Abstract
During lysogenization of myxophage Mx8, phage DNA can be integrated into the attB site of the Myxococcus xanthus chromosome through site-specific recombination. We previously demonstrated that the Mx8 attP site is located within the coding sequence of the Mx8 intP gene. Hence, the integration of Mx8 into the M. xanthus chromosome results in the conversion of the 112-amino-acid C-terminal segment of the IntP protein into a 13-amino-acid C-terminal segment of a new protein, IntR. To examine whether IntR is active for Mx8 excision, we have constructed a series of plasmids carrying various lengths of the intP-attP or intR-attR regions as well as the lacZ gene. The integrated Mx8 was excised at a high frequency, indicating that IntR is active for the excision. For Mx8 excision, a gene designated xis was shown to be required in addition to intR.
During lysogenization of most temperate phages, their genomic DNA is integrated into the specific attachment site of the host chromosome by a mechanism originally proposed by Campbell (2). For example, phage λ is integrated into the attB site of the Escherichia coli chromosome by conservative site-specific recombination between the λ attP site and host attB site (1). The product of the λ int gene and the host integration host factor protein are required for integration. Upon induction of the SOS response λ phage is excised from the host chromosome. In this case the product of the λ xis gene is also required in addition to λ Int and integration host factor. On the λ genome, the xis and int genes overlap by 23 nucleotides and the attP site follows the int gene.
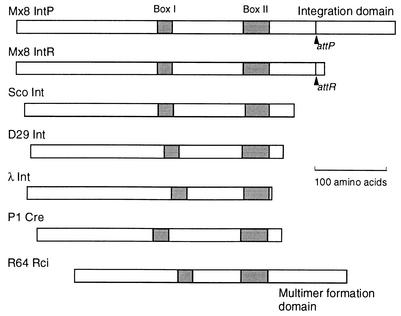
While the various site-specific recombinases of the integrase family have evolved diversely, all carry the conserved box I and box II motifs (13, 18) (see Fig. 5). An arginine residue in box I and histidine, arginine, and tyrosine residues in box II are completely conserved, and they form an active center for phosphotransfer reaction. The conserved tyrosine residue in box II forms a phosphodiester bond with the 3′ end of the recombining DNA. The processes of site-specific recombination were analyzed by X-ray crystallography for the Cre-loxP system (6-8). The loxP Holliday junction intermediate bound to four Cre molecules has been demonstrated.
FIG. 5.
Specific functions of the C-terminal domains of Mx8 IntP and R64 Rci recombinases. Structures of seven site-specific recombinases of the integrase family are compared. Sco, S. coelicolor. Only smaller forms of Mx8 IntP and IntR are illustrated. Locations of the box I and box II motifs in each recombinase are indicated by stippled bars. Locations of the attP and attR sites in Mx8 IntP and IntR, respectively, are also indicated. Only Mx8 IntP and R64 Rci carry additional C-terminal domains with specific functions.
Myxococcus xanthus is a unique gram-negative bacterium that can undergo multicellular development involving cell-to-cell interactions (for a review, see reference 4). When depleted of nutrients, cells on a solid medium aggregate to form mounds, which then convert to fruiting bodies. Rod-shaped vegetative cells change to round or ovoid myxospores. Myxophage Mx8 is a generalized transducing phage of M. xanthus (17, 23). Mx8 can be integrated into the M. xanthus chromosome by site-specific recombination during lysogeny (19). This recombination system has been used to introduce recombinant plasmids into the M. xanthus chromosome (22).
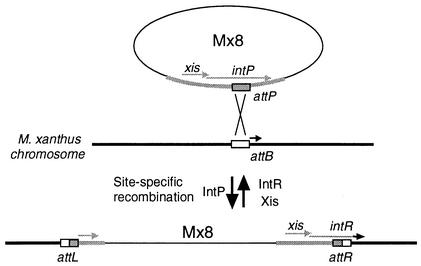
In a previous study (25), we have investigated the mechanism of Mx8 integration into the M. xanthus chromosome. The Mx8 attP site, M. xanthus chromosome attB site, and attL and attR phage-host junctions were cloned and sequenced. Based on sequence alignments of the attP, attB, attL, and attR sites, a strictly conserved 29-bp sequence was identified among the four sequences. The intP gene of Mx8 encodes an integrase family site-specific recombinase of 533 amino acid residues. Later it was found that the translation of the Mx8 intP gene can also be initiated by an alternative initiation codon located 123 bp upstream of the initiation codon that we have identified, yielding a 574-amino-acid protein (15, 16). The site-specific recombination system of Mx8 is unique, since the attP site is located within the intP coding sequence, as schematically illustrated in Fig. 1. Therefore, the integration of phage Mx8 into the M. xanthus chromosome attB site results in the conversion of the intP gene to the intR gene. As a result, the 112-residue C-terminal segment of the IntP protein is replaced by a 13-residue sequence of the IntR protein after Mx8 integration. The entire region of the intP gene, including the convertible C-terminal segment, was shown to be essential for the integration of Mx8 into the M. xanthus chromosome (25).
FIG. 1.
Schematic illustration of the integration and excision of Mx8 by site-specific recombination. In the Mx8 integration system, the attP site is located within the intP coding sequence (25). The integration of Mx8 into the M. xanthus chromosome results in the conversion of the intP gene to a new gene, intR. As a result of this conversion, the 112-amino-acid C-terminal segment of the IntP protein is replaced by a 13-amino-acid segment of the IntR protein. The IntP protein, including the variable C-terminal segment, is required for Mx8 integration, while the IntR and Xis proteins are required for excision.
It is of great interest to examine whether the intR gene is active for Mx8 excision. In the present work, we analyzed the excision of Mx8 phage from the M. xanthus chromosome. It was found that the intR gene is sufficient for excision and that another gene, designated xis, is required for Mx8 excision.
MATERIALS AND METHODS
Bacteria, phage, and plasmids.
M. xanthus DZF1 sglA1 (12) was used. E. coli JM83 Δ(lac-proAB) rpsL thi ara φ80d lacZΔM15 and JM109 recA1 Δ(lac-proAB) endA1 gyrA96 thi hsdR17 supE44 relA1/F′ traD36 proAB lacIqZΔM15 (26) were used for the construction of various plasmids. P1clr100 Cm was used for the introduction of various plasmids from E. coli into M. xanthus cells (22). The Mx8 TM1 strain was from laboratory stock (25). pMC1403 and pSI1403 (3, 11) were used as vectors. Various segments of intP-attP and intR-attR were obtained from pMP001 and pMP004, respectively (25). pP1inc and pMXL101 (24) were also used.
Culture conditions.
M. xanthus cells were grown in Casitone-yeast extract (CYE) medium (12) at 30°C. Solid medium contained 1.5% Bacto agar (Difco). Kanamycin sulfate (40 μg/ml) or oxytetracycline (6.25 μg/ml) was added for the selection of kanamycin-resistant (Kmr) or tetracycline-resistant (Tcr) M. xanthus cells, respectively. Development of M. xanthus was induced on CF agar plates (10) as described previously (12).
E. coli cells were grown in Luria-Bertani medium (21) at 37°C. Ampicillin (100 μg/ml), tetracycline (12 μg/ml), and kanamycin sulfate (50 μg/ml) were used for the selection of plasmid-harboring cells.
Recombinant DNA techniques.
Construction of plasmids, preparation of plasmid and chromosomal DNAs, transformation, Southern blot hybridization, and other methods for DNA manipulation were performed as described previously (21).
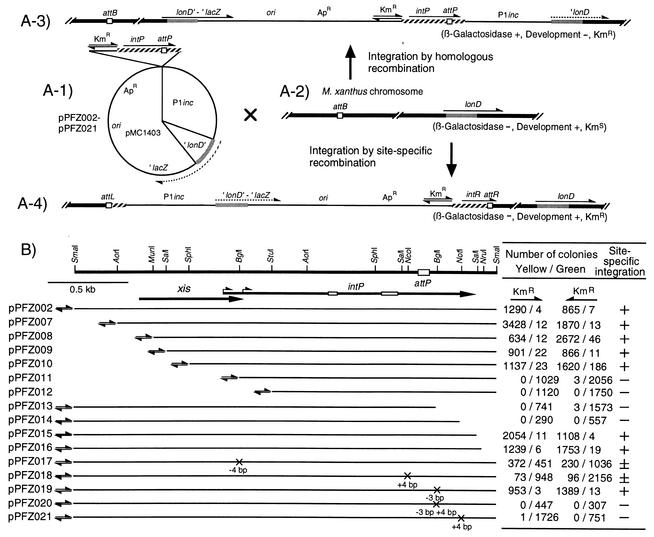
To construct plasmids pPFZ002 through pPFZ021 (Fig. 2A-1), a 1,368-bp AorI-BamHI internal segment of the M. xanthus lonD gene (encoding LonD residues 13 to 468) was first inserted into the SmaI-BamHI site of pMC1403. Next, a 5.4-kb EcoRI-KpnI fragment of the P1 phage incompatibility (P1inc) region was inserted. Finally, various lengths of the Mx8 attP-intP region (Fig. 2B) were excised from pMP001 DNA by using the indicated restriction enzymes and inserted into the resultant plasmid together with a 1.3-kb HindIII-SmaI Kmr fragment of Tn5. The Kmr fragment was inserted at both orientations in relation to the intP gene. pPFZ017, pPFZ018, pPFZ019, pPFZ020, and pPFZ021 carried a 4-bp deletion at the BglI site (position 1123 in the sequence under GenBank accession no. D86464), a 4-bp insertion at the NcoI site (position 2292), a 3-bp deletion at the BglI site (position 2486), replacement of AAC by TGCA at the BglI site (position 2486), and a 4-bp insertion at the NotI site (position 2657), respectively (25).
FIG. 2.
Integration of pPFZ series plasmids into the M. xanthus chromosome by site-specific or homologous recombination. (A-1) Structure of the pPFZ series plasmids containing various lengths of Mx8 intP-attP segments and the ′lonD-lacZ fusion gene without the N-terminal region. (A-2) Chromosomal structures of M. xanthus recipient cells around the attB site and the lonD locus. (A-3 and A-4) M. xanthus chromosomal structure in which the pPFZ series plasmids were integrated into the lonD locus by homologous recombination (A-3) or into the attB site by site-specific recombination (A-4). Hatched bars, Mx8 DNA fragment; open boxes, four att sites; solid bars, M. xanthus chromosome; stippled bars, a portion of lonD coding sequence; solid lines, vector and P1inc sequences; solid arrows, locations and orientations of active genes; dotted arrows, truncated genes. Integration of the pPFZ series plasmids into M. xanthus lonD by homologous recombination (A-3) results in reconstruction of an active lonD-lacZ fusion gene and inactivation of the lonD gene, while integration into attB by site-specific recombination (A-4) keeps the truncated ′lonD-lacZ fusion gene inactive and the lonD gene active. (B) Integration of the pPFZ series plasmids into attB or lonD of the M. xanthus chromosome. At the top, a restriction map of the 2.9-kb SmaI fragment of Mx8 is shown together with the attP core sequence (open box). Short and long arrows represent the coding sequences and orientations of the xis and intP genes, respectively. Two alternative initiation codons for intP are indicated by bent arrows. Open bars on the intP coding sequence represent box I and box II motifs. Solid lines below the map indicate DNA portions present in various pPFZ series plasmids. pPFZ013, pPFZ014, pPFZ018, pPFZ020, and pPFZ021 encode truncated IntP proteins lacking the C-terminal 87, 30, 152, 87, and 30 amino acids, respectively. pPFZ019 encodes modified IntP with conversion of the 446th lysine and 447th leucine (from the second initiation codon) by methionine. The orientations of the Kmr genes are indicated. Crosses on the lines indicate the mutations constructed by modifying various restriction sites. pPFZ series plasmids were introduced into M. xanthus cells by P1 transduction. The numbers of yellow and green Kmr colonies were counted after X-Gal treatment. Ability for site-specific integration was inferred, as follows: +, high frequency; ±, low frequency; −, deficient.
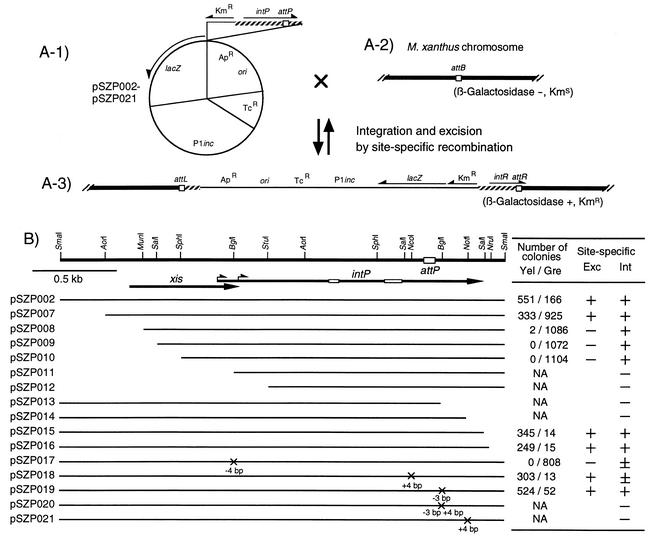
To construct plasmids pSZP002 through pSZP021 (Fig. 3A-1), a 4.5-kb DraI-PvuII segment of pSI1403 (a pMC1403 derivative carrying a translation initiation codon and the Shine-Dalgarno sequence for the lacZ gene) was first replaced by the P1inc fragment and the 1.4-kb EcoRI-BalI Tcr fragment of pBR322. Next, various lengths of the Mx8 attP-intP region with or without mutations (Fig. 3B) and the Tn5 Kmr fragment were inserted into the resultant plasmid.
FIG. 3.
Excision of integrated pSZP series plasmids from the M. xanthus chromosome. (A-1) Structure of the pSZP series plasmids containing a promoterless E. coli lacZ gene and various lengths of the Mx8 intP-attP segments. The lacZ gene is expressed by transcription from the Kmr gene. (A-2) The attB site of the M. xanthus chromosome without integration of the pSZP series plasmids. (A-3) Structure of the M. xanthus chromosome where the pSZP series plasmids were integrated into the attB site by site-specific recombination. (B) Excision of the pSZP series plasmids from the attB site of the M. xanthus chromosome. At the top, a restriction map of the 2.9-kb SmaI fragment of Mx8 is shown together with the attP core sequence (open box). Short and long arrows represent the coding sequences and orientations of the xis and intP genes, respectively. Open bars on the intP coding sequence represent box I and box II motifs. Solid lines below the map indicate DNA portions present in the pSZP series plasmids. Crosses on the line indicate the mutations constructed by modifying various restriction sites. After the pSZP series plasmids integrated into the attB site of the M. xanthus chromosome, their excision from attB was examined on CYE plates without kanamycin. Numbers of yellow (Yel) and green (Gre) colonies were counted after X-Gal treatment. NA, not applicable. Ability for site-specific excision (Exc) was inferred, as follows: +, high frequency; ±, low frequency; −, deficient. Ability for site-specific integration (Int) is also indicated.
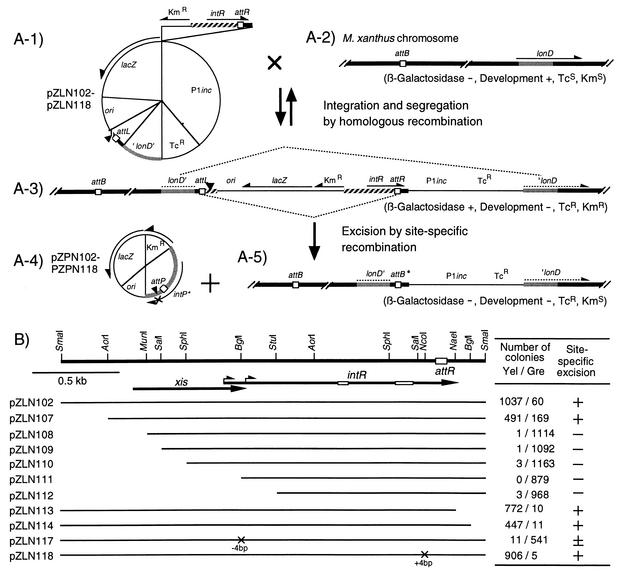
To construct plasmids pZLN002 through pZLN018 (Fig. 4A-1), the 4.5-kb DraI-PvuII segment of pSI1403 was first removed. Next, the 1.13-kb EcoRI-DraI fragment of the resultant plasmid was replaced by the P1inc fragment, the pBR322 Tcr fragment, an M. xanthus lonD internal segment, and a 0.72-kb SalI fragment containing the Mx8 attL region. The NotI site within the intP C-terminal region of attL had been filled in. Finally, various lengths of the Mx8 attR-intR region with or without mutations (Fig. 4B) and the Tn5 Kmr fragment were inserted into the resultant plasmid.
FIG. 4.
Excision of pZPN series plasmids from pZLN series plasmids integrated in the M. xanthus chromosome. (A-1) Structure of the pZLN series plasmids containing a promoterless lacZ gene, the Mx8 attL segment with a 4-bp insertion at the NotI site (solid triangle), a truncated M. xanthus lonD gene, and various lengths of Mx8 intR-attR segments. The lacZ gene is expressed by transcription from the Kmr gene. (A-2) Chromosomal structures of M. xanthus recipient cells around the attB site and the lonD gene. (A-3) Structure of the M. xanthus chromosome where pZLN series plasmids were integrated into the lonD gene by homologous recombination. (A-4) Structure of the pZPN series plasmids produced by excision through site-specific recombination between the attL and attR sites. pZPN series plasmids are deficient in site-specific integration into the M. xanthus chromosome, since they carry a 4-bp insertion at the NotI site which leads to the inactivation of the intP gene (see Fig. 2B). (A-5) Chromosome structure after excision of pZPN series plasmids. attB* represents a newly generated attB site. (B) Excision of pZPN series plasmids from the artificial attB site of the M. xanthus chromosome. At the top, a restriction map of the 2.66-kb SmaI fragment of the Mx8 attR junction is shown with the attR core sequence (open box). Short and long arrows represent the coding sequences and orientations of the xis and intR genes, respectively. Open bars on the intR coding sequence represent box I and box II motifs. Solid lines below the map indicate DNA portions present in various plasmids. Crosses on the line indicate the mutations constructed by modifying various restriction sites. After the pZLN series plasmids integrated into the lonD gene of the M. xanthus chromosome, excision of pZPN series plasmids was examined on CYE plates without kanamycin. Numbers of yellow (Yel) and green (Gre) colonies were counted after X-Gal treatment. Ability of site-specific excision was inferred, as follows: +, high frequency; ±, low frequency; −, deficient.
Assay for integration and excision activity of various lengths of intP-attP or intR-attR segments with or without mutations.
The pPFZ series plasmids were transferred from E. coli into M. xanthus cells by P1 transduction to compare their frequencies of integration into the lonD locus or into the attB site of the chromosome. M. xanthus cells infected with P1 transducing phages were plated on CYE agar plates containing kanamycin and incubated for 5 to 7 days. When Kmr colonies appeared, the plates were overlaid with CYE soft agar containing 100 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal) and incubated for 6 to 12 h. Yellow and green colonies were counted.
To determine the excision frequency, pSZP series plasmids were first introduced into M. xanthus cells by P1 transduction. Integration of pSZP series plasmids into the attB locus was confirmed by Southern blot analysis. M. xanthus cells carrying the pSZP series plasmids were grown overnight in CYE broth without kanamycin and plated on CYE agar without kanamycin. After colonies appeared, the plates were overlaid with CYE soft agar containing X-Gal as described above. Yellow and green colonies were counted.
The excision frequency was further determined by using pZLN series plasmids which carried artificial phage segments flanked by attL and attR sequences. pZLN series plasmids were introduced into M. xanthus cells by P1 transduction. Integration of pZLN series plasmids into the lonD locus was confirmed by Southern blot analysis. M. xanthus cells carrying pZLN series plasmids were grown overnight in CYE broth with oxytetracycline and plated on CYE agar with oxytetracycline. After colonies appeared, the plates were overlaid with CYE soft agar containing X-Gal. Yellow and green colonies were counted.
RESULTS
Site-specific integration of intP-attP segments into the M. xanthus chromosome.
In a previous study (25), we qualitatively determined the integration activities of various intP-attP segments by examining the formation of Kmr colonies when test plasmids carrying the intP-attP region were introduced into M. xanthus cells. To quantitatively estimate the frequency of site-specific integration, pPFZ series plasmids were constructed (Fig. 2A-1). These plasmids carried a 1,368-bp AorI-BamHI internal segment (corresponding to codons 13 to 468) of the M. xanthus lonD gene (24), to which the E. coli lacZ gene was fused in frame. They also carried various intP-attP segments with or without the Kmr promoter. pPFZ series plasmids can be integrated into the M. xanthus chromosome in two ways. First, they can be integrated into the lonD locus of the M. xanthus chromosome by homologous recombination (Fig. 2A-3). In the recombinant M. xanthus cells, the active lonD-lacZ fusion gene, which was previously shown to be expressed during vegetative growth (24), is constructed, whereas the cells are deficient in development. Second, when the intP-attP segment is active for integration, the pPFZ series plasmids can be integrated into the attB site of the M. xanthus chromosome by site-specific recombination (Fig. 2A-4). In this case, the recombinant cells are deficient in β-galactosidase activity but proficient in development. Thus, the frequency of site-specific recombination can be estimated as a relative ratio to that of homologous recombination.
pPFZ series plasmids were introduced into M. xanthus cells from E. coli cells by P1 transduction, and Kmr transductants were selected. Since the pPFZ series plasmids cannot replicate in M. xanthus cells, Kmr recombinants carry pPFZ series plasmids that have integrated into the M. xanthus chromosome by either homologous recombination or site-specific recombination as described above. To determine whether each transductant carries a pPFZ series plasmid at the lonD locus or at the attB site, the β-galactosidase activity of Kmr colonies was determined by pouring soft agar containing X-Gal onto the plate. After a few hours, yellow and green colonies were found. Southern blot analysis indicated that pPFZ002 integrated into the attB site in M. xanthus cells from the yellow colonies, whereas it integrated into the lonD locus in cells from the green colonies. In addition, the cells from the yellow and green colonies were found to be proficient and deficient for development, respectively. The numbers of the yellow and green colonies were counted (Fig. 2B). In pPFZ002 to pPFZ010, pPFZ015, and pPFZ016, yellow colonies were 300- to 9-fold more numerous than green colonies, indicating that these plasmids integrated predominantly into the attB site of the M. xanthus chromosome by site-specific recombination. Plasmids pPFZ011 through pPFZ014 produced practically no yellow colonies, indicating that these plasmids integrated only into the lonD locus by homologous recombination. Frameshift mutations within the intP coding sequence weakly or strongly reduced the activity for site-specific recombination, while pPFZ019, encoding IntP with one amino acid deleted, integrated predominantly into the attB site. The direction of the external Kmr promoter did not affect the integration frequencies. These results are consistent with our previous observation that the entire IntP protein, including the convertible C-terminal segment, is required for site-specific integration of Mx8 (25).
Site-specific excision of the integrated plasmids from the M. xanthus chromosome.
To examine the excision activity of Mx8, pSZP series plasmids were constructed (Fig. 3A-1). These plasmids carried various intP-attP segments as well as an intact lacZ gene transcribed from the Kmr promoter. Hence, M. xanthus cells carrying integrated pSZP series plasmids produce β-galactosidase (Fig. 3A-3). If pSZP series plasmids are excised from the chromosome of M. xanthus cells, however, M. xanthus cells become deficient in β-galactosidase activity.
pSZP series plasmids were introduced into M. xanthus cells by P1 transduction, and Kmr transductants were selected. Plasmids pSZP002 through pSZP010 as well as pSZP015 and pSZP016 were integrated into the attB site of the M. xanthus chromosome at high frequencies, while pSZP017 and pSZP018 were integrated at low frequencies (Fig. 3B). In contrast, the remaining pSZP series plasmids could not be integrated into the M. xanthus chromosome. These results for integration activity are consistent with the results in Fig. 2B. M. xanthus cells carrying integrated pSZP series plasmids were grown overnight in CYE medium without kanamycin and plated on a CYE plate. After colonies formed, β-galactosidase activity was determined as described above (Fig. 3B). From M. xanthus cells carrying integrated pSZP002, pSZP007, pSZP015, pSZP016, pSZP018, and pSZP019 plasmids, yellow colonies appeared at high frequencies. In contrast, from M. xanthus cells carrying integrated pSZP008, pSZP009, pSZP010, and pSZP017 plasmids, practically no yellow colonies appeared. These results indicate that some pSZP series plasmids were excised from the M. xanthus chromosome at a high frequency, while the other pSZP series plasmids were not. pSZP008, pSZP009, pSZP010, and pSZP017 integrated into the attB site of the M. xanthus chromosome but could not be excised, suggesting that a gene designated xis (Fig. 3B) is required for the excision of Mx8. The Mx8 xis gene may start from a GTG initiation codon located at the nucleotide 436 of the previously described sequence (GenBank accession no. D86464), and it encodes a 241-amino-acid basic protein with a calculated molecular weight of 25,324.
To further examine Mx8 excision, pZLN series plasmids were constructed (Fig. 4A-1). These plasmids contained artificial Mx8 segments flanked by the attL and attR sites, in which the intact lacZ gene transcribed from the Kmr promoter was inserted. To exclude the possibility of reintegration of the excised DNA element, the NotI site within the coding sequence for the IntP C-terminal segment was filled in by DNA polymerase I Klenow fragment. pZLN series plasmids carried various lengths of intR-attR segments (Fig. 4B). They can be integrated into the lonD locus of the M. xanthus chromosome by homologous recombination (Fig. 4A-3). The entire plasmid or a portion of the integrated pZLN series plasmids can be excised from the M. xanthus chromosome in two ways. First, the entire pZLN series plasmids can be excised from the M. xanthus chromosome by homologous recombination (Fig. 4A-1). Second, since the DNA segment flanked by the attL and attR sites constitutes an artificial Mx8 phage, the pZPN series plasmids (Fig. 4A-4) can be excised from the M. xanthus chromosome by the Mx8 site-specific recombination system when the intR segment is active for excision. In this case, a portion of the pZLN series plasmids, including the Tcr gene, remained on the M. xanthus chromosome (Fig. 4A-5). The excised pZPN series plasmids cannot be reintegrated into the attB site of the M. xanthus chromosome by site-specific recombination, since the reconstituted intP gene carries a frameshift mutation at the NotI site.
pZLN series plasmids were introduced into M. xanthus cells by P1 transduction, and Kmr transductants were selected. When M. xanthus cells carrying the integrated pZLN series plasmids were grown in CYE medium without antibiotics, very few segregants exhibiting tetracycline sensitivity were produced, indicating that the frequency of segregation of pZLN series plasmids by homologous recombination is very low. M. xanthus cells carrying integrated pZLN series plasmids were grown overnight in CYE medium with tetracycline and plated on a CYE plate with tetracycline. After colonies appeared, their β-galactosidase activities were determined as described above (Fig. 4B). From M. xanthus cells carrying integrated pZLN102, pZLN107, pZLN113, pZLNP114, and pZLN118 plasmids, yellow colonies appeared at high frequencies, whereas from those carrying pZLN117, yellow colonies appeared at a very low frequency. The plasmid DNA fraction was extracted from M. xanthus cells carrying integrated pZLN102, pZLN107, pZLN113, pZLNP114, and pZLN118 plasmids and was used to transform E. coli cells. From transformed E. coli cells, pZPN series plasmids (Fig. 4A-4) were recovered, indicating that DNA recombination between the attL and attR sites of the integrated pZLN series plasmids had occurred. In contrast, from M. xanthus cells carrying integrated pZLN108, pZLN109, pZLN110, pZLN111, and pZLN112, practically no yellow colonies appeared. These results confirm that the pZPN series plasmids are excised from the M. xanthus chromosome by site-specific recombination at a high frequency and that the xis gene is required for Mx8 excision.
DISCUSSION
In the present study, we have determined the frequencies of site-specific integration and excision of myxophage Mx8 into and from the attB site of the M. xanthus chromosome. Construction of pPFZ series plasmids enabled us to estimate the ability for site-specific integration of various lengths of intP-attP segments as a frequency relative to that of homologous recombination. The frequency of Mx8 integration was found to be up to 300-fold higher than that of homologous recombination. Higher frequencies of Mx8 integration have been reported previously (5, 22). With one exception, the results of the present study are in accord with those of our previous study (25), in which site-specific integration was determined by the formation of M. xanthus Kmr colonies after P1 transduction.
The result for pPFZ018 was unexpected: pPFZ018 exhibited a low level of site-specific integration, while mutants producing longer IntP fragments (pPFZ013, pPFZ014, pPFZ020, and pPFZ021) exhibited no site-specific integration. Low site-specific integration of pPFZ018 may be explained as follows. The Mx8 IntP protein consists of two segments, the N-terminal integrase segment and the C-terminal integration accessory domain, which are linked around the NcoI site in which the frameshift mutation was introduced in pPFZ018 (Fig. 5). The Mx8 IntP N-terminal integrase segment corresponds to the entire sequence of most integrases of other phages. The 381-amino-acid N-terminal segment (from the second initiation codon up to the NcoI site) of Mx8 IntP shares 18 to 20% sequence identity with the entire integrases of Streptomyces coelicolor, Bacillus subtilis, and phage D29 and contains the box I and box II motifs conserved within the integrase family of site-specific recombinases (Fig. 5). The IntP C-terminal domain is specific for Mx8 among phage integrases and was shown to be required for integration but not for excision in the present study. In pPFZ018, the translation of the truncated IntP protein stops with eight additional amino acids following the NcoI site. This protein may exhibit full excision activity and low integration activity. In pPFZ013, pPFZ014, pPFZ020, and pPFZ021, however, the truncated IntP proteins carry fragments of the C-terminal domain, which may disturb Mx8 integration but not excision.
While the presence of an additional C-terminal domain is specific for Mx8 IntP among the various phage integrases, an additional C-terminal domain has also been identified in the shufflon-specific Rci recombinase, which belongs to the integrase family of site-specific recombinases (13, 14). Plasmid R64 bears a multiple inversion system designated shufflon, in which four DNA segments invert independently and in groups by the function of Rci. Shufflon-specific recombination sites (sfx), consisting of a central 7-bp spacer sequence and left and right 12-bp arms, were shown to be asymmetric: only the spacer sequence and right arm sequence are conserved among various R64 sfxs, whereas the left arm sequences are not conserved and are not related to the right arm sequence (9). Two Rci molecules are thought to bind to the sfx right and left arms in sequence-specific and non-sequence-specific manners, respectively. The Rci C-terminal domain is suggested to play a key role in non-sequence-specific Rci binding to the sfx left arm (9).
The present experiments using the pSZP series and pZLN series plasmids revealed that the integrated Mx8 is excised from the M. xanthus chromosome at a high frequency. For the Mx8 excision, IntP was not required and IntR was sufficient. The xis gene was required for Mx8 excision, as in the case of other phage integration-excision systems (1). The high frequency of Mx8 excision was unexpected, since Mx8 integration into the M. xanthus chromosome has been reported to be stable (5, 22). One possible reason for this discrepancy is that the Mx8 TM1 strain used in the present experiments happened to carry a mutation. Salmi et al. (20) independently sequenced the Mx8 int region and found that the Mx8 TM1 strain carries a 89-bp deletion just upstream of the xis gene in comparison to wild-type Mx8. A good Shine-Dalgarno sequence, GAGGT, was formed 5 bp upstream of the putative initiation codon of the xis gene as a result of the 89-bp deletion in the Mx8 TM1 strain. In the wild-type Mx8 strain, the corresponding Shine-Dalgarno sequence, GGGGT, is poor. Higher expression of the xis gene may increase the excision frequency in Mx8 TM1.
Acknowledgments
We are grateful to K. Takayama for critical reading of the manuscript.
This work was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Azaro, M. A., and A. Landy. 2002. λ integrase and λ Int family, p. 118-148. In N. L. Craig, R. Craigie, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. ASM Press, Washington, D.C.
- 2.Campbell, A. M. 1962. Episomes. Adv. Genet. 11:101-145. [Google Scholar]
- 3.Casadaban, M. J., J. Chou, and S. N. Cohen. 1980. In vitro gene fusions that join an enzymatically active β-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translation initiation signals. J. Bacteriol. 143:971-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dworkin, M., and D. Kaiser (ed.). 1993. Myxobacteria II. American Society for Microbiology, Washington, D.C.
- 5.Gill, R. E., and L. J. Shimkets. 1993. Genetic approaches for analysis of myxobacterial behavior, p. 129-155. In M. Dworkin and D. Kaiser (ed.), Myxobacteria II. American Society for Microbiology, Washington, D.C.
- 6.Gopaul, D. N., F. Guo, and G. D. van Duyne. 1998. Structure of the Holliday junction intermediate in Cre-loxP site-specific recombination. EMBO J. 17:4175-4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo, F., D. N. Gopaul, and G. D. van Duyne. 1997. Structure of Cre recombinase complexed with DNA in a site-specific recombination synapse. Nature 389:40-46. [DOI] [PubMed] [Google Scholar]
- 8.Guo, F., D. N. Gopaul, and G. D. van Duyne. 1999. Asymmetric DNA bending in the Cre-loxP site-specific recombination synapse. Proc. Natl. Acad. Sci. USA 96:7143-7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gyohda, A., N. Furuya, N. Kogure, and T. Komano. 2002. Sequence-specific and nonspecific binding of the Rci protein to the asymmetric recombination sites of the R64 shufflon. J. Mol. Biol. 318:975-983. [DOI] [PubMed] [Google Scholar]
- 10.Hagen, D. C., A. P. Bretscher, and D. Kaiser. 1978. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev. Biol. 64:284-296. [DOI] [PubMed] [Google Scholar]
- 11.Horiuchi, T., M. Taoka, T. Isobe, T. Komano, and S. Inouye. 2002. Role of fruA and csgA genes in gene expression during development of Myxococcus xanthus: analysis by two-dimensional gel electrophoresis. J. Biol. Chem. 277:26753-26760. [DOI] [PubMed] [Google Scholar]
- 12.Inouye, M., S. Inouye, and D. R. Zusman. 1979. Gene expression during development of Myxococcus xanthus: pattern of protein synthesis. Dev. Biol. 68:579-591. [DOI] [PubMed] [Google Scholar]
- 13.Komano, T. 1999. Shufflons: multiple inversion systems and integrons. Annu. Rev. Genet. 33:171-191. [DOI] [PubMed] [Google Scholar]
- 14.Komano, T., A. Kubo, and T. Nisioka. 1987. Shufflon: multi-inversion of four contiguous DNA segments of plasmid R64 creates seven different open reading frames. Nucleic Acids Res. 15:1165-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magrini, V., C. Creighton, and P. Youderian. 1999. Site-specific recombination of temperate Myxococcus xanthus phage Mx8: genetic elements required for integration. J. Bacteriol. 181:4050-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magrini, V., M. L. Storms, and P. Youderian. 1999. Site-specific recombination of temperate Myxococcus xanthus phage Mx8: regulation of integrase activity by reversible, covalent modification. J. Bacteriol. 181:4062-4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin, S., E. Sodergren, T. Masuda, and D. Kaiser. 1978. Systematic isolation of transducing phages for Myxococcus xanthus. Virology 88:44-53. [DOI] [PubMed] [Google Scholar]
- 18.Nunes-Düby, S. E., H. J. Kwon, R. S. Tirumalai, T. Ellenberger, and A. Landy. 1998. Similarities and differences among 105 members of the Int family of site-specific recombinases. Nucleic Acids Res. 26:391-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orndorff, P., E. Stellwag, T. Starich, M. Dworkin, and J. Zissler. 1983. Genetic and physical characterization of lysogeny by bacteriophage MX8 in Myxococcus xanthus. J. Bacteriol. 154:772-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salmi, D., V. Magrini, P. L. Hartzell, and P. Youderian. 1998. Genetic determinants of immunity and integration of temperate Myxococcus xanthus phage Mx8. J. Bacteriol. 180:614-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 22.Shimkets, L. J., and S. J. Asher. 1988. Use of recombination techniques to examine the structure of csg locus of Myxococcus xanthus. Mol. Gen. Genet. 211:63-71. [DOI] [PubMed] [Google Scholar]
- 23.Stellwag, E., J. M. Fink, and J. Zissler. 1985. Physical characterization of the genome of the Myxococcus xanthus bacteriophage MX-8. Mol. Gen. Genet. 199:123-132. [DOI] [PubMed] [Google Scholar]
- 24.Tojo, N., S. Inouye, and T. Komano. 1993. The lonD gene is homologous to the lon gene encoding an ATP-dependent protease and is essential for the development of Myxococcus xanthus. J. Bacteriol. 175:4545-4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tojo, N., K. Sanmiya, H. Sugawara, S. Inouye, and T. Komano. 1996. Integration of bacteriophage Mx8 into the Myxococcus xanthus chromosome causes a structural alteration at the C-terminal region of the IntP protein. J. Bacteriol. 178:4004-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]