Abstract
Objective
To analyze the results of a prospective study of 176 patients with Zollinger-Ellison syndrome (ZES) (138 sporadic, 38 MEN1) undergoing 207 operations over a 17-year period.
Summary Background Data
The existence of lymph node (LN) primary gastrinoma causing ZES is controversial.
Methods
Three groups of patients were compared: LN only resected, cured, and no relapse (likely LN primary); same criteria but relapse (unlikely LN primary); and duodenal primary and LN metastases (Duo-LN).
Results
Forty-five (26%) had only LN(s) as the initial tumor found. Twenty-six of the 45 (58%) fit the definition of a likely LN primary because they were apparently cured postresection. At 10.4 ± 1.2 years, 69% of the 26 patients with likely LN primary tumors have remained cured and have LN primaries. In the 8 of 26 with recurrent ZES, it occurred at 5 ± 1 years, and 3 had duodenal gastrinoma that had been missed. Ten percent (13/138) of all patients with sporadic ZES and 0% (0/38) with ZES and MEN1 remained cured with only a LN tumor removed. In patients with sporadic gastrinomas no clinical, laboratory, or radiographic localization feature differed among patients with likely LN primary (n = 16) and those with unlikely LN primary (n = 6) or those with Duo-LN (n = 37). In the likely LN primary group, the largest LN was 2.2 ± 0.2 cm, the number of LNs removed was 1.3 ± 0.1 (25% ≥1 LN), and 78% were in the gastrinoma triangle, which also did not differ from the other 2 groups. Disease-free survival was similar in the likely LN primary group, patients with Duo-LN, and those with pancreatic primaries.
Conclusions
These results support the conclusion that primary LN gastrinomas occur and are not rare (approximately 10% of sporadic cases). These results suggest that a proportion (25%) of these tumors are either multiple or malignant. Because no clinical, laboratory, or tumoral characteristic distinguishes patients with LN primary tumors, all patients with ZES undergoing surgery should have an extensive exploration to exclude duodenal or pancreatic tumors and routine removal of lymph nodes in the gastrinoma triangle.
The existence of primary lymph node (LN) gastrinomas is controversial. 1–9 Even though a literature review 1 in 1994 identified 63 cases of apparent lymph node primary gastrinoma, their existence and, if they occur, their characteristics remain unclear. This has occurred because most reports are case studies, follow-up is limited, and surveillance of disease activity postresection is often not systematically done. Furthermore, recent studies show that a secretin provocative test is essential to rule out recurrence postresection, especially in patients with normal fasting serum levels of gastrin, 10,11 and this was frequently not done. Therefore, continued disease-free status after only removal of an LN gastrinoma, a criterion for diagnosing primary LN tumors, was often not fulfilled. 12
To address the issue of the possible existence of lymph node primary gastrinoma and their characteristics, we analyzed our results in a large series of patients with possible LN primaries who were found in our prospective study of patients with Zollinger-Ellison syndrome (ZES) undergoing surgery for potential cure.
METHODS
Since 1981 at the National Institutes of Health and 1997 at the University of California San Francisco, all patients with ZES who underwent surgical exploration for possible cure were eligible for this study. As of May 2002, 176 patients underwent surgical exploration and were included in this protocol.
The diagnosis of ZES was based on acid secretory studies, measurement of fasting serum level of gastrin, as well as the results of secretin and calcium provocative tests. 10 Criteria for MEN1 in a patient with ZES have been described elsewhere. 13,14 Basal acid output was determined for each patient using methods described previously. 15 Doses of oral gastric antisecretory drug were determined as described previously. 16
Time from onset of the disease to exploration was determined for all patients as described previously. 10,17 Time of diagnosis of MEN1 or ZES was the time the diagnosis was first established by appropriate laboratory studies or when a physician established the diagnosis based on clinical presentation. 17,18
Specific Protocol
To be eligible for this study, all patients referred with a diagnosis of possible ZES underwent an evaluation to establish the diagnosis of ZES 10,18 and studies to determine the suitability of surgical exploration for cure. 14,19 These latter studies included tumor localization studies, studies to determine the presence or absence of MEN1 13,14 (because only patients with MEN1 and ZES with tumors ≥ 2.5 cm underwent surgical exploration 14), and studies to determine the presence of other disease that might make surgery contraindicated.
Of the 176 patients undergoing surgical exploration for possible cure, patients with a possible LN primary gastrinoma were analyzed in detail in this study. A possible LN primary was as defined previously 1,19 as occurring in a patient whose only extrahepatic tumor resected was in LNs and who was disease-free postresection. Disease-free (cure) was defined as a normal fasting serum gastrin level, negative secretin test, and no tumor on imaging studies. 10,19,20 Patients underwent evaluation for disease-free status immediately postoperatively (i.e., 2 weeks postresection), within 3 to 6 months postresection, and then yearly. 10,19,20 Yearly evaluations included conventional imaging studies (CT, ultrasound, MRI, and angiography, if necessary); somatostatin receptor scintigraphy (SRS) since 1994; assessment of disease status (acid secretory studies, fasting gastrin determinations x 3, secretin provocative test); and assessment of endocrine status (parathyroid, pituitary, adrenal function). 10,19,20
Patients with possible LN primaries were stratified into two groups depending on the results of the long-term follow-up studies: group 1 (likely LN primary), who remained disease-free with no relapses; and group 2 (unlikely LN node primary) who relapsed during follow-up. Relapse was defined as described previously 10,19 as occurring if a patient was initially disease-free postresection and then became not disease-free by developing an elevated fasting serum gastrin level, positive secretin test, or positive imaging result on a follow-up visit. 10,19 Results from the two groups of patients with possible lymph node primaries were compared to patients with duodenal gastrinomas with LN metastases (Duo-LN), a group that they could easily be mistaken for.
The localization and the extent of pancreatic endocrine tumors were evaluated in all patients as described elsewhere 21–23 by using upper gastrointestinal endoscopy and conventional imaging studies (CT scan, MRI, transabdominal ultrasound, selective abdominal angiography, and bone scanning). Somatostatin receptor scintigraphy was performed since 1994 using [111In-DTPA-DPhe1]-octreotide (6 mCi) with whole-body, planar, and SPECT views, as described previously. 24,25 At exploration, an extensive search for endocrine tumors was performed. 19,20,22,26 Briefly, palpation and intraoperative ultrasound with a 10-MHz real-time transducer was performed 19 after an extended Kocher maneuver. Since 1987, endoscopic transillumination of the duodenum was performed 27 and a 3-cm longitudinal duodenotomy was centered in the anterolateral surface of the descending (second) duodenum to search for duodenal tumors. 19
Tumors in the pancreatic head were enucleated. Tumors in the pancreatic body and tail were enucleated if possible; otherwise, they were resected. Distal pancreatectomy was not routinely performed and was done only if multiple pancreatic body and/or tail tumors were present that could not be enucleated. 19,28 If multiple pancreatic head tumors were present that could not be enucleated, a pancreaticoduodenectomy was performed if the patient had given prior consent. A detailed inspection for peripancreatic, periduodenal, or portohepatic LNs was carried out, and these were routinely removed. If liver metastases were present and localized, they were wedge-resected with a 1-cm margin, if possible; if this was not possible and they were localized, a segmental resection or lobectomy was performed. 29
Statistics
The Fisher exact test, the Student t test, the Mann-Whitney test, and analysis of variance were used. For a post hoc test, the Bonferroni/Dunn test was used. Values differing by P < .05 were considered significant. All continuous variables were reported as mean ± standard error of the mean. The probabilities of survival were calculated and plotted according to the Kaplan-Meier method. 30
RESULTS
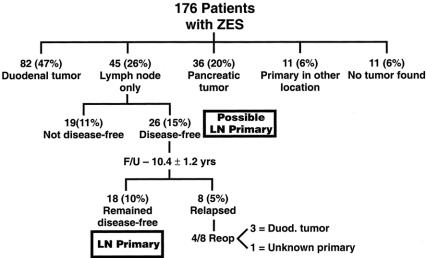
One hundred seventy-six patients with ZES underwent attempted curative resection during the 21-year study period; of these, 138 patients (78%) did not have MEN1 and 38 patients (22%) had MEN1. The 176 patients underwent 207 exploratory laparotomies; 149 patients had 1 exploration, 25 patients had 2, and 2 patients had 3 and 5 laparotomies, respectively. Forty-five patients (26%) had only an LN gastrinoma as the only extrahepatic tumor initially resected and were candidates for having an LN primary gastrinoma. This compared to 82 patients (47%) having a duodenal primary, 36 patients (20%) with a pancreatic primary, 11 patients (6%) with primaries in a nonduodenal-pancreatic-LN location, and 11 patients (6%) with no tumor found (Fig. 1).
Figure 1. Distribution of patients with ZES dependent on surgical results. Of the 176 patients with ZES undergoing exploratory laparotomy, 45 had an LN only removed, and 26 of the 45 patients (15%) were disease-free postresection and had a possible LN primary. During follow-up with yearly assessment, 18 remained cured and had LN primaries. Of the eight patients with relapses, four were reoperated, with three having a duodenal primary found that was missed at the first operation. The sites of the 11 primaries in other locations was pyloric canal (n = 1), liver (n = 4), biliary tract (n = 2), jejunum (n = 1), ovary (n = 1), heart (n = 1), and lung (n = 1).
Of the 45 patients with an LN gastrinoma as the only extrahepatic tumor resected, 58% (26/45) had a possible LN primary tumor. They were disease-free postresection of only an LN tumor with a negative secretin test, normal fasting gastrin level, and no tumor on imaging studies. 1,19 The other 19 patients with LNs only resected at surgery were not disease-free, suggesting that a primary tumor was likely missed. This conclusion was supported by the findings at a second operation (1.5–9 years later) in 6 of these 19 patients, in which 5 patients had a duodenal gastrinoma found, and 1 a biliary tract primary gastrinoma.
In the 26 patients with a possible LN primary, each was assessed yearly postresection for disease-free status. During a mean follow-up of 10.9 ± 1.2 years (range 1.2–19.9 years), 18 of the patients remained disease-free and are considered to have an LN primary tumor. All of these patients have been disease-free for at least 1 year postresection, 94% for at least 2 years, 72% for at least 5 years, 56% for at least 10 years, and 22% for at least 15 years (Fig. 2). In the eight patients with LNs only resected who were disease-free postresection and then developed a recurrence, the recurrences were detected a mean of 5.4 ± 0.9 years postresection (range 2–10 years). Four of the eight patients with recurrences (patients 21, 23, 24, 26) had a second exploration when tumor localization studies became positive. 31 In three of these patients a duodenal gastrinoma was found and in the fourth patient, with MEN1, a pancreatic tail tumor was found but the gastrin staining was negative, showing the gastrinoma primary was still unknown.

Figure 2. Duration of follow-up after resection of a possible LN primary tumor. The 26 patients disease-free after resection of an LN tumor only were evaluated yearly and assessed for disease activity. Shown is the time from resection to the present for the 18 patients who remained disease-free and the time to recurrence for the 8 patients who developed a recurrence (increased fasting gastrin, positive secretin test or imaging studies). 10 The location of primary tumor found in the four patients with recurrence who were reoperated is listed.
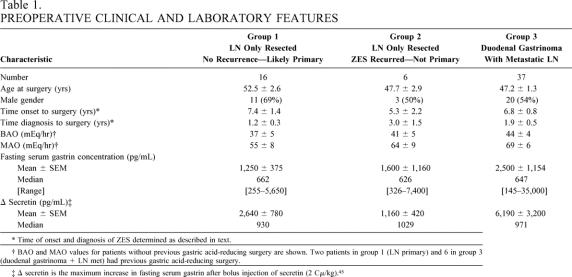
To determine whether any laboratory or clinical feature had predictive value in identifying patients with LN primaries, the characteristics of three groups of patients with sporadic ZES (without MEN1) were compared (Tables 1–3). Specifically, patients with a likely LN primary without relapse (n = 16) (group 1), unlikely LN primary with relapse (n = 6) (group 2), or with a duodenal primary with metastatic LNs (n = 37) (group 3), which could mimic an LN primary if the duodenal tumor had been missed, were compared. There were no significant differences among the three groups in age at surgery, gender distribution, disease duration from onset or diagnosis before surgery, basal or maximal acid output, fasting serum levels of gastrin, or the magnitude of the increase in fasting gastrin postsecretin (i.e., delta secretin).
Table 1. PREOPERATIVE CLINICAL AND LABORATORY FEATURES
* Time of onset and diagnosis of ZES determined as described in text.
† BAO and MAO values for patients without previous gastric acid-reducing surgery are shown. Two patients in group 1 (LN primary) and 6 in group 3 (duodenal gastrinoma + LN met) had previous gastric acid-reducing surgery.
‡ Δ secretin is the maximum increase in fasting serum gastrin after bolus injection of secretin (2 Cμ/kg). 45
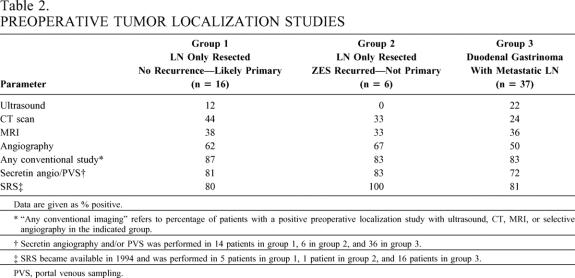
Table 2. PREOPERATIVE TUMOR LOCALIZATION STUDIES
Data are given as % positive.
* “Any conventional imaging” refers to percentage of patients with a positive preoperative localization study with ultrasound, CT, MRI, or selective angiography in the indicated group.
† Secretin angiography and/or PVS was performed in 14 patients in group 1, 6 in group 2, and 36 in group 3.
‡ SRS became available in 1994 and was performed in 5 patients in group 1, 1 patient in group 2, and 16 patients in group 3.
PVS, portal venous sampling.
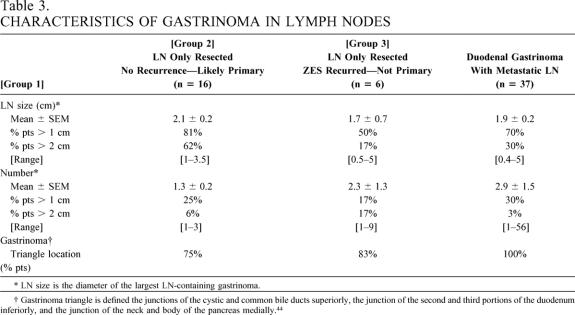
Table 3. CHARACTERISTICS OF GASTRINOMA IN LYMPH NODES
* LN size is the diameter of the largest LN-containing gastrinoma.
† Gastrinoma triangle is defined the junctions of the cystic and common bile ducts superiorly, the junction of the second and third portions of the duodenum inferiorly, and the junction of the neck and body of the pancreas medially. 44
Conventional preoperative tumor localization studies (ultrasound, CT, MRI, selective abdominal angiography) did not differ in sensitivity for localizing a tumor preoperatively in patients with LN primaries (group 1), without LN primary (group 2), or with a duodenal primary with a metastatic LN (group 3). In general for each, the relative sensitivities were angiography > MRI, CT > ultrasound. Localization results of functional tumor localization by measuring gastrin gradients, either after secretin injection 32 or by portal venous sampling, 33 and tumor images by SRS, did not differ in the three groups. Each of these localization modalities was more sensitive than the other conventional imaging studies.
There were no characteristics of the primary LN gastrinoma (group 1) that distinguished it from patients without an LN primary tumor (groups 2 and 3) who had metastatic gastrinoma in LNs either from an unknown primary (group 2) or a duodenal primary (group 3). Specifically, in terms of the size of the largest LN tumor, percentage with tumors larger than 1 cm or larger than 2 cm, number of LN tumors, or percentage with more than 1 LN tumor or more than 2 LN tumors, patients with an LN only resected who remained disease-free (LN primary, group 1) did not differ from those without a LN primary, including those who relapsed (group 2) or those with a duodenal primary with a metastatic LN (group 3). Twenty-five percent of patients with an LN node primary had multiple LNs resected (range 1–3), and in 75% the LNs were in the gastrinoma triangle; these results are not significantly different from the two nonprimary LN groups (groups 2 and 3).
The effect of LN size and number containing tumor, as well as tumor location, on disease-free survival was investigated using Kaplan-Meier analysis (Fig. 3). Whether only one LN was found or more than one LN and the patient was disease-free immediately postresection had no significant effect on the long-term disease-free survival rate (P = .32). Whether the largest LN was less than 2 cm in diameter or more than 2 cm also did not have a significant effect on long-term disease-free survival (P = .095). Lastly, the disease-free survival for the 26 patients with a possible LN primary who were disease-free immediately postresection did not differ from patients with a duodenal primary gastrinoma with LN metastases or with a pancreatic primary only, who were disease-free postresection (Table 4).
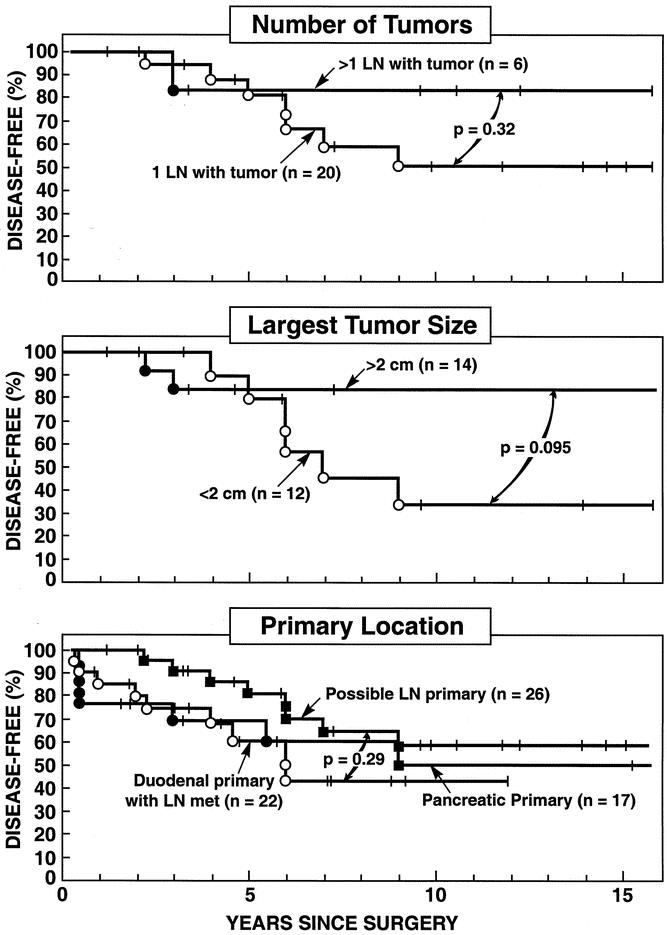
Figure 3. Disease-free survival for patients with or without an LN primary. Data are plotted in the form of Kaplan-Meier for patients with a possible LN primary (all three panels), duodenal primary with LN metastases (lower panel), or with a pancreatic primary (lower panel).
Table 4. DISEASE-FREE SURVIVAL
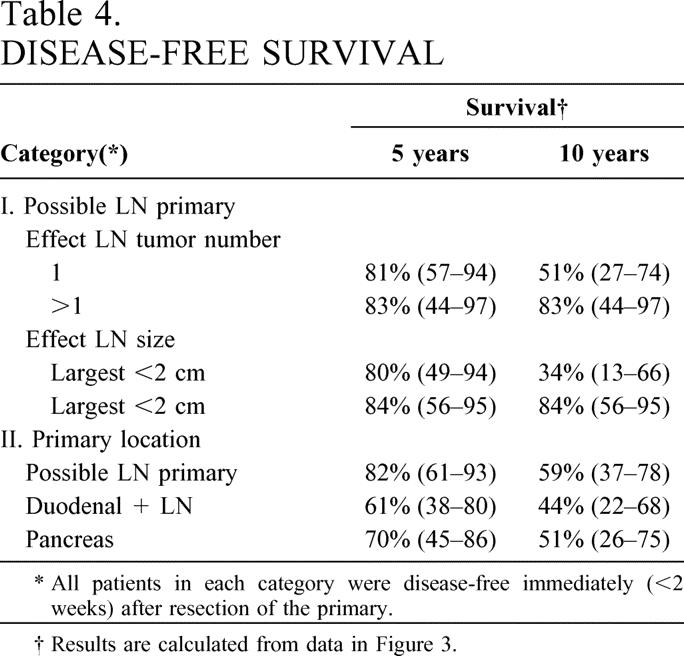
* All patients in each category were disease-free immediately (<2 weeks) after resection of the primary.
† Results are calculated from data in Figure 3.
DISCUSSION
The existence of LN gastrinomas remains controversial. 1,3,5–9 If they occur, almost nothing is known about their frequency, natural history, rate of long-term curability postresection, or preoperative and/or operative factors that predict their occurrence and help the surgeon identify them.
The purpose of the present study was to provide information on each of these issues by analyzing these factors in a group of patients with likely primary LN gastrinomas identified from a large prospective study of patients with ZES. 14,18,34 While our results do not prove the existence of primary LN gastrinomas, a number of findings certainly provide strong support for their existence. The most important finding is the identification of a group of patients who had only LN gastrinomas resected and remained disease-free for up to 20 years. Almost three quarters of this group were disease-free for more than 5 years, 56% for more than 10 years, and 22% for more than 15 years. In a number of the case reports in the literature 1,3 of possible LN primaries, the question of whether long-term cure actually occurred could be raised either because postoperative follow-up was relatively short or because rigorous studies to establish disease-free status were not performed. A recent study 10 demonstrates it is essential to perform both fasting gastrin determinations after stopping antisecretory drugs as well as secretin provocative testing to unequivocally establish disease activity. Furthermore, one study, 35 but not another, 10 suggests serial imaging is also essential to establish disease-free status in patients postresection of a gastrinoma. The present study had none of these limitations. Patients were followed yearly and long-term (up to 20 years), and at each evaluation serial fasting gastrin levels, secretin provocative testing, and multiple imaging studies (ultrasound, CT, MRI, and since 1994 SRS) were performed. One could argue that a microscopic primary tumor in an unknown location could still exist, and we have no data that can completely refute this. However, long-term disease-free survival greater than 10 years with negative yearly assessments by both a sensitive tumor marker for gastrinoma (i.e., gastrin) as well as a negative provocative test (i.e., secretin) argue for the likelihood that the LN gastrinoma was the primary. This conclusion is further supported by the fact that the recurrence rate after identification of patients with a possible LN primary (disease-free immediately postresection) was not significantly different from that of patients with pancreatic primaries or duodenal gastrinomas with LN metastases.
Our data, suggesting LN primaries occur, are consistent with results of two recent pathology studies reporting that chromogranin or synaptophysin-positive cells can be found in abdominal LNs of patients without gastrinomas. 2,4 In one of these studies 2 20% of the LNs in the gastrinoma triangle contained synaptophysin-positive cells and 15% contained gastrin-containing cells in autopsy cases without ZES, whereas none of the LNs from the axilla or inguinal area contained positive staining. The histologic studies provide support for the hypothesis that entrapment of neuroendocrine cells 36 during development could lead to the development of the primary LN gastrinomas we report in the present study.
Our results support the conclusion that primary LN gastrinoma is not rare, as it occurred in 10% of our patients. This frequency makes these gastrinomas approximately one-fifth as frequent as duodenal gastrinomas, half as frequent as pancreatic gastrinomas, and 1.5-times more frequent than primary gastrinomas in nonduodenal-pancreatic or LN sites such as in the biliary tract, liver, heart, lung, ovary, gastric antrum, or omentum. 34,37 These results support the conclusion that it is important for the surgeon to be aware of the occurrence of primary LN gastrinomas and supports the practice of routinely removing LNs at surgical resection in patients with ZES. 38
Our study demonstrates that approximately one third (8/26) of patients identified immediately postoperatively as fulfilling the criteria for having a possible primary LN gastrinoma 1 in fact do not have such a primary with careful follow-up. Each of these eight patients had a recurrence of disease activity manifested by an elevated fasting serum gastrin concentration while off all antisecretory drugs, a positive secretin test, or positive tumor imaging. This conclusion was supported by the surgical findings at a second laparotomy in three of the patients who were found to have a small duodenal gastrinoma that was likely missed at the initial surgery.
Unfortunately, our analysis revealed that no preoperative clinical parameter (age, disease duration, gender) or laboratory result (fasting serum gastrin level, basal or maximal acid output level, or an increase in fasting gastrin level postsecretin) distinguished patients with or without primary LN gastrinomas. Specifically, none of the preoperative clinical or laboratory parameters distinguished patients with an LN only resected who were disease-free immediately postresection, who continued to remain disease-free and thus likely had a primary LN tumor, from those who had recurrence and had an occult primary in another location. Furthermore, similar results were found with a comparison to patients with duodenal gastrinomas with LN metastases. This comparison was made because duodenal gastrinomas are small (frequently <5 mm) and missed at exploration, whereas the accompanying LNs are frequently larger and more easily found. 7,8,20,39–41 Therefore, a missed duodenal primary with detection of the LN is mostly likely to mimic an LN primary. In fact, three of our patients with possible LN primaries who relapsed had duodenal gastrinomas eventually found.
Similar to clinical and laboratory features, the results of preoperative imaging studies (conventional [ultrasound, CT, MRI, angiography], functional by measuring hormone gradients either by portal venous gastrin sampling or after secretin injection, or using somatostatin receptor scintigraphy with 111In-pentetreotide) did not distinguish LN primaries from patients with LN only tumors with occult other primaries or with duodenal tumors. It might have been hoped that SRS, the most sensitive imaging modality available to localize gastrinomas or their metastases, 23,24,42,43 might have distinguished patients with an LN primary or another single primary gastrinoma from patients with LN metastases and either a latent primary or a duodenal primary. However, our results are consistent with those of a recent study 23 that demonstrated SRS frequently (∼50%) misses primary duodenal gastrinomas less than 1 cm in diameter and therefore may only localize the accompanying positive LN.
Further, no characteristic of the LN-containing tumor also allowed a primary LN tumor to be distinguished from a metastatic LN. Specifically, neither LN size nor number had predictive value. Furthermore, LN location did not help in distinguishing a possible LN primary because they were found in the gastrinoma triangle 44 in more than 80% of the cases, whether they were a primary LN tumor, LN tumor associated with a occult primary, or associated with a duodenal primary. Therefore, there was no distinguishing feature of the LN at surgery that allowed the surgeon to suspect that it might be a primary LN tumor or a metastasis. Furthermore, in 25% of the cases of primary LN tumors, there was more than one LN involved. This suggests LN primary gastrinomas may occasionally be malignant or multiple. Therefore, when more than one LN is found to be positive for tumor, it should not necessarily be assumed that a primary is being missed and more aggressive resection is indicated. This finding further complicates the decision on when a proximal pancreaticoduodenotomy should be performed in a given patient. 19,34
In conclusion, this study provides support for the conclusion that primary LN gastrinomas exist and are not rare. Because no clinical, laboratory, or tumoral characteristic distinguishes these tumors, all patients with ZES undergoing surgical resection for possible cure should have an extensive exploration to exclude duodenal, pancreatic, or extrapancreatic tumor and have routine removal of LNs found, especially those in the gastrinoma triangle. 44,45
Discussion
Dr. James C. Thompson (Galveston, TX): One of the major problems facing people who take care of patients with rare diseases is achieving sufficient clinical experience needed in order to develop clinical wisdom. A confounding problem in this disease is that when it was first discovered only virulent cases, late in the clinical course, were recognized.
Drs. Jensen and Norton and Fraker and their colleagues at the NIH have done us all a great service in amassing large numbers of patients with the Zollinger-Ellison syndrome, collating their vast clinical data, and following them for long periods, noting carefully the patterns of response to different therapeutic regimens, and periodically publishing their findings, as we see today. Only through their meticulous and painstaking efforts have we begun to learn the natural history of the Zollinger-Ellison syndrome, and we are in their debt. As to the present fine contribution, I would say, first of all, that the authors can with full confidence remove the word “possible” from their title.
The first report of primary lymph node gastrinoma was, I believe, from McGuigan’s group in Gainesville, published in The New England Journal of Medicine, if my memory serves, in the late 1970s, three patients whose symptoms abated with simple lymph node removal.
When that paper came out, we had had similar experiences. All in all, we have had three cases out of 34 patients with the Zollinger-Ellison syndrome who had their tumors limited to lymph nodes. All of them had at least two lymph nodes involved. We reported that, I believe, in the SG&O.
The salient contribution here is that Dr. Norton and colleagues followed their patients for 1.5 to nearly 20 years and found cures to persist in 70% of those resected for cure. This, I believe, goes as far as you can go with a clinical syndrome in proving Koch’s postulates.
I am surprised that they found no pattern of disease to be different in those patients cured when their lymph nodes were removed versus those patients who were not cured. And I have to ask about this lack of difference. Was there truly no difference in tumor size? Have you tested the tumors for ploidy or other DNA characteristics? Have you tested the tumors for angiogenic factors? Have you tested them for genetic patterns of tumor enhancement or tumor suppression, especially those genetic programs that either enhance or flummox apoptosis?
Dr. Leslie H. Blumgart (New York, NY): Thank you very much, Dr. Norton, for a very interesting and fascinating contribution. I just wanted to ask one question. I noticed on one of your slides that you have 6% of patients in whom the primary occurred in a site other than pancreas, duodenum, or lymph nodes. Were any of those in the liver? I ask this since we had a recent experience of one patient who appears to have had a primary gastrinoma occurring within the liver. Do you believe that primary neuroendocrine tumors can arise within the liver? Certainly, carcinoid tumors can arise within the bile ducts. Do you think they can arise primarily within the liver?
Dr. Murry F. Brennan (New York, NY): It is a great pleasure for me to be asked to discuss a manuscript by Jeff Norton, and particularly to have the opportunity to review the manuscript ahead of time. Dr. Norton followed myself at the National Cancer Institute in 1981, and he has truly made remarkable contributions, as Dr. Thompson mentioned, to the management of the Zollinger-Ellison syndrome. This current experience, a 17-year experience describing 176 Zollinger-Ellison syndrome patients, would have made Drs. Zollinger and Ellison impressed, a real El Dorado of opportunity. Dr. Norton asked the very challenging question of whether primary lymph node gastrinomas exist, and argued persuasively that they do. So it is a brave man that would question Dr. Thompson, Dr. Norton, and my good colleague Dr. Blumgart.
Rarely fearful of controversy, I would like to raise a few questions, Dr. Norton, to make the definitive conclusions perhaps more tenuous than you would like us to believe.
The premise that lymph node primaries exist is based on an examination of patients who had the lymph nodes only resected and do not relapse subsequently. There are certainly theoretical objections to the possibility of primary lymph node gastrinoma. For example, what are the cells of origin within the lymph node? Have we seen neuroendocrine cells in the lymph nodes? I know of one reference, although it has been suggested on one occasion also that Merkel cell cancer and neuroendocrine cutaneous malignancy can occur primarily in lymph nodes.
This is remarkably parallel to the long-standing debate on whether malignant melanoma can occur de novo in lymph nodes. Most people believe not, although the occasional pigmented cell has been found in lymph nodes.
The identification of metastatic melanoma and neuroendocrine tumors, without a known primary, is well established, and these patients following resection have a 50% 5-year survival!
The other problem Dr. Norton described well: what is recurrence? Recurrence is the absence of gastrin, the absence of a positive secretin test, and the absence of positive imaging. That is a reasonable way to evaluate the presence or absence of recurrence. But how long do they not have to occur?
Impressively, 72% of the patients were followed for more than 5 years. But I remind that you in a recent manuscript from the Mayo Clinic, 65% of the patients with node-positive islet cell carcinoma, a similar if not more aggressive population than the gastrinoma, were alive at 5 years!
Five-year survival in the endocrine tumors is certainly not evidence of cure. This would appear to be supported by the fact that patients that did not recur had actually larger metastatic lymph nodes, although it may not be significant, than those that recurred, suggesting an effect of removing tumor burden, thereby delaying inevitable recurrence.
How would Dr. Norton explain the patient who has more than one positive node? Do you mean, Dr. Norton, that primary gastrinoma can occur in more than one lymph node at one time? Or are you suggesting that the second lymph node is a metastasis from the first?
So we begin with 45 patients who have lymph nodes only removed; 26 are followed postoperatively, with negative evaluation; 18 remain disease-free; of these 18, 72% have been followed 5 years, or 12 patients. As I mentioned, 50% of node-positive melanoma are alive at the end of 5 years, and 65% of node-positive islet cell tumors are alive at 5 years. If the same was true, Dr. Norton, of primary gastrinoma, this would leave six patients possibly of primary origin.
But 25% had more than one lymph node positive; i.e., four of the patients would have to have had multifocal primary lymph node involvement—truly a surprising event? If you allow me to exclude those four, we have at best two beyond 5 years. And four of the total we know recurred after 5 years.
I believe the argument that primary lymph node gastrinoma exists becomes more tenuous. It is studies such as yours that will help resolve this issue. But I would suggest biologically that the high probability that these patients have an unknown primary would seem to be greater or as great. And I would with great temerity, given who discussed this paper before me, challenge the definitive conclusions of my very good friend and suggest that it is a conclusion as yet ostensible rather than real.
Dr. Dana K. Andersen (Worcester, MA): I would ask Dr. Norton to specifically comment on the subgroup of MEN1 patients with nodal but not metastatic disease. If I understand these data, no MEN1 patient with lymph node disease was cured in this series. Therefore, I would like Dr. Norton to address whether the presence of lymph node involvement, either by preoperative imaging or by intraoperative frozen-section analysis, is or should be a contraindication to attempted removal of the pancreatic primary in the MEN1 patient.
Dr. Stephen B. Vogel (Gainesville, FL): I enjoyed Dr. Norton’s presentation and I would like to ask several questions directly related to the concept of primary lymph node gastrinoma. In our series of Zollinger-Ellison patients, we have performed three “blind” Whipple resections to include the gastrinoma triangle, and in each of the three cases a small tumor was found the wall of the duodenum. In our most recent case, a young lady had two large lymph nodes demonstrated on octreotide scan. Prior endoscopic ultrasound showed no duodenal lesions, and we were unable to palpitate any lesion in the duodenum at the time of surgery. In view of her young age (30 years), we decided to resect the head of the pancreas, and the final pathology demonstrated a 4-mm duodenal wall tumor. This tumor was not demonstrated by octreotide scan.
My question is whether you would recommend a resection of the head of the pancreas in any of your subsets of patients, either where no primary is found in the head of pancreas or duodenum, or in the group whose serum gastrin does not return to normal in the postoperative period. An alternative to this approach is yearly follow-up with both CT examination and octreotide scan. Have any of the patients with possible primary lymph node gastrinomas shown up at a later date with primaries in either the duodenum or the head of the pancreas? Thank you.
Dr. Jeffrey A. Norton (San Francisco, CA): I would like to thank each of the discussants for their thoughtful and insightful comments.
In response to Dr. Thompson, I am encouraged by the fact that your data are very similar to ours in that about 10% of patients had disease-free survival with only lymph nodes removed and all of them had more than one lymph node. So I think that your observations support our data, adding credence to the possibility of lymph node primary gastrinoma.
We didn’t do any provocative or other experimental studies to try to better determine characteristics of the lymph node primary tumor. I think that that is also an important question. In the near future, we are performing genetic and molecular biologic studies to try to answer these questions, but we don’t have the data as yet.
In response to Dr. Blumgart, we have also seen liver primary gastrinoma. That is also controversial. We think that these tumors may be bile duct primary gastrinomas.
In response to Dr. Brennan, there are two papers in the literature where there is proof of endocrine cells in lymph nodes around the head of the pancreas. In one (references 2 and 4 in the accompanying paper) from the Mayo Clinic, they sampled lymph nodes from Whipple resections for diagnoses other than gastrinoma. They did meticulous immunohistochemical studies for gastrin and found some gastrin-positive cells in normal lymph nodes. There is a second more recent study in which they examined similar specimens and stained for chromogranin A and also neuron-specific enolase as markers for ectopic endocrine cells in normal lymph nodes. Both studies suggest that there are nests of endocrine cells within lymph nodes.
I think that the major difference between gastrinoma and melanoma is that in gastrinoma, we have a very sensitive marker of tumor recurrence. We have shown unequivocally that the secretin test to stimulate serum gastrin levels is a very sensitive marker of recurrent gastrinoma. If that test is not abnormal and only a lymph node gastrinoma has been removed, we feel very confident that a lymph node primary tumor exists. I think that lymph node primary tumors do metastasize to other lymph nodes. The only other possibility is that lymph node gastrinomas occur in multiple sites. Those are the two possible answers to the question why more than one lymph node is involved.
In response to Dr. Andersen, in the MEN1 patients with ZES, none of the patients were cured. We have demonstrated in other studies that nodal metastases do not affect survival and the cure rate with nodal metastases is the same as found without nodal metastases. So I think that the presence of nodal metastases is not a reason to do a major cancer operation like a Whipple procedure to resect a primary tumor, unlike other tumors that are more aggressive. We and others have shown that these tumors are fairly benign and patients live a long time with them.
That leads to Dr. Vogel’s comments. It is always a concern that we are missing duodenal primary gastrinomas. We have opened the duodenum and meticulously explored it in every single patient since 1987. Pipeleers-Marichal et al. reported in The New England Journal (1990; 322:723–727) about small primary duodenal gastrinomas that are 2 to 4 mm in diameter, just as Dr. Vogel described. So I agree that it is possible that we are missing some of these small tumors.
We haven’t advocated a Whipple resection for patients with minimal disease because our 10-year actual survival for patients in this study is 95%. Further, if we have either a lymph node tumor removed or a primary tumor with a lymph node metastasis, there is no change in the actual survival.
We think that the rate-limiting step on survival is liver metastases. If we don’t have liver metastases, we have no deaths from gastrinoma. So that is why we haven’t advocated a Whipple. But I share your concern. If we could do Whipple procedures safely with minimal long-term morbidity, it would be worth studying in these patients.
References
- 1.Arnold WS, Fraker DL, Alexander HR, et al. Apparent lymph node primary gastrinoma. Surgery. 1994; 116: 1123–1130. [PubMed] [Google Scholar]
- 2.Herrmann ME, Ciesla MC, Chejfec G, et al. Primary nodal gastrinomas: an immunohistochemical study in support of a theory. Arch Pathol Lab Med. 2000; 124: 832–835. [DOI] [PubMed] [Google Scholar]
- 3.Kitagawa M, Hayakawa T, Kondo T, et al. Gastrinoma in a mesenteric lymph node. Am J Gastroenterol. 1989; 84: 660–662. [PubMed] [Google Scholar]
- 4.Perrier ND, Batts KP, Thompson GB, et al. An immunohistochemical survey for neuroendocrine cells in regional pancreatic lymph nodes: a plausible explanation for primary nodal gastrinomas? Surgery. 1995; 118: 957–965. [DOI] [PubMed] [Google Scholar]
- 5.Bhagavan BS, Slavin RE, Goldberg J, Rao RN. Ectopic gastrinomas and the Zollinger-Ellison syndrome. Hum Pathol. 1986; 17: 584–592. [DOI] [PubMed] [Google Scholar]
- 6.Delcore R Jr, Cheung LY, Friesen SR. Outcome of lymph node involvement in patients with Zollinger- Ellison syndrome. Ann Surg. 1988; 206: 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard TJ, Zinner MJ, Stabile BE, et al. Gastrinoma excision for cure. A prospective analysis. Ann Surg. 1990; 211: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson NW, Vinik AI, Eckhauser FE, et al. Extrapancreatic gastrinomas. Surgery. 1985; 98: 1113–1120. [PubMed] [Google Scholar]
- 9.Friesen SR. Are aberrant nodal gastrinomas pathogenetically similar to lateral aberrant thyroid nodules? Surgery. 1990; 107: 236–238. [PubMed] [Google Scholar]
- 10.Fishbeyn VA, Norton JA, Benya RV, et al. Assessment and prediction of long-term cure in patients with Zollinger-Ellison syndrome: the best approach. Ann Intern Med. 1993; 119: 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolfe MM, Jain DK, Edgerton JR. Zollinger-Ellison syndrome associated with persistently normal fasting serum gastrin concentrations. Ann Intern Med. 1985; 103: 215–217. [DOI] [PubMed] [Google Scholar]
- 12.McGuigan JE, Wolfe MM. Secretin injection test in the diagnosis of gastrinoma. Gastroenterology. 1980; 79: 1324–1331. [PubMed] [Google Scholar]
- 13.Benya RV, Metz DC, Venzon DJ, et al. Zollinger-Ellison syndrome can be the initial endocrine manifestation in patients with multiple endocrine neoplasia-type 1. Am J Med. 1994; 97: 436–444. [DOI] [PubMed] [Google Scholar]
- 14.Norton JA, Alexander HR, Fraker DL, et al. Comparison of surgical results in patients with advanced and limited disease with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. Ann Surg. 2001; 234: 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy P, Venzon DJ, Feigenbaum KM, et al. Gastric secretion in Zollinger-Ellison syndrome: correlation with clinical expression, tumor extent and role in diagnosis—A prospective NIH study of 235 patients and review of the literature in 984 cases. Medicine (Baltimore). 2001; 80: 189–222. [DOI] [PubMed] [Google Scholar]
- 16.Metz DC, Pisegna JR, Fishbeyn VA, et al. Control of gastric acid hypersecretion in the management of patients with Zollinger-Ellison syndrome. World J Surg. 1993; 17: 468–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber HC, Venzon DJ, Lin JT, et al. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995; 108: 1637–1649. [DOI] [PubMed] [Google Scholar]
- 18.Roy P, Venzon DJ, Shojamanesh H, et al. Zollinger-Ellison syndrome: clinical presentation in 261 patients. Medicine. 2000; 79: 379–411. [DOI] [PubMed] [Google Scholar]
- 19.Norton JA, Fraker DL, Alexander HR, et al. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999; 341: 635–644. [DOI] [PubMed] [Google Scholar]
- 20.Norton JA, Doppman JL, Jensen RT. Curative resection in Zollinger-Ellison syndrome: Results of a 10-year prospective study. Ann Surg. 1992; 215: 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orbuch M, Doppman JL, Strader DB, et al. Imaging for pancreatic endocrine tumor localization: recent advances. In: Mignon M, Jensen RT, eds. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management. Frontiers of Gastrointestinal Research. Basel Switzerland: S. Karger; 1995: 268–281.
- 22.Sugg SL, Norton JA, Fraker DL, et al. A prospective study of intraoperative methods to diagnose and resect duodenal gastrinomas. Ann Surg. 1993; 218: 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander HR, Fraker DL, Norton JA, et al. Prospective study of somatostatin receptor scintigraphy and its effect on operative outcome in patients with Zollinger-Ellison syndrome. Ann Surg. 1998; 228: 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibril F, Reynolds JC, Doppman JL, et al. Somatostatin receptor scintigraphy: its sensitivity compared with that of other imaging methods in detecting primary and metastatic gastrinomas: a prospective study. Ann Intern Med. 1996; 125: 26–34. [DOI] [PubMed] [Google Scholar]
- 25.Termanini B, Gibril F, Reynolds JC, et al. Value of somatostatin receptor scintigraphy: A prospective study in gastrinoma of its effect on clinical management. Gastroenterology. 1997; 112: 335–347. [DOI] [PubMed] [Google Scholar]
- 26.Fraker DL, Norton JA, Alexander HR, et al. Surgery in Zollinger-Ellison syndrome alters the natural history of gastrinoma. Ann Surg. 1994; 220: 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frucht H, Norton JA, London JF, et al. Detection of duodenal gastrinomas by operative endoscopic transillumination: a prospective study. Gastroenterology. 1990; 99: 1622–1627. [DOI] [PubMed] [Google Scholar]
- 28.MacFarlane MP, Fraker DL, Alexander HR, et al. A prospective study of surgical resection of duodenal and pancreatic gastrinomas in multiple endocrine neoplasia type 1. Surgery. 1995; 118: 973–980. [DOI] [PubMed] [Google Scholar]
- 29.Norton JA, Doherty GD, Fraker DL, et al. Surgical treatment of localized gastrinoma within the liver: A prospective study. Surgery. 1998; 124: 1145–1152. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958; 53: 457–481. [Google Scholar]
- 31.Jaskowiak NT, Fraker DL, Alexander HR, et al. Is reoperation for gastrinoma excision in Zollinger-Ellison syndrome (ZES) indicated? Surgery. 1996; 120: 1057–1063. [DOI] [PubMed] [Google Scholar]
- 32.Thom AK, Norton JA, Doppman JL, et al. Prospective study of the use of intraarterial secretin injection and portal venous sampling to localize duodenal gastrinomas. Surgery. 1992; 112: 1002–1008. [PubMed] [Google Scholar]
- 33.Miller DL, Doppman JL, Metz DC, et al. Zollinger-Ellison syndrome: technique, results and complications of portal venous sampling. Radiology. 1992; 182: 235–241. [DOI] [PubMed] [Google Scholar]
- 34.Jensen RT. Zollinger-Ellison syndrome. In: Doherty GM, Skogseid B, eds. Surgical Endocrinology: Clinical Syndromes. Philadelphia: Lippincott Williams & Wilkins; 2001: 291–344.
- 35.Diepstraten A, Driessen WM, Jansen JB, et al. Fallibility of gastrin level as an indicator of complete excision of a gastrinoma. Br J Surg. 1990; 77: 1403–1405. [DOI] [PubMed] [Google Scholar]
- 36.Passaro E Jr, Howard TJ, Sawicki MP, et al. The origin of sporadic gastrinomas within the gastrinoma triangle: a theory. Arch Surg. 1998; 133: 13–16. [DOI] [PubMed] [Google Scholar]
- 37.Jensen RT, Gardner JD. Gastrinoma. In: Go VLW, DiMagno EP, Gardner JD, et al., eds. The Pancreas. Biology, Pathobiology and Disease. New York: Raven Press; 1993: 931–978.
- 38.Norton JA. Neuroendocrine tumors of the pancreas and duodenum. Curr Probl Surg. 1994; 31: 1–156. [DOI] [PubMed] [Google Scholar]
- 39.Norton JA, Doppman JL, Collen MJ, et al. Prospective study of gastrinoma localization and resection in patients with Zollinger-Ellison syndrome. Ann Surg. 1986; 204: 468–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson NW, Pasieka J, Fukuuchi A. Duodenal gastrinomas, duodenotomy, and duodenal exploration in the surgical management of Zollinger-Ellison syndrome. World J Surg. 1993; 17: 455–462. [DOI] [PubMed] [Google Scholar]
- 41.Thompson NW, Vinik AI, Eckhauser FE. Microgastrinomas of the duodenum. A cause of failed operations for the Zollinger-Ellison syndrome. Ann Surg. 1989; 209: 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krenning EP, Kwekkeboom DJ, Oei HY, et al. Somatostatin-receptor scintigraphy in gastroenteropancreatic tumors. Ann NY Acad Sci. 1994; 733: 416–424. [DOI] [PubMed] [Google Scholar]
- 43.Jensen RT. Presence of somatostatin receptors on gastro-enteropancreatic endocrine tumors (GEPs): impact on clinical management with somatostatin receptor imaging and other uses of somatostatin analogues. In: Lamberts SWJ, ed. Octreotide: The Next Decade. Bristol, UK: Bioscientific Ltd; 1999: 149–178.
- 44.Stabile BE, Morrow DJ, Passaro E Jr. The gastrinoma triangle: operative implications. Am J Surg. 1984; 147: 25–31. [DOI] [PubMed] [Google Scholar]
- 45.Frucht H, Howard JM, Slaff JI, et al. Secretin and calcium provocative tests in the Zollinger-Ellison syndrome: A prospective study. Ann Intern Med. 1989; 111: 713–722. [DOI] [PubMed] [Google Scholar]
Footnotes
Presented at the 114th Annual Session of the Southern Surgical Association, December 1–4, 2002, Palm Beach, Florida.
Correspondence: Jeffrey A. Norton, MD, Professor of Surgery, University of California San Francisco, 533 Parnassus Ave., U-371, Box 0790, San Francisco, CA 94143.
E-mail: nortonj@surgery.ucsf.edu
Accepted for publication December 2002.