B, Bertrand D. J Neurosci. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chavez-Noriega L E, Gillespie A, Stauderman K A, Crona H J, Claeps B O N, Elliot K J, Reid R T, Rao T S, Velicelebi G, Harpold M M, et al. Neuropharmacology. 2000;39:2543–2560. doi: 10.1016/s0028-3908(00)00134-9. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharyya S, Puri S, Miledi R, Panicker M M. Proc Natl Acad Sci USA. 2002;99:14470–14475. doi: 10.1073/pnas.212517999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusano K, Miledi R, Stinnakre J. J Physiol (London) 1982;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burton R F. Comp Biochem Physiol B. 1983;76:663–671. doi: 10.1016/0305-0491(83)90375-9. [DOI] [PubMed] [Google Scholar]
- 24.Lewis T M, Harkness P C, Sivilotti L G, Colquhoun D, Millar N S. J Physiol. 1997;505:299–306. doi: 10.1111/j.1469-7793.1997.299bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fucile S, Barabino B, Palma E, Grassi F, Limatola C, Mileo A M, Alemà S, Ballivet M, Eusebi F. NeuroReport. 1997;8:2433–2436. doi: 10.1097/00001756-199707280-00005. [DOI] [PubMed] [Google Scholar]