Abstract
Phototropin 1 (phot1) is a Ser/Thr photoreceptor kinase that binds two molecules of flavin mononucleotide as its chromophores and undergoes autophosphorylation in response to blue light. Phot1 is plasma membrane associated and, as with phot2, has been shown to function as a photoreceptor for phototropism, blue light–induced chloroplast movement, and blue light–induced stomatal opening. Phot1 likely also plays a redundant role with phot2 in regulating the rate of leaf expansion. Understanding the mechanism(s) by which phot1 initiates these four different responses requires, at minimum, knowledge of where the photoreceptor is located. Therefore, we transformed a phot1 null mutant of Arabidopsis with a construct encoding translationally fused phot1–green fluorescent protein (GFP) under the control of the endogenous PHOT1 promoter and investigated its cellular and subcellular distribution. This PHOT1-GFP construct complements the mutant phenotype, restoring second positive curvature. Phot1 is expressed strongly in dividing and elongating cortical cells in the apical hook and in the root elongation zone in etiolated seedlings. It is localized evenly to the plasma membrane region in epidermal cells but is confined largely to the plasma membrane region of the transverse cell walls in the cortical cells of both root and hypocotyl. It is found at both apical and basal ends of these cortical cells. In light-grown plants, phot1–GFP is localized largely in the plasma membrane regions adjacent to apical and basal cell end walls in the elongating inflorescence stem, where the photoreceptor is expressed strongly in the vascular parenchyma and leaf vein parenchyma. Phot1 also is localized to the plasma membrane region of leaf epidermal cells, mesophyll cells, and guard cells, where its distribution is uniform. Although phot1 is localized consistently to the plasma membrane region in etiolated seedlings, a fraction becomes released to the cytoplasm in response to blue light. Possible relationships between observed phot1 distribution and the various physiological responses activated by blue light are discussed.
INTRODUCTION
Blue light induces a wide variety of plant responses, many of which are mediated by phototropin 1 (phot1), phototropin 2 (phot2), or both (Briggs and Huala, 1999; Christie and Briggs, 2001; Briggs and Christie, 2002). For example, the shoots of young seedlings grow toward the light, whereas roots grow away from it. Chloroplasts become oriented to minimize self-shading under low-intensity blue light (accumulation response), presumably to maximize photosynthesis. By contrast, they become oriented to maximize mutual shading under high-intensity blue light (avoidance response), presumably to minimize photodamage. Stomata are induced to open in response to blue light, allowing CO2 entry for photosynthesis and water loss to drive transpiration. Blue light also induces a rapid inhibition of shoot elongation in etiolated seedlings. Only recently were the phototropins identified as photoreceptors that mediate all four of these responses (Briggs and Christie, 2002).
Phot1 was identified originally as a 120-kD membrane-associated protein showing blue light–dependent phosphorylation. A range of studies indicated that it was a component in the signal transduction pathway for phototropism (Briggs et al., 2001), but efforts to purify it were not successful. Ultimately, the cloning and sequencing of its gene established that its C-terminal domain is a classic Ser/Thr kinase (Huala et al., 1997). Later work established that the blue light–activated phosphorylation was likely autophosphorylation and that the protein itself was the photoreceptor (Christie et al., 1998), using two molecules of flavin mononucleotide as its chromophores (Christie et al., 1999).
These chromophores are bound within two conserved domains (Christie et al., 1999) that have been designated LOV1 and LOV2 (Huala et al., 1997) because they are found in proteins that are regulated by light, oxygen, or voltage. LOV domains are a subgroup of PAS domains, which are involved in protein–protein interaction and ligand binding (Taylor and Zhulin, 1999). Activation by light results in the formation of a covalent adduct between the C(4a) carbon of flavin mononucleotide and a highly conserved Cys found in both LOV domains (Salomon et al., 2000, 2001; Crosson and Moffat, 2002).
A second phototropin, phot2 (Jarillo et al., 1998), also has been demonstrated to play a role in phototropism, but only at high fluence rates of blue light (Sakai et al., 2001). Both phot1 and phot2 can mediate blue light–activated chloroplast accumulation (Sakai et al., 2001). However, phot2 alone mediates the avoidance response to high light (Jarillo et al., 2001; Kagawa et al., 2001). Recently, Kinoshita et al. (2002) showed that both phototropins serve redundantly as photoreceptors for blue light–activated stomatal opening. In addition, Folta and Spalding (2001) found that an Arabidopsis mutant lacking phot1 also lacked the blue light–induced rapid inhibition of hypocotyl growth. Phot1 also has been shown to regulate blue light–induced calcium uptake (Baum et al., 1999).
With the exception of light-activated stomatal opening (Schroeder et al., 2001), little is known regarding the steps immediately downstream from these photoreceptors. In the case of phototropism, blue light–induced lateral transport of auxin leading to differential growth of the illuminated and shaded sides of the organ generally is believed to be the downstream basis of phototropic curvature (Iino, 2001). Cytoskeletal elements are involved in both chloroplast relocation responses (Kadota and Wada, 1992; Tlalka and Gabrys, 1993; Sato et al., 2001) and stomatal opening (Hwang et al., 1997). All three of these responses to blue light almost certainly involve the plasma membrane. Thus, it was of interest to determine both the cellular and subcellular localization of the photoreceptors that mediate these responses.
In etiolated seedlings, both cosedimentation with known plasma membrane marker enzymes (Gallagher et al., 1988; Hager and Brich, 1993) and aqueous two-phase partitioning (Palmer et al., 1993; Short et al., 1993; Salomon et al., 1996; Sharma et al., 1997) conclusively locate unphosphorylated phot1 to the plasma membrane. Because phot1 is highly hydrophilic and has no obvious membrane-spanning domains, it is clearly not an integral membrane protein. Experiments with right-side-out plasma membrane vesicles indicate that it is associated only with the inner surface of the plasma membrane (Short et al., 1993). However, nothing is known about its subcellular localization in etiolated seedlings given brief light treatments or in light-grown plants.
At this time, we know only that phot2 activity is associated with a membrane fraction (Christie and Briggs, 2002), although given that its role is partially redundant with that of phot1, it seems likely that it is also localized specifically to the plasma membrane. Three known consequences of blue light treatment—the phosphorylation and activation of a plasma membrane proton-ATPase in guard cells (Kinoshita and Shimizaki, 1999), anion channel activation (Folta and Spalding, 2001), and the uptake of extracellular calcium (Baum et al., 1999)—are all consistent with plasma membrane localization of the relevant photoreceptor(s).
Auxin transporters, potentially directly or indirectly involved with phototropism, are known to be localized to the plasma membrane. Several putative auxin transporters, both efflux carriers (Gälweiler et al., 1998; Müller et al., 1998; Steinmann et al., 1999; Friml et al., 2002) and an auxin permease (Bennett et al., 1996; Swarup et al., 2001), have been identified, and their tissue, cellular, and subcellular localization have been investigated.
In view of the recent identification of the phototropins as photoreceptors mediating both chloroplast movement and stomatal opening, phenomena presumably unrelated to auxin transport, it is important to ascertain the localization of the phototropins in leaf mesophyll and guard cells. Likewise, both the regulation of leaf expansion by blue light (Van Volkenburgh, 1999) and solar tracking by mature leaves of some species (Koller, 2000) may involve phototropins, raising additional questions about the localization of these photoreceptors in other leaf tissues and cells.
Because the phototropins regulate diverse physiological responses, one might expect different distribution patterns related to their different functions. In the present study, we report the cellular and subcellular distribution of phot1 in various organs and cells of Arabidopsis, both etiolated and light grown, using a phot1–green fluorescent protein (GFP) fusion protein as a reporter.
RESULTS
The phot1-GFP Fusion Protein Complements a Null phot1 Mutant and Exhibits Blue Light–Activated Autophosphorylation
To investigate the cellular and subcellular localization of phot1, we constructed a gene transfer vector that expresses a translationally fused PHOT1-GFP gene under the control of the full-length PHOT1 promoter. Because the PHOT1-GFP fusion gene is expressed under the control of the native PHOT1 promoter, we expected the distribution of the GFP signal in the transgenic plants to correspond with the cellular distribution of endogenous phot1.
We first characterized the 5′ untranslated region of the PHOT1 mRNA by rapid amplification of cDNA ends. A single rapid amplification of cDNA ends product was obtained, and direct sequencing suggested that phot1 transcription starts 15 bp upstream of the initiation codon. To include all of the elements that might be required to control the gene expression of PHOT1, we introduced both 3′ and 5′ noncoding sequences into the PHOT1-GFP fusion gene, as illustrated in Figure 1A.
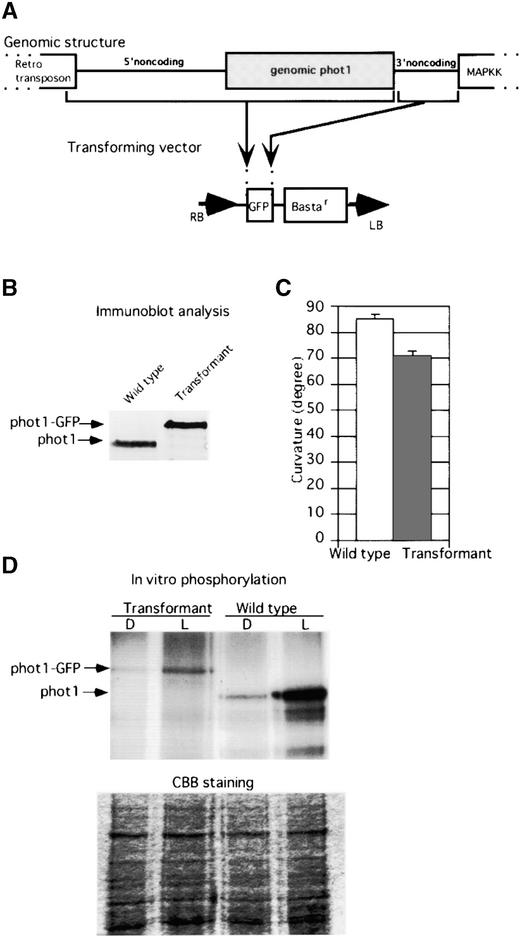
Figure 1.
Plasmid Construction and Analysis of Transformants.
(A) Construction of the gene transfer vector. The start of the genomic PHOT1 DNA from the end of the retrotransposon to the 3′ end of the phot1 structural gene was ligated to the 5′ end of the GFP gene so that the PHOT1 gene was fused translationally with the GFP gene. The 3′ noncoding region between PHOT1 and the downstream mitogen-activated protein kinase kinase (MAPKK) gene was ligated to the 3′ end of the GFP gene. This construct was used to transform the phot1 null mutant nph1-5 (Huala et al., 1997). LB, left border; RB, right border.
(B) Immunoblot analysis of phot1 in the wild type and of the phot1-GFP fusion protein in a transformant. Thirty micrograms of whole protein was loaded onto each lane.
(C) Comparison of phototropic curvatures of wild-type and null mutant phot1-5 (nph1-5) transformed with the construct encoding the phot1-GFP fusion protein. Curvatures were measured after 24 h of blue light (2 μmol·m−2·s−1).
(D) In vitro phosphorylation analysis of membrane protein from wild-type or transformant seedlings. The microsomal fraction was phosphorylated with 32P-ATP in the presence (L) or absence (D) of light. CBB, Coomassie blue–stained gel to show equal protein loading for the phosphorylation assay.
The resulting construct was used to transform the Arabidopsis null phot1 mutant phot1-5 (nph1-5; Huala et al., 1997) by the Agrobacterium tumefaciens–mediated method (Cutler et al., 2000). The seedlings were screened by low-power fluorescence microscopy (Cutler et al., 2000), and several independent T3 transformants showing strong green fluorescence were obtained (Figure 2A). All of the results described below were obtained with the single transformant line LEGT2-6, but the results were confirmed with several other transformants.
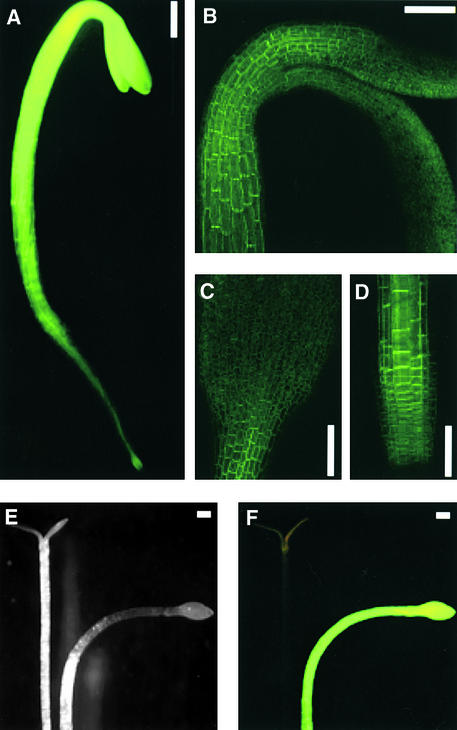
Figure 2.
Expression of the phot1-GFP Fusion Protein in 3-Day-Old Etiolated Seedlings.
(A) Image of the entire seedling obtained by fluorescence microscopy.
(B) Projection confocal image of the hypocotyl hook region.
(C) Projection confocal image of the cotyledon.
(D) Projection confocal image of the root tip.
(E) Phototropic response of the null mutant phot1-5 and the mutant transformed with the PHOT1-GFP construct as seen under white light.
(F) Phototropic response of the null mutant phot1-5 and the mutant transformed with the PHOT1-GFP construct as seen under blue light.
Bars = 300 μm.
We then monitored the expression of the phot1-GFP fusion protein in etiolated seedlings of the transformed phot1-5 mutants or of the native phot1 protein in wild-type seedlings by immunoblot analysis. As shown in Figure 1B, the transformant line selected expressed the fusion protein at a level comparable to that of wild-type phot1, and at the predicted size.
We next examined whether the fusion protein was still functional when undergoing blue light–activated autophosphorylation. For this purpose, we used a microsomal fraction from etiolated seedlings for the phosphorylation assay. As shown in Figure 1D, light-dependent phosphorylation of a protein of the predicted size was detected in the microsomal fraction of the transgenic plants. The Coomassie blue–stained gel shown at bottom indicates equal loading.
To determine whether the phot1-GFP fusion protein would complement the null mutant and restore normal phototropic sensitivity, we determined the second positive curvature response of the mutant transformants compared with the untransformed phot1-5 mutant and wild-type seedlings. The 3-day-old seedlings were illuminated with unilateral blue light for 24 h, and the curvatures were measured. The fluence rate used (2 μmol·m−2·s−1) is insufficient to induce phot2-mediated hypocotyl curvature (Sakai et al., 2001).
The transformants showed strong green fluorescence under blue light and curved strongly toward the light source, whereas the untransformed mutant failed to show green fluorescence and failed to curve under the illumination conditions used (Figures 2E and 2F). The relative phototropic responses of wild-type and mutant transformant seedlings are illustrated in Figure 1C. The transformants were only slightly less sensitive than the wild type under the light conditions used. Because the expression level of the phot1-GFP fusion protein in the transformant was similar to that of the wild-type phot1 protein and the phot1-GFP protein complemented the null mutant, we hypothesize that the GFP signals from the transformant reflect the distribution of endogenous phot1.
Localization of phot1-GFP in the Phototropically Sensitive Regions of Etiolated Hypocotyls
The strongest green fluorescence in the etiolated shoots (Figure 2A) was located in the elongation zones of the hypocotyls (Figure 2B) and in the basal region of the cotyledons (Figure 2C). The subcellular localizations of phot1-GFP in epidermal and cortical cells of the apical hook and hypocotyl are shown together as a projection image (Figure 3A) and separately as single optical sections (Figures 3B and 3C). In epidermal cells, phot1-GFP was localized in close proximity to the plasma membrane (Figure 3B) and was distributed evenly adjacent to the entire cell wall. Phot1-GFP also was localized in close proximity to the plasma membrane in the cortical cells (Figure 3C) but was located predominantly adjacent to the end walls. The signal from the cortical cells also was consistently stronger than that from the epidermis.
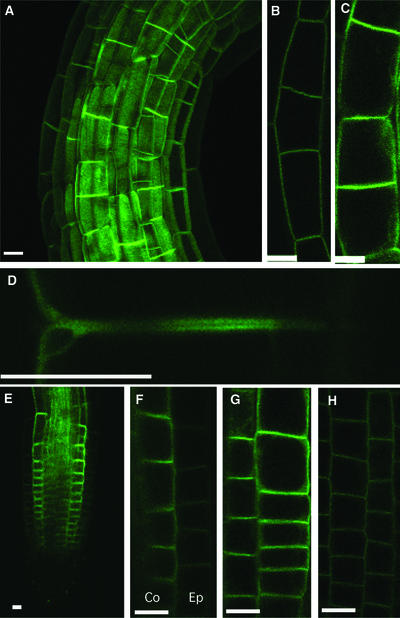
Figure 3.
Confocal Images of the phot1-GFP Signal in Etiolated Seedlings.
(A) Projection image of the apical hook.
(B) Single optical section of the hook epidermis.
(C) Single optical section of cortical cells.
(D) Enlargement of a single optical section of two adjacent cortical cell end walls showing signal from the basal end of the top cell and the apical end of the bottom cell.
(E) Single optical section of the root elongation zone.
(F) Single optical section showing root cortical (Co) and adjacent epidermal (Ep) cells. Note the weakness of the signal from the epidermal cells and the absence of signal from the cortical cells adjacent to the vascular cylinder.
(G) Single optical section of root cortical cells. Note the enhanced signal from the end walls.
(H) Single optical section of root epidermal cells. Note the weakness of the signal and the even distribution over all of the walls.
Bars = 50 μm.
By contrast, when Arabidopsis seedlings were transformed with the GFP gene alone driven by the 35S promoter, the GFP fluorescence was distributed uniformly throughout the cytoplasm (data not shown). To determine whether the strong signals detected adjacent to the end walls were possibly an optical artifact, we compared this image with the image of a known plasma membrane marker–GFP fusion protein, PIP2A (Cutler et al., 2000). With this marker, there was no preferential localization to the cortical cell end walls (data not shown).
The auxin efflux carrier PIN1 is localized to the plasma membrane adjacent to the basal cell end walls of vascular parenchyma cells (distal to the shoot apex) in Arabidopsis inflorescence stems (Gälweiler et al., 1998; see below) and in the respective elongation zones of Arabidopsis root epidermal cells (Müller et al., 1998). In both cases, the distribution is asymmetric, with the efflux carriers located almost exclusively at the basipetal ends of these cells. Given the likely participation of the blue light–activated alteration of auxin transport in phototropism, it was of interest to determine whether the phot1 protein showed the same distribution. As shown in Figure 3D, phot1 was found at both apical and basal ends of the cortical cells in the etiolated hypocotyl.
Localization of phot1-GFP in the Phototropically Sensitive Regions of Etiolated Roots
The distribution of the phot1-GFP fusion protein in the elongation zone of roots of etiolated seedlings is shown in Figures 2D and 3E to 3H. As with the hypocotyl, the fluorescence signal in roots was strongest in the elongation zone (Figures 2D and 3E) and much stronger in internal tissues than in the epidermis (Figures 3F to 3H). Indeed, we detected very little signal from the root epidermis except in the region of the elongation zone, where the signal was weak. We also failed to detect fluorescence in the root cap. In the epidermis of the growing region, the signal was not distributed polarly but was found evenly along the entire epidermal cell wall (Figure 3H). As with the hypocotyl, however, the fluorescence signal in the internal cells was strongest adjacent to the cell end walls.
No signal was detected along the walls of cortical cells adjacent to the endodermis (Figures 3F and 3G), a situation similar to that found for PIN2 (Müller et al., 1998). As with the hypocotyl, GFP fluorescence was detected at both apical and basal ends of the cortical cell walls, a pattern similar to that found for PIN2. However, the PIN2 signal in the root epidermis is strong and highly localized to the basal cell ends, in contrast to the phot1 signal, which was weak and distributed evenly adjacent to all of the walls.
Localization of phot1-GFP in Mature Leaves
In addition to its role in activating stomatal opening and chloroplast movement, blue light activates at least two other responses in leaves: leaf expansion (Van Volkenburgh and Cleland, 1990; Van Volkenburgh et al., 1990; Blum et al., 1994; Van Volkenburgh, 1999) and, in certain species, solar tracking (Voerkel, 1934; Yin, 1938). Therefore, it was of interest to determine the distribution of phot1 in leaves.
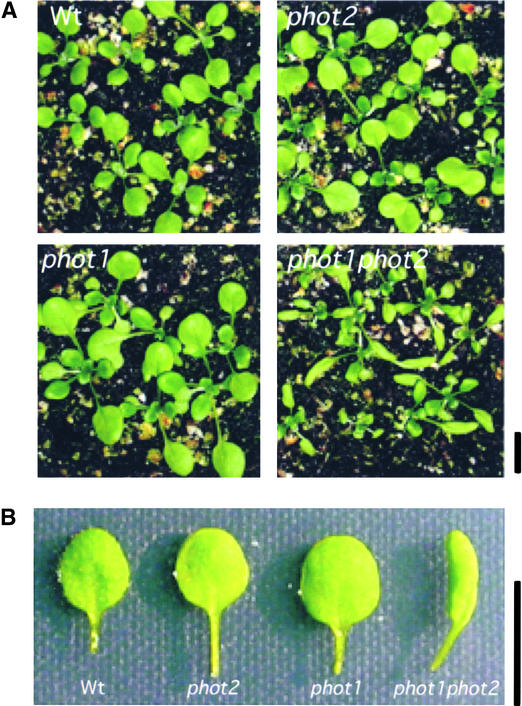
The expression and distribution of the phot1-GFP fusion protein in the leaf epidermis are shown in Figure 4A, and those in the mesophyll cells are shown in Figure 4B. Given the fact that blue light is known to stimulate epidermal growth through processes other than photosynthesis (Van Volkenburgh and Cleland, 1990; Van Volkenburgh et al., 1990; Van Volkenburgh, 1999), it is not surprising to find the phot1-GFP fusion at the plasma membrane in the epidermal cells. Indeed, at a young stage, the leaves of the phot1 phot2 double mutant were far smaller than those of the wild type and were strongly curled, a situation not found in the single mutants (Figures 5A and 5B). Sakai et al. (2001) also noted that leaves of the double mutant show abnormal curling. These results suggest the localization of phot1 in epidermal cells and provide evidence that both phototropins may redundantly regulate leaf expansion just as they function redundantly in other responses.
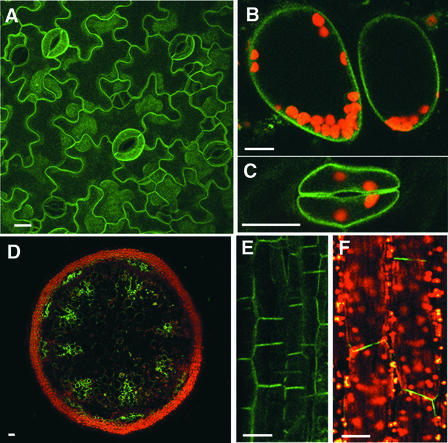
Figure 4.
Confocal Images of the phot1-GFP Signal from Various Arabidopsis Organs and Tissues.
Chloroplast autofluorescence is indicated in red. Bars = 50 μm.
(A) Projection image of confocal images obtained from the leaf epidermis. Note the uniform distribution of signal along all of the epidermal cell walls.
(B) Single optical section of mesophyll cells in a transverse section of a leaf.
(C) Single optical section of a guard cell pair. Note the signal along all of the walls, including the stomatal pore.
(D) Single optical section of a transverse section of the inflorescence stem. Note the signal in the xylem parenchyma and rudimentary cambial regions and in the phloem parenchyma outside of the vascular bundles.
(E) Projection confocal image of several optical sections of the xylem parenchyma in a vertical section of the inflorescence stem. Note the bipolar distribution of signal.
(F) Single optical section of subepidermal cells of the midvein of a mature leaf. The axis of the vein is vertical in the figure. Note the strong signal from the end walls.
Figure 5.
Three-Week-Old Light-Grown Wild-Type and phot Mutant Seedlings of Arabidopsis.
(A) Whole seedlings.
(B) Individual leaves.
Note the curled leaf of the phot1 phot2 double mutant. Wt, wild type. Bars = 1 cm.
Likewise, given the fact that the chloroplast aggregation response is likely cell autonomous (Kagawa and Wada, 1996), it is not surprising to find the fusion protein in the photosynthetic mesophyll cells underlying the epidermis (Figure 4B). In both cell types, green fluorescence was distributed evenly along the plasma membrane.
The strongest signals detected in the leaf epidermis arose from the guard cells (Figures 4A and 4C), which is not surprising given the recent evidence that phot1 serves as one of the photoreceptors for blue light–activated stomatal opening (Kinoshita et al., 2002). In this case as well, the phot1-GFP fusion protein was closely associated with the plasma membrane. This observation is not surprising given the evidence that the blue light–induced swelling response of guard cells is known to be cell autonomous (Zeiger and Hepler, 1977) and that the guard cell plasma membrane is involved intimately in stomatal regulation (Schroeder et al., 2001).
The leaf lamina of several plant species become oriented at right angles to the direction of incident blue light. On the basis of experiments with polarized light, Koller et al. (1990) hypothesized that the responsible photoreceptor was localized at the end walls of parenchyma cells along the main leaf veins. Although mature Arabidopsis leaves show an extremely weak orientation response with respect to light direction (data not shown), we nevertheless investigated the distribution of the phot1-GFP fusion protein along the leaf veins.
The phot1-GFP fusion protein was expressed in elongated parenchyma cells underlying the epidermis along the leaf veins (Figure 4F). It was found predominantly adjacent to the cell end walls lying at right angles to the long axis of the vein. Thus, the distribution is consistent with the predicted localization of the photoreceptor for leaf movement in response to a change in the direction of incident light. Therefore, it seems likely that phot1 may regulate this response. As in the other responses, phot1 and phot2 may be at least partially redundant.
Localization of phot1 in the Inflorescence Stem
Like the etiolated hypocotyl, the growing inflorescence stem shows a positive phototropic response to unilateral light (Fukaki et al., 1996). Therefore, we investigated the distribution of the phot1-GFP fusion protein in this elongating tissue. As shown in Figure 4D, strong green fluorescence was detected in the region of the xylem parenchyma and rudimentary cambium. Smaller groups of parenchyma cells to the outside of the vascular bundle also showed strong green fluorescence.
This pattern is similar to that seen for the PIN proteins in the inflorescence stem. However, PIN1 is expressed only in the xylem parenchyma and cambium (Gälweiler et al., 1998), and PIN3 is expressed only outside the vascular bundles (Friml et al., 2002). Figure 4E shows a single optical section (longitudinal) of the xylem parenchyma. As with the cortical cells of the root and hypocotyl elongation zones, the phot1-GFP fusion protein showed strong bipolar distribution.
Effect of Blue Light Treatment on the Subcellular Localization of phot1-GFP
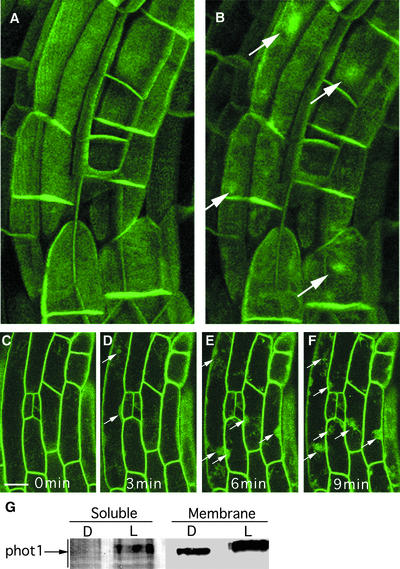
We next investigated whether blue light treatment in any way affected the subcellular distribution of the phot1-GFP fusion protein. Because GFP fluorescence is excited by blue light, the confocal laser used to scan the sample provides a blue light stimulus. Figure 6A shows a projection image of the apical hook of a dark-grown seedling scanned for the first time. Twenty minutes later, the sample was scanned again (Figure 6B). Although GFP fluorescence was confined initially to the region of the plasma membrane, significant GFP fluorescence can be seen in the cytoplasm in both epidermal and cortical cells at the time of the second scan (arrows).
Figure 6.
Light-Dependent Dissociation of a Fraction of the phot1-GFP Fusion Protein from the Region of the Plasma Membrane.
(A) Projection confocal image of cortical cells in the apical hook of an etiolated seedling.
(B) Projection confocal image of the same cells 20 min after the initial scan. The light signal is the laser scan itself. Note the appearance of GFP fluorescence in the cytoplasm (arrows).
(C) to (F) Confocal images of single optical sections of epidermal cells from an etiolated seedling scanned every minute, with the images used taken every 3 min. Note the appearance of GFP fluorescence in the cytoplasm (arrows).
(G) Immunoblot of the soluble and microsomal fractions of 3-day-old etiolated seedlings before (D) or 1 h after (L) light treatment (100 μmol·m−2·s−1 white light).
Bars = 50 μm.
Figures 6C to 6F show confocal images of a single optical section of the epidermis scanned every minute with images taken at 3-min intervals. Within the first 3 min, fluorescence was detected in the cytoplasm. As is clear from Figures 6C to 6F, the repeated laser scanning had no detectable effect on the overall fluorescence, suggesting that the multiple scans used here did not cause significant photobleaching of the GFP.
To determine whether the cytoplasmic GFP signal represents the full-length phot1-GFP protein or is the consequence of phot1-GFP degradation and release from the plasma membrane, we examined phot1 protein levels in soluble and membrane fractions from dark control or white light–irradiated wild-type seedlings by immunoblot analysis. Most of the phot1 was detected in the membrane fraction in both samples (Figure 6G). However, although the protein was not detectable in the soluble fraction in the dark control, a small amount appeared in the soluble fraction after light treatment. The phot1 found in the soluble fraction after light treatment was the same size as that found in the membrane fraction after light treatment, indicating that the GFP signal detected in the cytoplasm after light treatment (Figure 5) likely arises from the full-length phot1-GFP fusion protein.
A decrease in the mobility of wild-type phot1 after a 1-h light treatment compared with the dark control was detected in both membrane and soluble fractions (Figure 6G), indicating that both protein samples were phosphorylated (Short et al., 1993). These results suggest that a fraction of the phot1-GFP fusion protein becomes dissociated from the plasma membrane in a blue light–dependent manner. Similar light-dependent dissociation of phot1-GFP from the plasma membrane region also was observed in cells from the elongating zone of roots of etiolated seedlings after light treatment (the laser of the confocal microscope was the blue light source) and in leaf epidermal cells adapted in the dark for 24 h before blue light treatment (data not shown). Although GFP fluorescence appeared somewhat localized to nucleus-shaped structures, frequently more than one such area per cell was present. However, it is premature to assign this fusion protein to any region other than the cytoplasm.
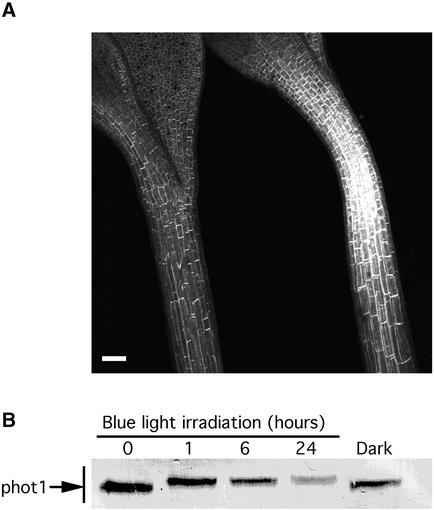
The soluble phot1-GFP released by light treatment disappeared after prolonged light exposure (8 h of blue light) or prolonged dark treatment (3 h) after irradiation by the confocal microscope laser. In both cases, the cellular and subcellular distribution of GFP fluorescence returned to the pattern seen in the dark controls. However, after continuous light (24 h of blue light, 20 μmol·m−2·s−1), the relative amount of phot1-GFP fluorescence observed was reduced greatly (Figure 7A). Thus, continuous light appears to downregulate the fusion protein in some manner.
Figure 7.
Downregulation of phot1 after Extended Blue Light Treatment (24 h at a Fluence Rate of 20 μmol·m−2·s−1).
(A) Projection image of confocal sections of 3-day-old seedlings before (right) and after (left) 24 h of blue light treatment. Bar = 300 μm.
(B) Immunoblot analysis of total protein extracted from 3-day-old wild-type seedlings at 0, 3, 6, and 24 h after the onset of the blue light treatment.
Figure 7B shows an immunoblot analysis of whole protein extracts of wild-type seedlings treated for various times with continuous blue light. The phot1 signal decreased strongly in response to blue light, whereas it decreased only slightly in the dark. Thus, the loss of phot1-GFP fluorescence in the transformant after 24 h of blue light was comparable to the loss of phot1 protein in wild-type seedlings after 24 h of blue light. Hence, the loss of fluorescence observed likely represents blue light–induced loss of the phot1-GFP protein itself rather than blue light–induced bleaching of the GFP.
The strong fluorescence observed in the guard cells of light-grown plants (Figures 4A and 4C) also indicates that the GFP moiety was stable in the light. In summary, blue light has a rapid effect on a small fraction of the fusion protein, causing it to leave the plasma membrane region, and a much slower effect, resulting in a much reduced level of the protein after prolonged blue light treatment. PHOT1 transcript levels also were reduced after the 24-h blue light treatment (data not shown), likely at least partially the cause of the reduction in phot1 protein.
DISCUSSION
We introduced a phot1-GFP fusion gene under the control of the PHOT1 promoter into the Arabidopsis phot1 null mutant nph1-5 (Huala et al., 1997) and obtained several independent transformations. These T3 transgenic plants expressed a full-length phot1-GFP fusion protein, and the expression level of the fusion protein in the transformants was similar to that of the native phot1 protein in wild-type seedlings. The cellular and subcellular distribution of phot1-GFP also was similar from one transformant to another. We found strong expression in the elongation zones of both roots and hypocotyls, regions in which the light signal for phototropism is likely perceived.
The tissue distribution found in the present study is consistent with that found for light-induced kinase activity of the 120-kD plasma membrane protein in growing regions of pea epicotyls (Short and Briggs, 1990) and coleoptiles (Hager and Brich, 1993; Palmer et al., 1993). Thus, the GFP signals obtained from the transformants likely reflect the tissue localization of endogenous phot1. The fusion protein complemented the phot1-5 null mutation in restoring second positive curvature (Figures 1C and 2F) and also underwent blue light–activated autophosphorylation (Figure 1D). In both cases, the transformant was less sensitive than the wild type. It is possible that the C-terminal GFP moiety may slightly inhibit the phot1 function of the fusion protein.
The phot1-GFP fusion protein showed strong localization to the end walls of cortical cells in the growing and phototropically sensitive regions of etiolated roots and hypocotyls and the inflorescence stem (Figures 2B to 2D, 3A, 3C, and 3E to 3G). Elongated parenchyma cells beneath the epidermis above the leaf veins also showed distinct end wall localization (Figure 4F). However, in all other cell types examined, including leaf epidermis, guard cells, palisade parenchyma, and both hypocotyl and root epidermis, the distribution appeared to be uniform adjacent to all cell walls (see below).
Perhaps these differences should not be surprising when one considers the diversity of responses mediated by phot1. A subcellular distribution of the photoreceptor appropriate for one response might not be appropriate for another. A photoreceptor that regulates auxin transport could require a different subcellular location than one regulating chloroplast position, leaf expansion, or guard cell turgor.
The partial congruence of phot1 with the products of the PIN1, PIN2, and PIN3 genes is tantalizing. Given the apparent relationship of phototropism and auxin transport, one might expect some colocalization. There are some striking similarities: strong localization adjacent to the end walls in cortical cells of elongating tissue, etiolated hypocotyls and roots, and parenchymatous cells of the inflorescence stem; specific localization to the xylem parenchyma and rudimentary cambium in the inflorescence stem; and presence in the root cortical cells adjacent to the epidermis but not adjacent to the endodermis.
However, there are major differences even in these cases. In the inflorescence stem, PIN1 is restricted to the xylem parenchyma and cambium (Gälweiler et al., 1998) and PIN3 is restricted to the parenchyma outside of the vascular bundles (Friml et al., 2002), whereas phot1 was clearly expressed in both tissues. Although PIN1 is localized only to basipetal cell walls in both the inflorescence stem (Gälweiler et al., 1998) and the roots (protophloem only; Swarup et al., 2001), end wall localization was consistently bipolar for phot1 (Figure 3D). Likewise, whereas PIN2 shows a distribution in the root cortical cells identical to that of phot1, adjacent to both end walls and lacking along the wall adjacent to the endodermis, it shows strong expression and a strongly polar distribution in the root epidermal cells. Phot1 was expressed very weakly in those same cells and was distributed uniformly.
These differences might be expected given the fact that phot1 is the photoreceptor for several processes and not just for phototropism. However, even in the case of phototropism, it is premature to do more than note the similarities and differences in the distribution of phot1 and the PIN proteins in the relevant tissues. It is possible that phot1 may interact directly or indirectly with more than one of them, and there are other PIN proteins to be characterized. Further work will be required to determine the relationship of phot1 with any of these auxin transporters. To clarify the situation, it also will be essential to determine the subcellular distribution of the proteins encoded by any other PIN genes and by PHOT2.
Experiments with polarized blue light provided strong evidence for the fixed orientation of the photoreceptors for both chloroplast accumulation and avoidance movements in the protonemata of the fern Adiantum capillus-veneris, consistent with localization of the relevant photoreceptors to the plasma membrane (Yatsuhashi et al., 1985). In Arabidopsis, however, the fixed orientation of the photoreceptors involved in chloroplast movements has been demonstrated only for the avoidance response (Kagawa and Wada, 2000), which is known to be mediated exclusively by phot2 (Jarillo et al., 2001; Kagawa et al., 2001). The present study shows that phot1 in Arabidopsis is localized adjacent to the plasma membrane in mesophyll cells, as might be predicted from the earlier experiments with Adiantum. From polarized light experiments (Kagawa and Wada, 2000), one would expect this same distribution for phot2.
As mentioned above, Zeiger and Hepler (1977) provided evidence that the guard cells themselves harbored the photoreceptor that caused them to expand on blue light treatment. It has been known for some time that blue light activates proton secretion by guard cells (Assmann et al., 1985). More recently, Kinoshita and Shimizaki (1999) showed that in guard cells, blue light activates plasma membrane proton-ATPase activity by phosphorylation. Hence, the localization of phot1 adjacent to the plasma membrane is completely consistent with the earlier observations. Given the redundancy of the two phototropins, one might expect the same subcellular distribution of phot2 in guard cells.
To date, the photoreceptor for blue light–activated leaf expansion has not been identified. The results presented here indicate that this response may be mediated by both phototropins acting redundantly. Given the facts that the leaf epidermis physically limits leaf growth (Becraft, 1999; Van Volkenburgh, 1999) and that phot1 is distributed evenly along the plasma membrane of Arabidopsis leaf epidermal cells, it might be expected that leaf growth is yet another process regulated by the phototropins.
As shown in Figure 5A, young leaves of the phot1 phot2 double mutant were smaller than either their wild-type counterparts or the leaves of either single mutant and were strongly curled. Thus, the situation for leaf expansion is very similar to that for stomatal opening. Single mutants do not show an obvious phenotype, because either photoreceptor can mediate the response. It is only when both are missing that a strong phenotype emerges. The difference between the wild type and the double mutant is much less obvious for older plants. Thus, it is likely that the phototropins regulate the rate of leaf expansion rather than the absolute amount of growth.
It appears to be a property of some plant photoreceptors that they can move from cell compartment to cell compartment upon photoexcitation (Nagy et al., 2001). As shown in Figure 6, phot1 is no exception. Over the course of a few minutes, a fraction of the phot1-GFP fusion protein left the plasma membrane region for the cytoplasm in dark-grown seedlings. The fact that only a fraction of the photoreceptor left the plasma membrane suggests that the larger remaining fraction may be more tightly associated and perhaps interacts with a different membrane component.
This shift from plasma membrane to cytoplasm does not seem to be associated with degradation and a consequent downregulation of photosensitivity, at least in the short term. The cytoplasmic fraction appears to be the same size as the membrane fraction and shows a mobility shift, indicating that it is phosphorylated on multiple sites, as is the membrane fraction. It remains to be determined if this light-activated shift in subcellular localization plays a signaling role, and if so, with respect to which responses.
Given the multiple roles played by the two phototropins, it is not surprising that different tissues and cells show different distribution patterns. However, the results of the present study confirm predictions regarding the cellular and subcellular locations of the blue light receptors that activate various responses and should be helpful in elucidating subsequent steps in each of the signal transduction pathways involved. However, the story will be incomplete without similar studies involving the localization of phot2. Such studies are in progress.
METHODS
Plasmid Construction and Transformation
To identify the transcription start site, rapid amplification of cDNA ends was performed with 5 μg of total RNA using the rapid amplification of cDNA ends kit (Clontech, Palo Alto, CA) as directed by the manufacturer. The stop codon of the genomic PHOT1 gene was replaced with a HindIII restriction site with the DNA primers 5′-AGA-AGATCTGCAAACAAATGTTTTTAAGCTTTTGGACTAAACGTTTATCG-3′ and 5′-CGATAAACGTTTAGTCCAAAAGCTTAAAAACATTTGTTTGCA-GATCTTCT-3′ using a kit for site-directed mutagenesis (Stratagene).
The resulting genomic PHOT1 gene, including the PHOT1 structural gene (5404 bp) and 4727 bp of upstream sequence (including the 5′ untranslated region and presumably the entire promoter), was ligated to the 5′ end of the green fluorescent protein (GFP) gene in the gene transfer vector pEIN(2) (S. Cutler, unpublished data) so that the GFP gene would be translated along with the PHOT1 structural gene in the same frame. The PHOT1 3′ noncoding sequence (1193 bp) was introduced at the 3′ end of the GFP gene. The resulting vector was transformed into Arabidopsis thaliana by the Agrobacterium tumefaciens–mediated method (Cutler et al., 2000). T3 seedlings were used for the studies described here.
Isolation and Microscopic Analysis of Transgenic Arabidopsis
Second positive phototropic curvature was assayed as described elsewhere (Lacève et al., 1999) by illuminating 3-day-old etiolated seedlings with unilateral blue light (2 μmol·m−2·s−1) for 24 h, and the resulting curvatures were measured. Sample sizes were 25 (control) or 27 (transformant) seedlings.
Immunoblot Analysis and in Vitro Phosphorylation
To determine the amount of phot1 remaining in 3-day-old etiolated wild-type seedlings after various times in continuous unilateral blue light (2 μmol·m−2·s−1), whole protein extracts were subjected to immunoblot analysis. Whole protein was extracted from the seedlings by homogenizing the tissue with the following extraction buffer: 4% SDS, 2% (v/v) 2-mercaptoethanol, 2 mM phenylmethylsulfonyl fluoride, and 0.1 M Tris-HCl, pH 8.5, followed by incubation at 80°C for 3 min. The homogenate was centrifuged (16,000g for 10 min at room temperature), and the soluble fraction was used for immunoblot analysis.
For immunoblot analysis, the proteins were separated by SDS-PAGE, blotted onto a membrane, and detected with anti-phot1 antibody as described previously (Christie et al., 1998). For analysis of the light-activated movement of phot1 from the plasma membrane into the cytoplasm by protein gel blotting, the microsomal and soluble fractions prepared as described elsewhere (Sakai et al., 2001) were precipitated with 80% acetone, washed in 80% acetone, and resuspended in an equal volume of phosphorylation buffer before SDS-PAGE. For phosphorylation analysis, 10 μg of membrane protein (without acetone treatment) was subjected to the autophosphorylation assay described previously (Sakai et al., 2001).
Visualization of the Distribution of GFP Fluorescence in Transgenic Seedlings
The procedures used were essentially those described by Cutler et al. (2000). Seeds from transformed plants were germinated on agar medium containing Murashige and Skoog (1962) salts. Seedlings then were screened for GFP fluorescence with a Leica (Deerfield, IL) dissecting microscope provided with a mercury lamp and filters for epifluorescence. Seedlings showing green fluorescence were placed on microscope cover slips, and images were obtained with a Nikon inverted fluorescence microscope (Tokyo, Japan) equipped with appropriate Nikon water immersion objective lenses and a Bio-Rad MRC 1024 confocal head.
Seedlings showing green fluorescence were transferred back onto nutrient agar and then to soil after 2–3 weeks for seed production. Seeds from the various fluorescent lines were germinated and retested by confocal microscopy for green fluorescence. Whole seedlings were used to obtain the images shown in Figures 2, 3, 5, and 6, and hand sections were used to obtain the images shown in Figure 4.
Upon request, all novel material described in this article will be made available in a timely manner for noncommercial research purposes. No restrictions or conditions will be placed on the use of any materials described in this article that would limit their use for noncommercial research purposes.
Acknowledgments
Seeds of the Arabidopsis phot1 (cav1-1) single mutant and the phot1 (nph1-5) phot2 (cav1-1) double mutant were the kind gift of Masamitsu Wada. We are grateful to John M. Christie for his careful review of the manuscript. We also are grateful to David Ehrhardt for his indispensable help with confocal microscopy. Sean Cutler provided the pEIN(2) gene transfer vector. We are extremely grateful for this support. This work was supported by grants IBN 9601164 and MCB 0091384 from the National Science Foundation. This is Carnegie Institution of Washington Department of Plant Biology Publication No. 1542.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.003293.
References
- Assmann, S.M., Simoncini, L., and Schroeder, J.I. (1985). Blue light activates electrogenic ion pumping in guard cell protoplasts of Vicia faba. Nature 318, 285–287. [Google Scholar]
- Baum, G., Long, J.C., Jenkins, G.I., and Trewavas, A.J. (1999). Stimulation of the blue light phototropic receptor causes a transient increase in cytosolic Ca2+. Proc. Natl. Acad. Sci. USA 96, 13554–13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becraft, P.W. (1999). Development of the leaf epidermis. Curr. Top. Dev. Biol. 45, 1–40. [DOI] [PubMed] [Google Scholar]
- Bennett, M.J., Marchant, A., Green, H.G., May, S.T., Ward, S.P., Nillner, P.A., Walker, A.R., Schulz, B., and Feldman, K.A. (1996). Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science 273, 948–950. [DOI] [PubMed] [Google Scholar]
- Blum, D.E., Neff, M.M., and Van Volkenburgh, E. (1994). Light-stimulated cotyledon expansion in the blu3 and hy4 mutants of Arabidopsis thaliana. Plant Physiol. 105, 1433–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs, W.R., and Christie, J.M. (2002). Phototropin 1 and phototropin2: Two versatile plant blue-light receptors. Trends Plant Sci. 7, 204–210. [DOI] [PubMed] [Google Scholar]
- Briggs, W.R., Christie, J.M., and Salomon, M. (2001). Phototropins: A new family of flavin-binding blue light receptors in plants. Antiox. Redox Signaling 3, 775–788. [DOI] [PubMed] [Google Scholar]
- Briggs, W.R., and Huala, E. (1999). Blue-light receptors in higher plants. Annu. Rev. Cell Dev. Biol. 15, 33–62. [DOI] [PubMed] [Google Scholar]
- Christie, J.M., and Briggs, W.R. (2001). Blue light sensing in higher plants. J. Biol. Chem. 276, 11457–11460. [DOI] [PubMed] [Google Scholar]
- Christie, J.M., and Briggs, W.R. (2002). Phototropin LOV domains exhibit distinct roles in regulating photoreceptor function. Plant J., in press. [DOI] [PubMed]
- Christie, J.M., Reymond, P., Powell, G., Bernasconi, P., Raibekas, A.A., Liscum, E., and Briggs, W.R. (1998). Arabidopsis NPH1: A flavoprotein with the properties of a photoreceptor for phototropism. Science 282, 1698–1701. [DOI] [PubMed] [Google Scholar]
- Christie, J.M., Salomon, M., Nozue, K., Wada, M., and Briggs, W.R. (1999). LOV (light, oxygen, voltage) domains of the blue-light photoreceptor phototropin (nph1): Binding sites for the chromophore flavin mononucleotide. Proc. Natl. Acad. Sci. USA 96, 8779–8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson, S., and Moffat, K. (2002). Photoexcited structure of a plant photoreceptor domain reveals a light-driven molecular switch. Plant Cell 14, 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler, S., Ehrhardt, D.W., Griffitts, J.S., and Somerville, C.R. (2000). Random GFP::cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at high frequency. Proc. Natl. Acad. Sci. USA 97, 3718–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folta, K.M., and Spalding, E.P. (2001). Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J. 26, 471–478. [DOI] [PubMed] [Google Scholar]
- Friml, J., Wisniewska, J., Benková, E., Mendgen, K., and Palme, K. (2002). Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415, 806–809. [DOI] [PubMed] [Google Scholar]
- Fukaki, H., Fujisawa, H., and Tasaka, M. (1996). SGR1, SGR2, and SGR3: Novel genetic loci involved in shoot gravitropism in Arabidopsis thaliana. Plant Physiol. 110, 945–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher, S., Short, T.W., Ray, P.M., Pratt, L.H., and Briggs, W.R. (1988). Light-mediated changes in two proteins found associated with plasma membrane fractions from pea stem sections. Proc. Natl. Acad. Sci. USA 85, 8003–8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gälweiler, L., Guan, C., Müller, A., Wisman, E., Mendgen, K., Yephremov, A., and Palme, K. (1998). Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282, 2226–2230. [DOI] [PubMed] [Google Scholar]
- Hager, A., and Brich, M. (1993). Blue-light-induced phosphorylation of a plasma-membrane protein from phototropically sensitive tips of maize coleoptiles. Planta 189, 567–576. [Google Scholar]
- Huala, E., Oeller, P.W., Liscum, E., Han, I.-S., Larsen, E., and Briggs, W.R. (1997). Arabidopsis NPH1: A protein kinase with a putative redox-sensing domain. Science 278, 2120–2123. [DOI] [PubMed] [Google Scholar]
- Hwang, J.-U., Suh, H., Yi, J., and Lee, Y. (1997). Actin filaments modulate both stomatal opening and inward K+-channel activities in guard cells of Vicia faba L. Plant Physiol. 115, 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino, M. (2001). Phototropism in higher plants. In Photomovement, D.-P Häder and M. Lebert, eds (New York: Elsevier Science), pp. 659–811.
- Jarillo, J.A., Ahmad, M., and Cashmore, A.R. (1998). NPL1 (accession No. AF053941): A second member of the NPH serine/threonine kinase family of Arabidopsis. Plant Physiol. 117, 719. [Google Scholar]
- Jarillo, J.A., Gabrys, H., Capel, J., Alonso, J.M., Ecker, J.R., and Cashmore, A.R. (2001). Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 410, 952–954. [DOI] [PubMed] [Google Scholar]
- Kadota, A., and Wada, M. (1992). Photoorientation of chloroplasts in protonemal cells of the fern Adiantum as analysed by use of a video-tracking system. Bot. Mag. Tokyo 105, 265–279. [Google Scholar]
- Kagawa, T., Sakai, T., Suetsugu, N., Oikawa, K., Ishiguro, S., Kato, T., Tabata, S., Okada, K., and Wada, M. (2001). Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high-light avoidance response. Science 291, 2138–2141. [DOI] [PubMed] [Google Scholar]
- Kagawa, T., and Wada, M. (1996). Phytochrome- and blue-light-absorbing pigment-mediated directional movement of chloroplasts in dark-adapted cells of the fern Adiantum as analyzed by microbeam irradiation. Planta 198, 488–493. [Google Scholar]
- Kagawa, T., and Wada, M. (2000). Blue light-induced chloroplast relocation in Arabidopsis thaliana as analyzed by microbeam irradiation. Plant Cell Physiol. 41, 84–93. [DOI] [PubMed] [Google Scholar]
- Kinoshita, T., Doi, M., Suetsugu, N., Kagawa, T., Wada, M., and Shimizaki, K.-I. (2002). phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414, 656–660. [DOI] [PubMed] [Google Scholar]
- Kinoshita, T., and Shimizaki, K.-I. (1999). Blue light activated the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J. 18, 5548–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller, D. (2000). Plants in search of sunlight. Adv. Bot. Res. 33, 36–131. [Google Scholar]
- Koller, D., Ritter, S., Briggs, W.R., and Schäfer, E. (1990). Action dichroism in perception of vectorial photo-excitation in the solar-tracking leaf of Lavatera cretica L. Planta 181, 184–190. [DOI] [PubMed] [Google Scholar]
- Lacève, G., Leymarie, J., Olney, M.O., Liscum, E., Christie, J.M., Vavasseur, A., and Briggs, W.R. (1999). Arabidopsis contains at least four independent blue-light-activated signal transduction pathways. Plant Physiol. 120, 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, A., Guan, C., Gälweiler, L., Tänzler, P., Huijser, P., Marchant, A. Parry, G., Bennett, M., Wisman, E., and Palme, K. (1998). AtPIN1 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 17, 6903–6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Nagy, F., Kircher, S., and Schäfer, E. (2001). Intracellular trafficking of photoreceptors during light-induced signal transduction in plants. J. Cell Sci. 114, 475–480. [DOI] [PubMed] [Google Scholar]
- Palmer, J.M., Short, T.W., Gallagher, S., and Briggs, W.R. (1993). Blue light-induced phosphorylation of a plasma membrane-associated protein in Zea mays L. Plant Physiol. 102, 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai, T., Kagawa, T., Kasahara, M., Swartz, T.E., Christie, J.M., Briggs, W.R., Wada, M., and Okada, K. (2001). Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc. Natl. Acad. Sci. USA 98, 6969–6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon, M., Christie, J.M., Knieb, E., Lempert, U., and Briggs, W.R. (2000). Photochemical and mutational analysis of the FMN binding domains of the plant blue light receptor, phototropin. Biochemistry 39, 9401–9410. [DOI] [PubMed] [Google Scholar]
- Salomon, M., Eisenreich, W., Dürr, H., Schleicher, E. Knieb, E., Massey, V., Müller, F., Bacher, A., and Richter, G. (2001). An optomechanical transducer in the blue light receptor phototropin from Avena sativa. Proc. Natl. Acad. Sci. USA 98, 12357–12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon, M., Zacherl, M., and Rüdiger, W. (1996). Changes in blue-light-dependent protein phosphorylation during the early development of etiolated seedlings. Planta 199, 336–342. [DOI] [PubMed] [Google Scholar]
- Sato, Y., Wada, M., and Kadota, A. (2001). Choice of tracks, microtubules and/or actin filaments for chloroplast photo-movement is differentially controlled by phytochrome and a blue light receptor. J. Cell Sci. 114, 269–279. [DOI] [PubMed] [Google Scholar]
- Schroeder, J.I., Allen, G.J., Hugouvieux, V., Kwak, J.M., and Waner, D. (2001). Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 627–658. [DOI] [PubMed] [Google Scholar]
- Sharma, V.K., Jain, P.K., Maheshwari, S., and Khurana, J.P. (1997). Rapid blue-light-induced phosphorylation of plasma-membrane-associated proteins in wheat. Phytochemistry 44, 775–780. [Google Scholar]
- Short, T.W., and Briggs, W.R. (1990). Characterization of a rapid, blue light-mediated change in detectable phosphorylation of a plasma membrane protein from etiolated pea (Pisum sativum L.) seedlings. Plant Physiol. 92, 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short, T.W., Reymond, P., and Briggs, W.R. (1993). A pea plasma membrane protein exhibiting blue light-induced phosphorylation retains photosensitivity following Triton solubilization. Plant Physiol. 101, 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann, T., Geldner, N., Grebe, M., Mangold, S., Jackson, C.L., Paris, S., Gälweiler, L., Palme, K., and Jürgens, G. (1999). Coordinated polar localization of the auxin efflux carrier PIN1 by GNOM AFR GEF. Science 286, 316–318. [DOI] [PubMed] [Google Scholar]
- Swarup, R., Friml, J., Marchant, A., Ljung, K., Sandberg, K., Palme, K., and Bennett, M. (2001). Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev. 15, 2648–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, B.T., and Zhulin, I.B. (1999). PAS domains: Internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 63, 479–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlalka, M., and Gabrys, H. (1993). Influence of calcium on blue-light-induced chloroplast movement in Lemna trisulca L. Planta 189, 491–498. [Google Scholar]
- Van Volkenburgh, E. (1999). Leaf expansion: An integrating plant behavior. Plant Cell Environ. 22, 1463–1473. [Google Scholar]
- Van Volkenburgh, E., and Cleland, R.E. (1990). Light-stimulated cell expansion in bean (Phaseolus vulgaris L.) leaves. I. Growth can occur without photosynthesis. Planta 182, 72–76. [DOI] [PubMed] [Google Scholar]
- Van Volkenburgh, E., Cleland, R.E., and Watanabe, M. (1990). Light-stimulated cell expansion in bean (Phaseolus vulgaris L.) leaves. II. Quantity and quality of light required. Planta 182, 77–80. [DOI] [PubMed] [Google Scholar]
- Voerkel, S. (1934). Untersuchungen über de phototaxis der Chloroplasten. Planta 21, 156–205. [Google Scholar]
- Yatsuhashi, H., Kadota, A., and Wada, M. (1985). Blue- and red-light action in photoorientation of chloroplasts of Adiantum protonemata. Planta 165, 43–50. [DOI] [PubMed] [Google Scholar]
- Yin, H.C. (1938). Diaphototropic movement of leaves in Malva neglecta. Am. J. Bot. 25, 1–6. [Google Scholar]
- Zeiger, E., and Hepler, P.K. (1977). Light and stomatal function: Blue light stimulates swelling of guard cell protoplasts. Science 196, 887–889. [DOI] [PubMed] [Google Scholar]