Abstract
Despite the identification of 1,000 mutations in the cystic fibrosis gene product CFTR, there remains discordance between CFTR genotype and lung disease phenotype. The study of CFTR, therefore, has expanded beyond its chloride channel activity into other possible functions, such as its role as a regulator of gene expression. Findings indicate that CFTR plays a role in the expression of RANTES in airway epithelia. RANTES is a chemokine that has been implicated in the regulation of mucosal immunity and the pathogenesis of airway inflammatory diseases. Results demonstrate that CFTR triggers RANTES expression via a mechanism that is independent of CFTR's chloride channel activity. Neither pharmacological inhibition of CFTR nor activation of alternative chloride channels, including hClC-2, modulated RANTES expression. Through the use of CFTR disease-associated and truncation mutants, experiments suggest that CFTR-mediated transcription factor activation and RANTES expression require (i) insertion of CFTR into the plasma membrane and (ii) an intact CFTR C-terminal PDZ-interacting domain. Expression of constructs encoding wild-type or dominant-negative forms of the PDZ-binding protein EBP50 suggests that EBP50 may be involved in CFTR-dependent RANTES expression. Together, these data suggest that CFTR modulates gene expression in airway epithelial cells while located in a macromolecular signaling complex at the plasma membrane.
Cystic fibrosis (CF) is a common, lethal, autosomal-recessive genetic disease (reviewed in reference 30) caused by defective function of the cftr gene product, the cystic fibrosis transmembrane conductance regulator (CFTR) (25). The disease is characterized by abnormal chloride transport in all affected tissues, including the lung, pancreas, gastrointestinal tract, liver, sweat glands, and male reproductive ducts. In the lung, the defect in chloride transport is coupled with hyperabsorption of sodium. Together, these transport defects lead to the generation of thick and dehydrated mucus, subsequent chronic bacterial infections, bronchiectasis, and progressive airway destruction. The pulmonary manifestations of CF are responsible for substantial morbidity and more than 90% of CF-related mortality.
To date, approximately 1,000 mutations have been identified in the CFTR protein as reported by the CF Genetic Analysis Consortium (www.genet.sickkid.on.ca/cftr/). Despite classification and analysis of the various CFTR mutations, there remains discordance between CFTR genotype and disease phenotype (14). Specifically, the degree of correlation between CFTR genotype and clinical presentation varies and is the lowest for pulmonary disease. Such discordance has prompted investigators to examine alternative functions of CFTR. CFTR functions as a cyclic AMP (cAMP)-regulated, low-conductance chloride channel. Recent evidence, however, suggests that CFTR may also function as a regulator of other conductances, such as that of the outwardly rectifying chloride channel (ORCC) and the epithelial sodium channel (ENaC) (reviewed in reference 27). Other proposed functions of CFTR include regulation of vesicle trafficking and ATP release. CFTR may function as a chloride channel in intracellular compartments, including the endoplasmic reticulum and the trans-Golgi complex, and participate in the acidification of these compartments to facilitate vesicle trafficking and posttranslational modifications (22, 23). In addition, recent findings indicate that CFTR facilitates the transport of ATP out of an epithelial cell and into the extracellular space (29). Lastly, we and others have demonstrated that CFTR regulates the expression of several genes, including RANTES (32), interleukin 8 (IL-8) (6, 38), IL-10 (2), and inducible nitric oxide synthase (35).
RANTES (regulated upon activation, normal T cell expressed, and presumably secreted) is a chemokine that promotes the transmigration of monocytes, CD45 RO+ memory T lymphocytes, and eosinophils selectively (reviewed in reference 36). It is expressed and secreted by a variety of cell types, such as airway epithelial cells, and has been implicated in the pathogenesis of airway inflammatory diseases, including asthma (36). Importantly, recent evidence indicates that RANTES can enhance antigen-specific mucosal immune responses in mice (12). It should be noted that, with regard to RANTES expression in the CF lung in vivo, Koller and coworkers have observed significantly reduced levels of RANTES protein in the bronchoalveolar lavage fluid of CF patients compared with bronchoalveolar lavage fluid obtained from asthmatics (10). It was reported recently that CF airway epithelial cells express little or no RANTES protein or mRNA compared with their non-CF counterparts (32). In addition, it was demonstrated recently that insertion of wild-type (WT) CFTR into CF airway epithelial cells to correct the CFTR defect restored RANTES expression at the transcriptional level via an NF-κB-dependent mechanism (32). Interestingly, Kube et al. reported that CF airway epithelial cells fail to express RANTES in response to Pseudomonas aeruginosa, the predominant bacterium observed in CF patients (11). Data presented in this report demonstrate that CFTR triggers RANTES expression via a mechanism that is independent of CFTR's chloride channel activity. Interestingly, these results suggest that the C terminus of CFTR, including its PDZ-interacting domain, is necessary for CFTR-dependent RANTES gene expression. Further, our findings suggest that the PDZ-binding protein EBP50 may be involved in CFTR-dependent RANTES expression. Together, these data indicate that CFTR may modulate gene expression in airway epithelial cells while located in a macromolecular complex at the plasma membrane.
MATERIALS AND METHODS
Cell culture.
Experiments employed IB3-1 cells (bronchial; ΔF508/W1282X compound heterozygote; a gift from Pam Zeitlin, Johns Hopkins University, Baltimore, Md.) (44). Cells were grown as unpolarized monolayers on Vitrogen 100 (Collagen Corp., Palo Alto, Calif.)-coated flasks; Vitrogen 100 contains a mixture of collagen types I and IV. Cells were cultured in LHC-8 medium (Biofluids, Inc., Rockville, Md.) containing 5% fetal calf serum, 1% penicillin-streptomycin, and 0.2% amphotericin B (Fungizone). Cells were cultured in the presence and absence of tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) (each at 100 ng/ml; R & D Systems) and/or sodium butyrate (5 mM; Sigma Chemical Co., St. Louis, Mo.) as indicated.
Transient transfection of IB3-1 cells.
IB3-1 cells were transfected transiently with Lipofectamine Plus (Gibco BRL, Grand Island, N.Y.) as described previously (32). Specifically, cells were transfected with pSV-β-galactosidase reporter plasmid (Promega Corp., Madison, Wis.) and various constructs as indicated. The CFTR-encoding constructs utilized were pRSV-WT-CFTR (9) (a gift from Erik M. Schwiebert, University of Alabama at Birmingham), pGFP-WT-CFTR, pGFP-ΔF508, pGFP-G551D, and pGFP-ΔTRL (18) (all pGFP constructs were generous gifts from Bruce A. Stanton, Dartmouth Medical School). The mock control for pRSV-WT-CFTR was pcDNA3.1 while the mock control for all pGFP-CFTR constructs was pEGFP-C1. The RANTES promoter constructs utilized included 1.4R and ΔAP-1 (16) (gifts from Hiro Moriuchi, National Institutes of Health). EBP50-encoding constructs included FLAG-WT-EBP50 (full-length) and FLAG-DN-EBP50 (PDZ1 domain only; amino acids 1 to 97) (8) (gifts from R. Brian Doctor, University of Colorado Health Sciences Center). Transfected cells were analyzed for relative transfection efficiency by the β-Gal reporter assay system (Promega Corp.) according to the manufacturer's protocols.
Chloride efflux analysis of CFTR-mediated chloride transport.
CFTR-mediated chloride transport in transfected IB3-1 cells was monitored via chloride efflux as described previously (39).
Analysis of RANTES protein expression.
RANTES protein expression was analyzed via enzyme-linked immunosorbent assay (ELISA; R & D Systems) as described previously (32).
SPQ analysis of CFTR-mediated chloride transport.
CFTR-mediated chloride transport in transfected IB3-1 cells was also assayed with the halide-quenching dye SPQ [6-methoxy-N-(3-sulfopropyl)quinolinium]. Briefly, transfected cells were loaded with SPQ (Molecular Probes Inc., Eugene, Oreg.) and then mounted in a specially designed perfusion chamber for fluorescence measurements. Excitation of the SPQ dye was stimulated at 340 nm, and the emission wavelength was measured at >410 nm. All functional studies were performed at 37°C. First, cells were bathed in a quenching buffer (NaI; Sigma Chemical Co.) in order to quench SPQ fluorescence; fluorescence was normalized to this baseline fluorescence value. Second, the buffer was switched to a halide-free buffer (NaNO3; Sigma Chemical Co.) to dequench and assess basal chloride transport activity. Third, a cAMP agonist cocktail (200 μM chlorophenylthio [CPT]-cAMP, 10 μM forskolin, 100 μM 3-isobutyl-1-methylxanthine; Sigma Chemical Co.) was added in NaNO3 buffer in order to activate CFTR chloride channels in transfected cells. Lastly, NaI buffer was added to quench as well as wash out the cAMP response.
Immunoprecipitation of CFTR protein.
Transfected IB3-1 cells were monitored for CFTR protein expression via immunoprecipitation with a polyclonal antibody directed against the NBD-1 domain of CFTR (provided by the Gregory Fleming James Cystic Fibrosis Research Center at the University of Alabama at Birmingham) as described previously (24).
Biotinylation of CFTR surface protein.
Cell surface CFTR biotinylation was performed as described previously (24).
EMSAs.
To examine CFTR-mediated effects on NF-κB and AP-1 binding directly, electrophoretic mobility shift assays (EMSAs) were performed. Briefly, nuclear extracts from airway epithelial cells were prepared. Cells were grown in 100-mm-diameter dishes and then were stimulated with or without TNF-α and IFN-γ as indicated. After treatment, cells were washed with cold phosphate-buffered saline, harvested by scraping, and pelleted. Cells were resuspended in 1 ml of buffer A (10 mM KCl, 20 mM HEPES, 1 mM MgCl2, 1 mM dithiothreitol [DTT], 0.4 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM NaF, 1 mM Na3VO4), set on ice for 10 min, and pelleted at 1,000 × g for 10 min at 4°C. Cell pellets were resuspended and lysed in 0.5 ml of buffer A plus 0.1% Nonidet P-40, set on ice for 10 min, and centrifuged at 3,000 × g for 10 min at 4°C. The resulting pellet was resuspended in 1 ml of buffer B (10 mM HEPES, 400 mM KCl, 0.1 mM EDTA, 1 mM MgCl2, 1 mM DTT, 0.4 mM PMSF, 15% glycerol, 1 mM NaF, 1 mM Na3VO4) and set at 4°C for 30 min with constant gentle mixing. Nuclei were then pelleted at 40,000 × g for 30 min, and nuclear extracts were dialyzed for 18 h at 4°C against 1 liter of buffer C (20 mM HEPES, 200 mM KCl, 1 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, 0.4 mM PMSF, 15% glycerol, 1 mM NaF, 1 mM Na3VO4). Nuclear extracts were cleared by centrifugation at 14,000 × g for 15 min at 4°C. EMSA was performed with the following oligonucleotides as probes and/or competitors: the oligonucleotide NF-κB (5′-AGT TGA GGG TTT CCC AGG C-3′; Santa Cruz Biotechnology, Inc., Santa Clarita, Calif.), NF-κB mutant oligonucleotide (5′-AGT TGA GGC TTT CCC AGG C-3′; Santa Cruz Biotechnology, Inc.), AP-1 (5′-CGC TTG ATG ACT CAG CCG GAA-3′), and AP-1 mutant oligonucleotide (5′-CGC TTG ATG ACT TGG CCG GAA-3′). The gel shift reaction mixture was then prepared by incubating 32P-labeled oligonucleotide (250,000 cpm/reaction mixture) with 10 μg of nuclear extract in a volume of 20 μl containing 50 mM NaCl, 1 mM EDTA, 1 mM DTT, 10 mM Tris-Cl (pH 7.5), 5% glycerol, and 1 μg of poly(dI-dC) for 20 min at room temperature. For competition analysis, a molar excess (as indicated) of the indicated unlabeled DNA was included in the initial gel shift reaction mixture. For supershift analysis, 1 μl of antibody (directed against NF-κB subunits: NF-κB1 [p50/p105], NF-κB2 [p52/p100], RelA [p65], RelB, or c-Rel; directed against AP-1 subunits: c-Fos, c-Jun, or ATF2; Santa Cruz Biotechnology, Inc.) was added to the gel shift reaction mixture and then incubated for an additional 45 min at room temperature. Bound and free DNAs were resolved by electrophoresis through a 4% polyacrylamide gel at 190 V in 1× TGE buffer (50 mM Tris-Cl, 380 mM glycine, 2 mM EDTA). Dried gels were processed via autoradiography.
Detection of EBP50 protein via immunoblotting.
For detection of EBP50 protein expression in IB3-1 cells, EBP50-specific immunoblotting was performed. Briefly, whole-cell lysates were generated from IB3-1 cells, electrophoresed (125 μg), and transferred to a polyvinylidene difluoride membrane; a whole-cell lysate generated from kidney tissue (40 μg) was included as a positive control (R. Brian Doctor, University of Colorado Health Sciences Center [7]). The resulting blot was then immunoblotted with a polyclonal antibody directed against EBP50 (a gift from C. Chris Yun, Johns Hopkins University) or an isotype-matched immunoglobulin control and developed via chemiluminescence.
For the detection of FLAG-tagged EBP50 fusion proteins, whole-cell lysates were immunoprecipitated with an anti-FLAG polyclonal antibody (Santa Cruz Biotechnology, Inc.); immunoprecipitates were electrophoresed, transferred to a polyvinylidene difluoride membrane, and immunoblotted with an anti-EBP50 antibody as described above.
Statistical analysis.
Data are expressed as the means ± standard deviations of replicate determinations as indicated. Statistical significance was determined by analysis. A P value of ≤0.05 was considered significant.
RESULTS
WT-CFTR restores RANTES expression in CF airway epithelia in a dose-dependent fashion.
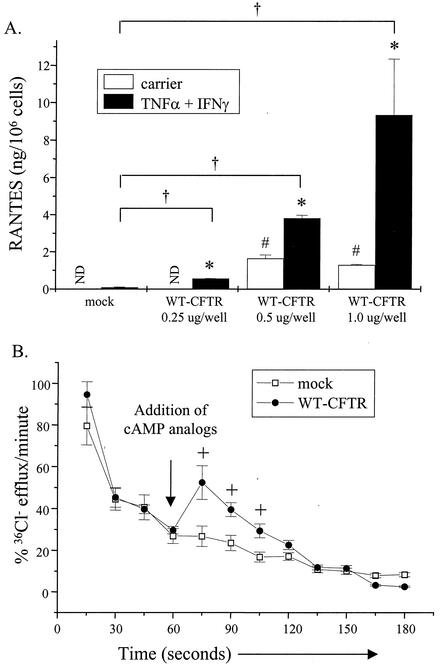
It was reported previously that primary CF airway epithelial cells and cell lines, which lack functional CFTR, do not express RANTES mRNA or protein in the presence or absence of TNF-α and IFN-γ (32). In addition, it was demonstrated that insertion of WT CFTR (WT-CFTR) into CF epithelial cells to correct the CFTR defect restored RANTES mRNA and protein expression (32). To extend these observations, we determined whether WT-CFTR would restore RANTES expression in a dose-dependent manner. Because the previous study demonstrated that WT-CFTR restores RANTES mRNA and protein expression in parallel (32), only protein expression results are presented here. For these experiments, cells of IB3-1, a CF airway epithelial cell line that is heterozygous for the CFTR mutation (W1282X/ΔF508 [44]), were utilized. Briefly, cells were transfected transiently with a construct encoding WT-CFTR or a mock control at increasing concentrations. Cells were then stimulated with and without TNF-α and IFN-γ and analyzed for RANTES protein expression. As shown in Fig. 1A, expression of WT-CFTR in IB3-1 cells restored RANTES expression in a dose-dependent manner. Cells transfected with WT-CFTR and then stimulated with TNF-α and IFN-γ expressed increasing amounts of RANTES protein that ranged between two- and fivefold greater than mock control levels. To ensure that IB3-1 cells complemented with WT-CFTR displayed CFTR-mediated chloride transport activity, transfected cells were monitored for changes in chloride transport via chloride efflux analysis. As shown in Fig. 1B, IB3-1 cells complemented with WT-CFTR displayed an increase in the amount of chloride efflux activity, compared with that of mock controls, in the presence of cAMP nucleotide analogs; neither TNF-α nor IFN-γ had any effect on this response (data not shown). These results indicate that WT-CFTR was expressed and functional in complemented IB3-1 cells.
FIG. 1.
WT-CFTR restores RANTES expression in a dose-dependent manner. (A) IB3-1 cells were transfected with constructs encoding β-galactosidase (pSV-β-galactosidase; 0.5 μg/well) and WT-CFTR (pRSV-WT-CFTR) or a mock control (pcDNA3.1) (each at 0.25, 0.5, and 1.0 μg/well). Cells were then stimulated in the presence and absence of TNF-α and IFN-γ (each at 100 ng/ml) for 18 h at 37°C. Supernatants were harvested and analyzed for RANTES protein expression via ELISA. Results are reported as nanograms per 106 cells (n = 3 to 5; ND, none detected; *, P ≤ 0.05 relative to respective carrier-treated control; #, P ≤ 0.05 relative to carrier-treated mock control; †, P ≤ 0.05 relative to TNF-α-IFN-γ-treated mock control). Results from cells transfected with the mock control at 1.0 μg/well are presented as representative of mock controls at each concentration tested. (B) In parallel, IB3-1 cells transfected with constructs encoding β-galactosidase (0.5 μg/well) and WT-CFTR (pRSV-WT-CFTR) or a mock control (pcDNA3.1) at the 1.0-μg/well concentration were treated with the cAMP analogs 8-bromo-cAMP and CPT-cAMP (each at 250 μM) and then monitored for changes in cAMP-dependent chloride transport via chloride efflux. Results are reported as percentages of 36Cl− efflux (at t0) per minute (n = 3; +, P ≤ 0.05 relative to mock control).
Chloride transport is not required for CFTR-mediated RANTES expression.
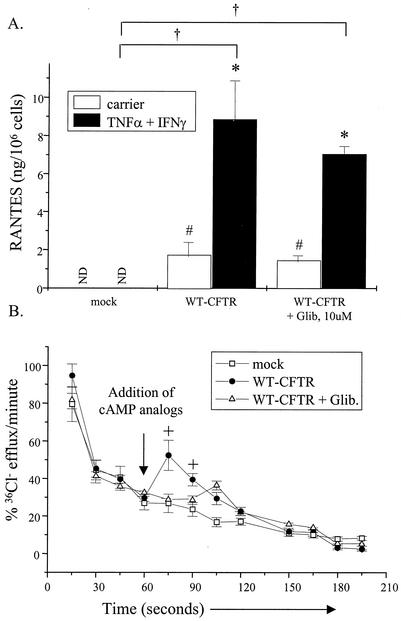
A possible mechanism that CFTR may utilize to restore RANTES expression in airway epithelia is one via its chloride channel activity. Specifically, the activation of CFTR's chloride channel activity may alter cell membrane potential and, in turn, trigger signal transduction events that ultimately stimulate RANTES expression. To determine whether changes in chloride channel activity altered RANTES expression in CF airway epithelia, two approaches were undertaken. First, IB3-1 cells were transfected transiently with constructs encoding WT-CFTR or a mock control. Transfected cells were then cultured with and without TNF-α and IFN-γ in combination with glibenclamide, a nonselective inhibitor of CFTR (31), and monitored for RANTES protein expression. The rationale for this approach was as follows: if CFTR's chloride channel activity was directly responsible for modulating RANTES expression in CF airway epithelia, then selective inhibition of CFTR chloride channel activity in WT-CFTR-expressing cells would inhibit RANTES production in these cells. As demonstrated in Fig. 2A, chronic treatment with glibenclamide did not block WT-CFTR restoration of RANTES protein expression. In contrast, chronic glibenclamide treatment inhibited cAMP-dependent chloride efflux in WT-CFTR-transfected cells (Fig. 2B).
FIG. 2.
CFTR-mediated chloride transport does not play a role in RANTES expression. (A) IB3-1 cells were transfected with constructs encoding β-galactosidase and WT-CFTR or a mock control (each at 1.0 μg/well) as described in the Fig. 1 legend. Following transfection, cells were cultured in the presence and absence of TNF-α and IFN-γ (each at 100 ng/ml) together with or without glibenclamide (10 μM) for 18 h at 37°C. Supernatants were harvested and analyzed for RANTES protein expression via ELISA. Results are reported as nanograms per 106 cells (n = 3; ND, none detected; *, P ≤ 0.05 relative to respective carrier-treated control; #, P ≤ 0.05 relative to carrier-treated mock control; †, P ≤ 0.05 relative to TNF-α-IFN-γ-treated mock control). (B) In parallel, transfected cells cultured in the presence and absence of glibenclamide as described above were treated with the cAMP analogs 8-bromo-cAMP and CPT-cAMP (each at 250 μM) and then monitored for changes in cAMP-dependent chloride transport via chloride efflux. Results are reported as percentages of 36Cl− efflux (at t0) per minute (n = 3; +, P ≤ 0.05 relative to mock control or glibenclamide-treated samples).
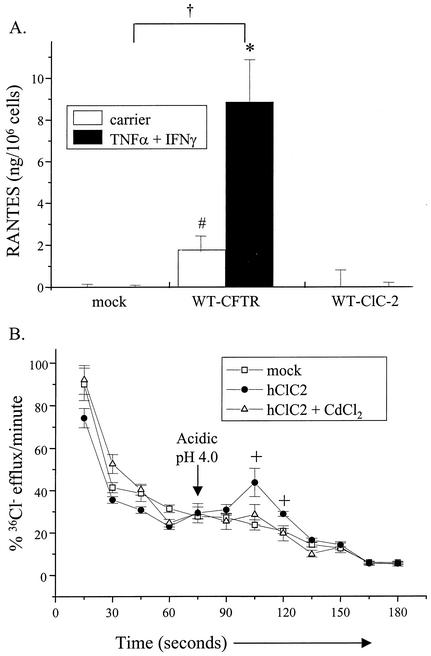
In the second approach, IB3-1 cells were transfected with the constructs encoding WT-CFTR, human ClC-2 (an alternative chloride channel), or a mock control. Transfected cells were then stimulated with and without TNF-α and IFN-γ and monitored for RANTES protein expression. The rationale for this approach was as follows: if increasing chloride channel activity, in general, modulates RANTES expression, then the insertion of another chloride channel such as ClC-2 into CF airway epithelia would increase RANTES production. ClC-2 is a relevant channel for these studies because it is expressed by human and rodent airway epithelial cells (20, 28). Figure 3A demonstrates that, unlike WT-CFTR, ClC-2 did not restore RANTES protein expression either in the presence or in the absence of TNF-α and IFN-γ. To demonstrate that ClC-2 expressed in transfected cells was functional, ClC-2-transfected cells were assessed for ClC-2 activity via external acidic pH-activated chloride efflux. As shown in Fig. 3B, ClC-2-transfected cells displayed an augmented pH-activated chloride efflux that was inhibited by cadmium chloride (CdCl2). This response is similar to that described elsewhere for IB3-1 clones transfected stably with a human ClC-2-encoding construct (28). Schwiebert and coworkers reported that IB3-1 cells overexpressing human ClC2 displayed basal ClC2 activity and that this activity was potentiated with decreased external pH (28). Together, these results suggest that chloride channel activity is not required for CFTR-dependent RANTES expression.
FIG. 3.
Chloride transport, in general, does not play a role in RANTES expression. (A) IB3-1 cells were transfected with constructs encoding β-galactosidase and WT-CFTR, ClC-2 (pRSV-hClC-2; 1.0 μg/well), or a mock control, as described in the Fig. 2 legend. Following transfection, cells were cultured in the presence and absence of TNF-α and IFN-γ and analyzed for RANTES protein expression as described in the Fig. 1 legend. Results are reported as nanograms per 106 cells (n = 3; *, P ≤ 0.05 relative to respective carrier-treated control; #, P ≤ 0.05 relative to carrier-treated mock control; †, P ≤ 0.05 relative to TNF-α-IFN-γ-treated mock control). (B) In parallel, transfected cells were treated with acidic pH (4.0) in the presence and absence of CdCl2 (10 μM) and then monitored for changes in cAMP-dependent chloride transport via chloride efflux as described above. Results are reported as percent 36Cl− efflux per minute (n = 3; +, P ≤ 0.05 relative to mock control or CdCl2-treated samples).
CFTR functional mutants do not facilitate RANTES expression.
To further examine the requirement for chloride channel activity in CFTR-dependent RANTES expression, IB3-1 cells expressing CFTR functional mutants were examined for differences in RANTES protein expression in the presence and absence of cytokines. For these experiments, we took advantage of the ΔF508, G551D, and ΔTRL CFTR mutations, which differ in their levels of chloride transport activity. ΔF508 is the most common mutation observed in CF. It contains a deletion of three nucleotides resulting in a missing phenylalanine at position 508 within the CFTR domain NBD-1. The consequence of this mutation is the expression of a CFTR protein that can conduct chloride but is absent from the plasma membrane due to aberrant intracellular processing and retention within the endoplasmic reticulum (1, 4, 22). In contrast, G551D is a missense mutation within NBD-1 resulting in the conversion of a glycine residue to an aspartic acid residue. Unlike ΔF508, the G551D mutation traffics normally to the plasma membrane; however, it is unable to function as a chloride channel (43). Its lack of chloride channel activity is thought to be the result of its inability to bind and/or hydrolyze ATP within the NBD-1 domain (13). The ΔTRL-CFTR mutant, which lacks the C-terminal PDZ-interacting domain, encodes a functional CFTR molecule that has been shown elsewhere to mislocalize to the lateral membrane (17).
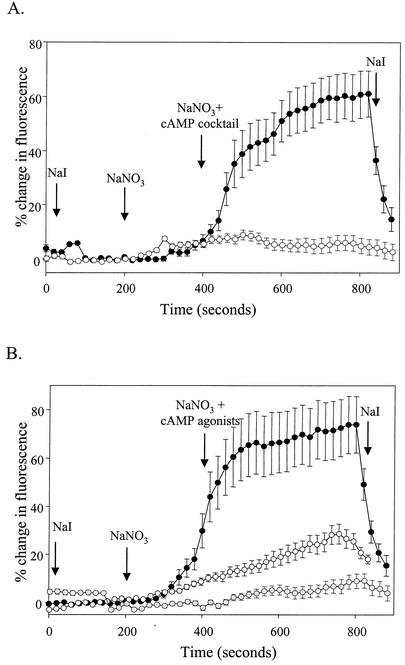
In these experiments, IB3-1 cells were transfected with constructs encoding WT-CFTR, ΔF508-CFTR, G551D-CFTR, ΔTRL-CFTR, or a mock control. Following transfection, cells were cultured in the presence and absence of TNF-α and IFN-γ and then monitored for chloride channel activity (Fig. 4) and RANTES protein expression (Fig. 5). To monitor CFTR-mediated chloride transport activity, transfected cells were assayed with the halide-quenching dye SPQ in the presence and absence of a cAMP agonist cocktail (5). As shown in Fig. 4, the cAMP agonist cocktail stimulated halide efflux in cells expressing WT-CFTR or ΔTRL-CFTR. In sharp contrast, cells expressing ΔF508-CFTR, G551D-CFTR, or the mock control were not responsive to the effects of cAMP agonists. The cytokines TNF-α and IFN-γ had no effect on CFTR-mediated chloride transport activity (data not shown).
FIG. 4.
Functional analysis of WT and mutant CFTR-transfected IB3-1 cells. IB3-1 cells were cotransfected with constructs encoding β-galactosidase (pSV-β-galactosidase; 0.5 μg/well) and mock control (pEGFP-C1; open circles) or WT-CFTR (pGFP-WT-CFTR; closed circles) (A) or G551D-CFTR (pGFP-G551D; gray circles), ΔF508-CFTR (pGFP-ΔF508; open circles), or ΔTRL-CFTR (pGFP-ΔTRL; closed circles) (B) (each at 1.0 μg/well). Following transfection, cells were analyzed for CFTR-mediated chloride transport via SPQ analysis. Results are reported as percent change in SPQ fluorescence relative to basal levels. Representative results from three independent experiments are shown.
FIG. 5.
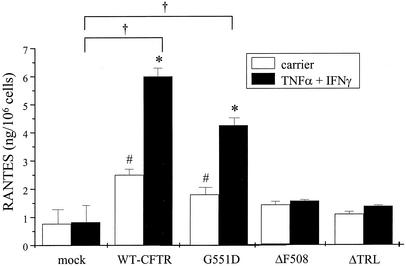
CFTR mutations affect RANTES expression differentially. IB3-1 cells were cotransfected with constructs encoding β-galactosidase and mock control, WT-CFTR, G551D-CFTR, ΔF508-CFTR, or ΔTRL-CFTR as described in the Fig. 4 legend and then cultured with or without TNF-α and IFN-γ as described in the Fig. 1 legend. Following stimulation, supernatants were harvested and analyzed for RANTES protein expression via ELISA. Results are reported as nanograms per 106 cells (n = 3 to 5; *, P ≤ 0.05 relative to respective carrier-treated control; #, P ≤ 0.05 relative to carrier-treated mock control; †, P ≤ 0.05 relative to TNF-α-IFN-γ-treated mock control).
With regard to RANTES protein expression, neither ΔF508-CFTR nor ΔTRL-CFTR restored RANTES expression in either the presence or the absence of cytokines (Fig. 5). In sharp contrast, G551D restored RANTES expression to a level slightly less than but comparable to that observed with WT-CFTR (Fig. 5). Collectively, these results support the findings presented above: namely, that chloride channel activity is not required for CFTR-dependent RANTES expression.
Insertion of CFTR into the plasma membrane is necessary for CFTR-mediated RANTES expression.
A second possible mechanism that CFTR may utilize to modulate RANTES expression is through protein-protein interactions with signaling adapter molecules and the subsequent initiation of signaling pathways that regulate RANTES gene expression. Such protein-protein interactions could facilitate the formation of a CFTR-associated macromolecular signaling complex at the plasma membrane. Because the CFTR mutants examined above have been reported to display different phenotypes with regard to surface expression, we examined the surface expression of each of these CFTR molecules in transfected cells in parallel with their effect on RANTES production shown above (Fig. 5); surface expression was monitored via biotinylation analysis. As shown in Fig. 6, biotinylation analysis revealed that WT-CFTR, G551D-CFTR, and ΔTRL-CFTR were each expressed at the cell surface; WT- and ΔTRL-CFTR surface expression levels were comparable while G551D-CFTR expression was detectable yet significantly less than that of the others. In contrast, ΔF508-CFTR was not detectable at the plasma membrane surface (Fig. 6). Because WT- and G551D-CFTR, but neither ΔTRL- nor ΔF508-CFTR, were able to restore RANTES expression, these results suggest that insertion of an intact CFTR molecule into the plasma membrane is necessary for CFTR-dependent RANTES expression.
FIG. 6.
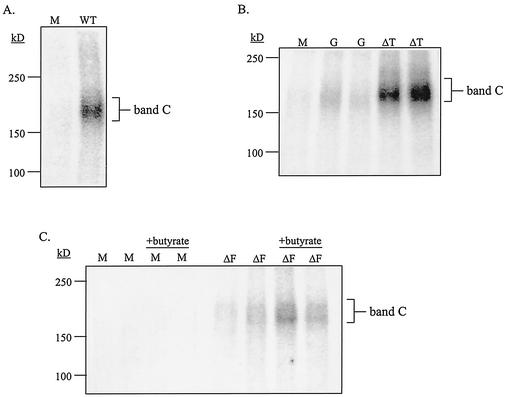
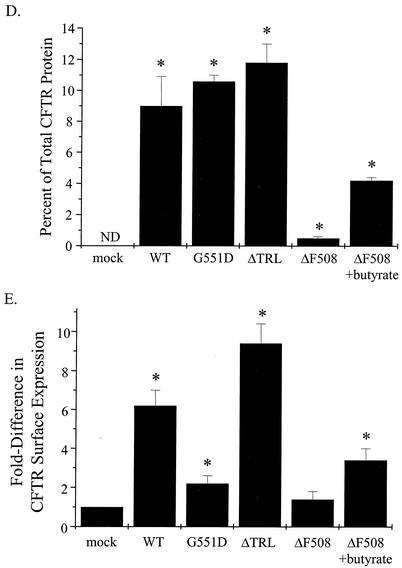
Analysis of surface expression of WT and mutant CFTR proteins. IB3-1 cells were cotransfected with constructs encoding β-galactosidase and mock control (M) or WT-CFTR (A), G551D-CFTR or ΔTRL-CFTR (B), or ΔF508-CFTR (C) as described in the Fig. 4 legend. Cells transfected with the ΔF508-CFTR construct were treated with and without sodium butyrate (5 mM) for 18 h at 37°C. Cells were analyzed for CFTR cell surface expression at 48 h posttransfection by a two-step cell surface periodate-long-chain hydrazide biotinylation procedure as described previously (24). Band C, the mature, fully glycosylated form of CFTR, is indicated; representative blots of biotinylated CFTR proteins are shown. (D) Percentage of CFTR at the cell surface versus total CFTR expressed under steady-state conditions (n = 3 to 5; *, P ≤ 0.05 relative to mock control; ND, none detected). (E) Fold difference in CFTR cell surface expression between WT- and mutant CFTR-expressing cells (n = 3 to 5; *, P ≤ 0.05 relative to mock control).
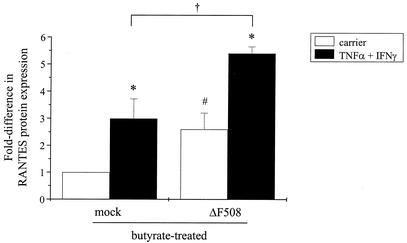
To determine directly whether insertion of CFTR into the plasma membrane was required for CFTR-mediated RANTES expression, we examined the ability of the ΔF508-CFTR processing mutant to restore RANTES protein expression in the presence of sodium butyrate and cytokines. Previous studies have demonstrated that butyrate treatment of cells expressing ΔF508-CFTR facilitates the transport of this mutant CFTR to the plasma membrane (19, 26). For these experiments, cells were transfected with constructs encoding ΔF508-CFTR or a mock control, cultured in the presence of sodium butyrate with and without TNF-α and IFN-γ, and then examined for changes in RANTES protein expression. Surface protein expression of ΔF508-CFTR in the presence of butyrate was confirmed via biotinylation analysis (Fig. 6). Results presented in Fig. 7 demonstrate that, in the presence of TNF-α-IFN-γ, butyrate treatment significantly enhanced RANTES protein expression in cells transfected with a construct encoding ΔF508-CFTR. These findings indicate that, when expressed at the plasma membrane, ΔF508-CFTR can restore RANTES production in the presence of cytokines. Moreover, these findings support our observation that insertion of CFTR into the plasma membrane is required for CFTR-mediated RANTES expression.
FIG. 7.
Butyrate treatment enhances RANTES protein expression in cells expressing ΔF508-CFTR. IB3-1 cells were cotransfected with constructs encoding β-galactosidase and ΔF508-CFTR or a mock control and then treated with sodium butyrate (5 mM) in the presence and absence of TNF-α-IFN-γ (each at 100 ng/ml) for 18 h at 37°C. Following treatment, supernatants were harvested and examined for changes in RANTES protein expression via ELISA. Results are reported as nanograms per 106 cells (n = 3 to 5; *, P ≤ 0.05 relative to respective carrier-treated control; #, P ≤ 0.05 relative to carrier-treated mock control; †, P ≤ 0.05 relative to TNF-α-IFN-γ-treated mock control).
The PDZ-binding protein EBP50 may be involved in CFTR-dependent RANTES expression.
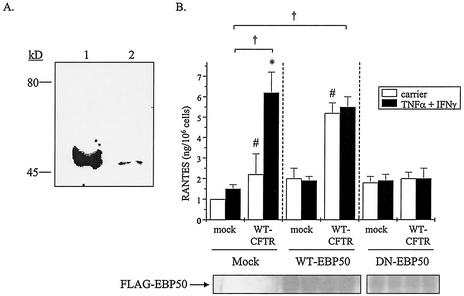
CFTR has been shown elsewhere to bind to PDZ-binding proteins, including EBP50, through its C-terminal PDZ-interacting domain (T-R-L) (17, 34). Because ΔTRL-CFTR did not restore RANTES expression in the presence of cytokines (Fig. 5), despite being inserted into the plasma membrane (Fig. 6), we examined the role of EBP50 in this response. First, EBP50 expression was examined in airway epithelial cells via immunoblotting with an antibody specific for EBP50 (7). In IB3-1 cells, the anti-EBP50 antibody detected a band of approximately 50 kDa that correlated with the positive control (Fig. 8A); the immunoglobulin control displayed no cross-reactivity (data not shown).
FIG. 8.
EBP50 may play a role in CFTR-dependent RANTES expression. (A) Whole-cell lysates were prepared from IB3-1 cells and analyzed for EBP50 protein expression via immunoblotting. Lane 1, kidney tissue lysate, positive control; lane 2, IB3-1 cell lysate. Representative results from three separate experiments are shown. (B) IB3-1 cells were transfected with constructs that encoded β-galactosidase, WT-CFTR, or a mock control (as described in the Fig. 2 legend) together with mock, WT-EBP50, or DN-EBP50 constructs (each at 1.0 μg/well). Following transfection, cells were cultured in the presence and absence of TNF-α-IFN-γ as described in the Fig. 1 legend, and supernatants were analyzed via ELISA. Cells were harvested and monitored for exogenous epitope (FLAG)-tagged EBP50 expression via immunoprecipitation and chemiluminescence analysis. Results are reported as nanograms per 106 cells (n = 3; *, P ≤ 0.05 relative to respective carrier-treated control; #, P ≤ 0.05 relative to carrier-treated mock control; †, P ≤ 0.05 relative to TNF-α-IFN-γ-treated mock control).
To examine the possible role of EBP50 in CFTR-dependent RANTES expression, IB3-1 cells were cotransfected with constructs encoding WT-CFTR or a mock control together with constructs encoding epitope-tagged WT or dominant-negative (DN) forms of EBP50. Molecular analysis has revealed that full-length EBP50 contains two PDZ-binding domains (PDZ-1 and PDZ-2). The WT-EBP50 construct encoded the full-length EBP50 molecule while the DN-EBP50 construct encoded the PDZ-1-binding domain only. Because DN-EBP50 contains the PDZ-1 motif only, it can bind to the C terminus of CFTR but not to downstream proteins, such as YAP-65, that trigger signal transduction events; such events are mediated via the PDZ-2-binding domain (15). Following transfection, cells were cultured in the presence and absence of TNF-α and IFN-γ and then analyzed for secreted RANTES protein expression; samples were also examined for relative expression of each epitope-tagged EBP50 molecule. As presented in Fig. 8B, expression of either WT- or DN-EBP50 had no effect on RANTES expression in mock-transfected cells. In contrast, cells that were transfected with WT-CFTR and WT-EBP50 constructs displayed enhanced RANTES protein expression in the absence of TNF-α-IFN-γ; expression appeared to be maximal since these cells were no longer responsive to the effects of TNF-α-IFN-γ. Cells transfected with WT-CFTR and DN-EBP50, however, expressed RANTES protein levels that were no greater than those of similarly treated mock controls (Fig. 8B). Equivalent exogenous expression levels of WT-EBP50 and DN-EBP50 molecules were detected in respective samples (Fig. 8B). These data suggest that EBP50 may play a role in CFTR-dependent RANTES expression.
Both CFTR and EBP50 appear to play a role in NF-κB activation.
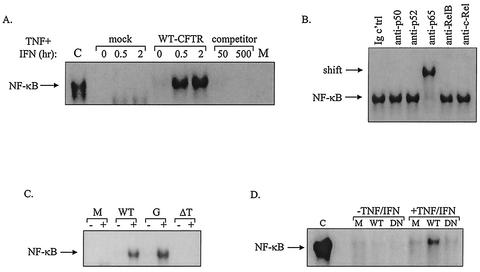
It has been reported previously that, in the presence of TNF-α and IFN-γ, WT-CFTR activates the RANTES promoter via an NF-κB-dependent mechanism (32). To examine directly the effects of WT and mutated forms of CFTR on the binding activity of NF-κB in airway epithelial cells, EMSAs were performed. The EMSA stabilizes DNA-protein interactions, facilitates the measurement of protein DNA-binding affinity, and, through the use of specific antibodies, permits identification of the transcription factor subunits participating in the DNA-protein complex. For these experiments, IB3-1 cells were transfected with constructs encoding WT-CFTR, G551D-CFTR, ΔTRL-CFTR, or a mock control and then cultured in the presence and absence of TNF-α and IFN-γ. Nuclear extracts were prepared, and EMSAs were performed.
Results presented in Fig. 9A demonstrate that IB3-1 cells complemented with WT-CFTR displayed enhanced NF-κB binding in the presence of TNF-α combined with IFN-γ compared with that of similarly treated mock controls. NF-κB binding was confirmed through EMSAs performed with unlabeled (competitor) and mutant NF-κB oligonucleotides. Shift analysis with antibodies directed against the various NF-κB subunits, including NF-κB1 (p50/p105), NF-κB2 (p52/p100), RelA (p65), RelB, and c-Rel, revealed that bound NF-κB complexes consisted solely of p65 subunits (Fig. 9B). As shown in Fig. 9C, cells transfected with constructs encoding G551D-CFTR displayed a pattern of enhanced NF-κB binding that resembled the pattern of NF-κB binding observed for cells transfected with constructs encoding WT-CFTR; in contrast, ΔTRL-CFTR did not promote the binding of NF-κB either in the presence or in the absence of TNF-α and IFN-γ (Fig. 9C).
FIG. 9.
CFTR and EBP50 mutations affect NF-κB binding differentially. (A) IB3-1 cells were cotransfected with constructs encoding β-galactosidase, WT-CFTR, or a mock control as described in the Fig. 4 legend. Following transfection, cells were cultured in the presence and absence of TNF-α and IFN-γ (each at 100 ng/ml) for 30 min or 2 h at 37°C and then prepared for EMSA. EMSAs were performed with a radiolabeled oligonucleotide containing a consensus NF-κB binding sequence. By utilizing cells transfected with constructs encoding β-galactosidase and WT-CFTR and then stimulated for 30 min with TNF-α and IFN-γ, analyses were also performed in the presence and absence of increasing concentrations of cold competitor (unlabeled NF-κB oligonucleotide), ranging from 0- to 500-fold excess, or a mutant NF-κB oligonucleotide (C, positive control, TNF-α-treated 9HTEo epithelial cells; M, mutant probe). (B) EMSAs were performed on cells transfected with constructs encoding β-galactosidase and WT-CFTR and then stimulated for 30 min with TNF-α and IFN-γ with antibodies directed against p50, p52, p65, RelB, and c-Rel NF-κB subunits. (C) IB3-1 cells were transfected with constructs encoding β-galactosidase, WT-CFTR, G551D-CFTR, or ΔTRL-CFTR; stimulated for 30 min with and without TNF-α-IFN-γ (denoted by + and −, respectively); and then prepared for EMSA. M, mock. (D) IB3-1 cells were cotransfected with constructs encoding β-galactosidase, WT-CFTR, and WT-EBP50, DN-EBP50, or a mock (M) control; stimulated for 2 h at 37°C with and without TNF-α-IFN-γ; and then prepared for EMSA. Representative results of three to six independent experiments are shown.
Because the data presented above suggest that EBP50 may be involved in CFTR-dependent RANTES expression, we determined whether EBP50 might also be involved in CFTR-dependent NF-κB activation. For these experiments, IB3-1 cells were transfected with constructs encoding WT-CFTR and WT-EB50, DN-EBP50, or a mock control and then cultured in the presence and absence of TNF-α and IFN-γ. Nuclear extracts were prepared, and EMSAs were performed. As presented in Fig. 9D, cells transfected with WT-CFTR and WT-EBP50 displayed enhanced NF-κB binding in the presence of TNF-α-IFN-γ. In contrast, cells transfected with WT-CFTR and DN-EBP50 displayed a reduction in NF-κB binding, indicating that expression of DN-EBP50 diminished this response (Fig. 9D). Together, these data suggest that both CFTR and EBP50 may be involved in NF-κB activation within CF epithelial cells.
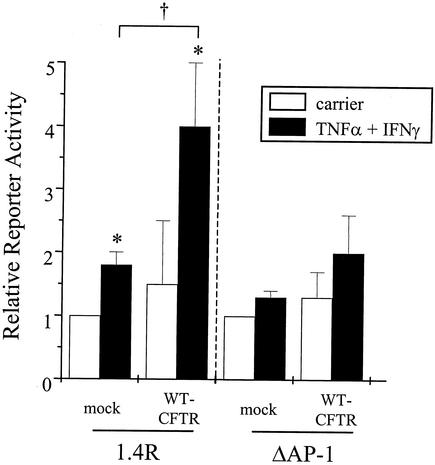
CFTR-mediated activation of the RANTES promoter is dependent upon AP-1.
Because the RANTES promoter is regulated by transcription factors other than NF-κB, including AP-1 (16), we determined whether CFTR-mediated modulation of RANTES promoter activation was also dependent upon AP-1. For these experiments, IB3-1 cells complemented with WT-CFTR were transfected transiently with RANTES promoter constructs fused to a luciferase reporter gene. Briefly, IB3-1 cells were transfected with reporter constructs encoding either an intact 1.4-kb 5′ noncoding sequence of the RANTES gene (1.4R) or a 1.4-kb RANTES promoter construct containing a site-directed mutant AP-1 binding site (ΔAP-1) together with WT-CFTR or mock control; following transfection, cells were cultured in the presence and absence of TNF-α and IFN-γ. As shown in Fig. 10, IB3-1 cells transfected with the 1.4R construct in the absence of WT-CFTR displayed low reporter activity in the presence or absence of TNF-α-IFN-γ. In contrast, cells transfected with the 1.4R construct together with WT-CFTR displayed approximately a fourfold increase in reporter activity in the presence of TNF-α and IFN-γ compared with unstimulated controls (Fig. 10). Importantly, IB3-1 cells transfected with both WT-CFTR and the ΔAP-1 construct were not responsive to the effects of TNF-α-IFN-γ (Fig. 10), indicating that CFTR-mediated activation of the RANTES promoter is dependent upon AP-1.
FIG. 10.
CFTR-mediated RANTES promoter activation is dependent upon AP-1. IB3-1 cells were cotransfected with constructs encoding β-galactosidase and WT-CFTR or a mock control as described in the Fig. 2 legend. In addition, cells were cotransfected with RANTES promoter constructs (1.4R and ΔAP-1; each at 0.5 μg/well) and then cultured in the presence and absence of TNF-α and IFN-γ as described in the Fig. 1 legend. Following stimulation, cells were harvested, counted, and processed for reporter activity. Results are reported as relative reporter activity compared with unstimulated mock controls (n = 3; *, P ≤ 0.05 relative to respective carrier-treated control; †, P ≤ 0.05 relative to TNF-α-IFN-γ-treated mock control).
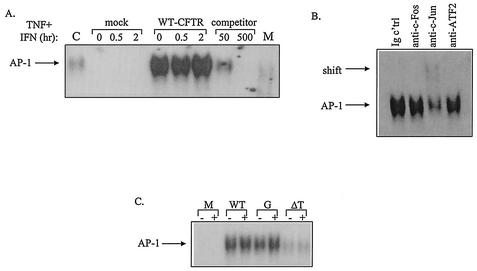
CFTR, but not EBP50, plays a role in AP-1 activation.
To examine directly the effects of WT or mutated CFTR on the binding activity of AP-1 in airway epithelial cells, EMSAs were performed. For these experiments, IB3-1 cells were transfected transiently with constructs encoding WT-CFTR, G551D-CFTR, ΔTRL-CFTR, or a mock control; cultured in the presence and absence of TNF-α and IFN-γ; and then prepared for EMSA. Results presented in Fig. 11A demonstrate that IB3-1 cells complemented with WT-CFTR displayed enhanced AP-1 binding in the absence of TNF-α and IFN-γ compared with similarly treated mock controls; cytokine treatment did not further amplify binding. AP-1 binding was confirmed through EMSAs performed with unlabeled (competitor) and mutant AP-1 oligonucleotides. Supershift analysis with antibodies directed against the various AP-1 subunits, including c-Fos, c-Jun, and ATF2, revealed that bound AP-1 complexes consisted solely of c-Jun subunits (Fig. 11B). As shown in Fig. 11C, cells transfected with constructs encoding G551D-CFTR displayed a pattern of enhanced NF-κB binding that resembled the pattern of NF-κB binding observed for cells transfected with constructs encoding WT-CFTR; in contrast, ΔTRL-CFTR did not promote the binding of NF-κB either in the presence or in the absence of TNF-α and IFN-γ (Fig. 11C).
FIG. 11.
CFTR mutations affect AP-1 binding differentially. (A) IB3-1 cells were cotransfected with constructs encoding β-galactosidase, WT-CFTR, or a mock control as described in the Fig. 4 legend. Following transfection, cells were cultured in the presence and absence of TNF-α and IFN-γ and then prepared for EMSA as described in the Fig. 9 legend. EMSAs were performed with a radiolabeled oligonucleotide containing a consensus AP-1 binding sequence (C, positive control, TNF-α-treated 9HTEo epithelial cells; M, mutant probe). (B) In addition, EMSAs were performed on cells cotransfected with constructs encoding β-galactosidase and WT-CFTR and then stimulated with TNF-α and IFN-γ with antibodies directed against c-Jun, c-Fos, and ATF2 subunits. (C) IB3-1 cells were transfected with constructs encoding β-galactosidase, WT-CFTR, G551D-CFTR, or ΔTRL-CFTR; stimulated with and without TNF-α-IFN-γ (denoted by + and −, respectively); and then prepared for EMSA as described in the Fig. 9 legend. Representative results of at least six independent experiments are shown. M, mutant.
Since the data presented above suggest that EBP50 may be involved in CFTR-dependent RANTES expression and NF-κB activation, we determined whether EBP50 might also be involved in CFTR-dependent AP-1 activation. For these experiments, IB3-1 cells were transfected with constructs encoding WT-CFTR and WT-EB50, DN-EBP50, or a mock control and then cultured in the presence and absence of TNF-α and IFN-γ. Nuclear extracts were prepared, and EMSAs were performed. Unlike the findings observed with regard to NF-κB binding presented above, expression of WT- and DN-EBP50 had no effect on AP-1 binding in the presence of WT-CFTR and TNF-α-IFN-γ (data not shown). Together, these data indicate that CFTR, but not EBP50, is involved in AP-1 activation within CF epithelial cells.
DISCUSSION
As described herein, there exist at least two possible mechanisms that CFTR may utilize to modulate gene expression in airway epithelia. First, activation of the CFTR chloride channel may affect cell membrane potential sufficiently in order to trigger signal transduction events. Cahalan and coworkers (21) first reported an example of this mechanism. These authors demonstrated that changes in the membrane potential of T lymphocytes altered calcium influx and, in turn, affected the activation of the transcription factor NFAT and subsequent IL-2 expression (21). The activities of transcription factors other than NFAT, including c-Fos, have also been linked to membrane depolarization and calcium influx (33). Data presented herein, however, demonstrate that neither selective inhibition of CFTR-mediated chloride transport nor expression of alternative chloride channels, such as ClC-2, affects RANTES expression in airway epithelia. Analyses of the CFTR mutants G551D- and ΔTRL-CFTR, which differ in their ability to transport chloride and restore RANTES expression, support these observations. Together, we believe that these data suggest that changes in chloride channel activity, either CFTR mediated or in general, do not trigger signal transduction events that modulate RANTES expression in CF airway epithelial cells.
Alternatively, CFTR may affect gene expression through protein-protein interactions with signaling adapter molecules to form a macromolecular signaling complex. Data obtained through the use of CFTR disease-associated (G551D and ΔF508) and truncation (ΔTRL) mutations presented herein support this mechanism. First, the ability of G551D, but not ΔF508, to restore RANTES expression to a level comparable with that observed with WT-CFTR suggests that CFTR molecules inserted into the plasma membrane restore RANTES expression. Second, butyrate treatment of cells expressing ΔF508-CFTR promoted CFTR expression to the plasma membrane surface and restored RANTES expression. Third, the inability of the ΔTRL mutant to restore RANTES expression, despite its insertion into the plasma membrane, indicates that the C-terminal PDZ-interacting domain is required for CFTR-dependent RANTES expression. Because biotinylation analyses revealed that the amount of CFTR expressed at the cell surface did not correlate with the degree of RANTES expression, it is clear that the ability of a CFTR molecule to restore RANTES expression is not dependent solely upon the amount of CFTR inserted into the plasma membrane.
It should be noted that, although ΔF508- and G551D-CFTR differ in their ability to restore RANTES expression, both of these CFTR mutations are associated with severe forms of CF. If the lack of RANTES expression is an important contributor to CF-associated airway inflammation, then it would be expected that neither ΔF508 nor G551D would support RANTES expression; however, this was not the response that was observed. With regard to the correlation of ΔF508- and G551D-CFTR mutations, RANTES expression, and severe CF pulmonary disease, we note that Mickle and Cutting have reported that, despite its being a monogenic disorder, the genotype-phenotype relationship in CF is complex (14). Moreover, these authors state that profound clinical variability is observed for CF patients with the classic form of CF, especially with regard to severity of lung disease. As such, we conclude that it is unclear whether RANTES expression correlates with severe CF pulmonary disease at present.
Data presented herein demonstrate that, in addition to RANTES expression, NF-κB and AP-1 transcription factor binding require insertion of CFTR into the plasma membrane as well as an intact CFTR C terminus. Recent reports suggest that CF cells expressing mutant forms of CFTR exhibit heightened NF-κB activation (40, 42). Specifically, reports by Prince and coworkers indicate that cells expressing the CFTR mutation ΔF508 or G551D as well as non-CFTR-expressing cells display increased NF-κB activation in response to a cytokine or P. aeruginosa stimulus compared with non-CF and “CFTR-corrected” cells; assessments were determined via measurements of NF-κB binding, NF-κB reporter construct activity, and NF-κB nuclear translocation (42). Blackwell and coworkers have reported similar findings (40). Although these reports attempt to link CFTR with activation of NF-κB directly, they fail to accurately correlate CFTR protein expression and chloride channel function with NF-κB activation in their analyses (40, 42). With regard to analysis of CFTR mutants and NF-κB activation, experiments were performed in a heterologous cell system (Chinese hamster ovary cells) and, therefore, do not represent events that may occur in human airway epithelial cells. Moreover, all reports focused solely on the NF-κB subunit p65 (RelA) (40, 42) despite the fact that, to date, five independent NF-κB subunits, including p50, p52, p65, RelB, and c-Rel, have been described. In contrast, results presented herein suggest that human airway epithelial cells expressing WT-CFTR or G551D-CFTR, but not ΔTRL-CFTR, exhibit enhanced NF-κB activation in the presence of TNF-α and IFN-γ compared with mock controls. All findings have been correlated with careful determinations of CFTR protein expression and chloride channel function, as monitored via immunoprecipitation and chloride transport analyses, respectively. Such conflicting results underscore the need for additional study of CFTR-mediated gene expression as outlined herein.
Although AP-1 is an important transcription factor for the regulation of numerous genes, the effects of CFTR on its activation have not been reported previously. Findings presented herein demonstrate that, like CFTR-mediated NF-κB activation, activation of AP-1 DNA binding required insertion of CFTR into the plasma membrane and an intact CFTR C terminus. In contrast with NF-κB, however, AP-1 activation was enhanced in the presence of WT-CFTR; cytokine stimulation did not further amplify AP-1 binding. These results indicate that CFTR-mediated AP-1 binding is constant in WT-CFTR-expressing airway epithelial cells and is not responsive to the effects of cytokines. Moreover, these results indicate that AP-1 binding precedes NF-κB binding in cytokine-stimulated, WT-CFTR-expressing airway epithelial cells. The consequence of such binding kinetics may be the priming of airway epithelial cells to respond to inflammatory stimuli, which promote NF-κB activation, for the expression of NF-κB-AP-1-dependent genes such as RANTES or other inflammatory mediators.
The requirement of an intact CFTR C terminus for RANTES expression and transcription factor binding in airway epithelial cells implies that interactions between CFTR's PDZ-interacting domain and PDZ-binding proteins are involved in these CFTR-dependent events. To this end, data presented herein suggest that EBP50 may play a role in CFTR-mediated RANTES expression and NF-κB activation; in contrast, EBP50 appeared to have no affect on CFTR-mediated AP-1 activation. Exogenous expression of WT-EBP50 in mock control cells had no effect on RANTES expression or NF-κB activation. In contrast, however, exogenous expression of WT-EBP50 in cells expressing WT-CFTR amplified RANTES expression in the presence and absence of cytokine-mediated stimulation; increased NF-κB binding was observed in the presence of cytokines only. Such a difference in the effect of exogenously expressed EBP50 on cytokine-mediated RANTES expression versus NF-κB activation may indicate that EBP50 is modulating the activity of other transcription factors, such as NFAT or NF-IL-6 (16), that regulate RANTES promoter activation in the presence of cytokines. Importantly, exogenous expression of DN-EBP50 in WT-CFTR-expressing cells blocked both cytokine-mediated RANTES expression and NF-κB binding. We believe that, together, these data implicate EBP50 in CFTR-mediated RANTES expression and NF-κB activation. These results, however, do not indicate that CFTR and EBP50 interact directly to facilitate this response, nor do they rule out the possibility that other PDZ-binding proteins (described below) may also play a role in this response.
EBP50 may be acting as an adapter protein to link CFTR to signaling pathways that activate NF-κB binding and, ultimately, RANTES expression. Recent evidence reported by Milgram and coworkers (34) demonstrates that EBP50 bound to CFTR may act as a scaffolding protein through its interactions with Yes-associated protein 65 (YAP-65) and, in turn, YAP-65's interactions with c-Yes, a nonreceptor tyrosine kinase (15). Milgram and coworkers hypothesize that the consequences of such protein-protein interactions at the apical membrane may regulate cellular processes, such as ion transport (15).
It is possible that, in the experiments whose results are presented in Fig. 9, exogenous expression of EBP50 may have outcompeted the binding of other proteins to the C terminus of CFTR; such proteins may also play a role in CFTR-mediated RANTES expression and transcription factor activation. To this end, several PDZ-binding proteins in addition to EBP50 have been reported elsewhere to bind the CFTR C terminus. For example, Frizzell and coworkers have demonstrated that the PDZ-binding motif at the CFTR terminus interacts with E3KARP, an EBP50-related protein (37). The authors suggest that E3KARP links CFTR to ezrin, a protein kinase A-anchoring and actin-binding protein, in order to stabilize CFTR in the epithelial apical membrane (37). In addition, Wang and colleagues have reported that CAP70, a novel PDZ-domain-containing protein, binds to the C terminus of CFTR and may serve as a linker molecule to facilitate CFTR multimerization (41). Lastly, Cheng and coworkers have shown that CAL (CFTR-associated ligand) binds to the CFTR C terminus via its PDZ-binding domain and modulates surface expression of CFTR (3).
CFTR has been implicated in the expression of several genes, including those for IL-8 (42), IL-10 (2), and inducible nitric oxide synthase (35) as well as RANTES (32). To our knowledge, this is the first report suggesting that CFTR modulates gene expression while located in a macromolecular signaling complex at the plasma membrane. Moreover, we believe that EBP50 may also participate in such a CFTR complex to activate transcription factors, such as NF-κB. Because this work has been performed exclusively in human airway epithelial cells, it is our opinion that the findings described in herein are directly relevant to the role of CFTR in epithelial function.
REFERENCES
- 1.Bear, C., F. Duguay, A. L. Naismith, N. Kartner, J. W. Hanrahan, and J. R. Riordan. 1991. Cl− channel activity in Xenopus oocytes expressing the cystic fibrosis gene. J. Biol. Chem. 266:19142-19145. [PubMed] [Google Scholar]
- 2.Bonfield, T. L., M. W. Konstan, P. Burfeind, J. R. Panuska, J. B. Hilliard, and M. Berger. 1995. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 13:257-261. [DOI] [PubMed] [Google Scholar]
- 3.Cheng, J., B. D. Moyer, M. Milewski, J. Loffing, M. Ikeda, J. E. Mickle, G. R. Cutting, M. Li, B. A. Stanton, and W. B. Guggino. 2002. A Golgi-associated PDZ domain protein modulates cystic fibrosis transmembrane regulator plasma membrane expression. J. Biol. Chem. 277:3520-3529. [DOI] [PubMed] [Google Scholar]
- 4.Cheng, S. H., R. J. Gregory, J. Marshall, S. Paul, D. W. Souza, G. A. White, C. R. O'Riordan, and A. E. Smith. 1990. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63:827-834. [DOI] [PubMed] [Google Scholar]
- 5.Clancy, J. P., F. E. Ruiz, and E. J. Sorscher. 1999. Adenosine and its nucleotides activate wild-type and R117H CFTR through an A2B receptor-coupled pathway. Am. J. Physiol. 276:C361-C369. [DOI] [PubMed] [Google Scholar]
- 6.DiMango, E., A. J. Ratner, R. Bryan, S. Tabibi, and A. Prince. 1998. Activation of NF-κB by adherent Pseudomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cells. J. Clin. Investig. 101:2598-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fouassier, L., C. Duan, A. P. Feranchak, C. Yun, E. Sutherland, F. Simon, J. G. Fitz, and R. B. Doctor. 2001. Ezrin-radixin-moesin-binding phosphoprotein 50 is expressed at the apical membrane of rat liver epithelia. Hepatology 33:166-176. [DOI] [PubMed] [Google Scholar]
- 8.Fouassier, L., C. Yun, J. G. Fitz, and R. B. Doctor. 2000. Evidence for ezrin-radixin-moesin-binding phosphoprotein 50 (EBP50) self-association through PDZ-PDZ interactions. J. Biol. Chem. 275:25039-25045. [DOI] [PubMed] [Google Scholar]
- 9.Fulmer, S. B., E. M. Schwiebert, M. M. Morales, W. B. Guggino, and G. R. Cutting. 1995. Two cystic fibrosis transmembrane conductance regulator mutations have different effects on both pulmonary phenotype and regulation of outwardly rectified chloride currents. Proc. Natl. Acad. Sci. USA 92:6832-6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koller, D. Y., M. Irmhild, J. Otto, R. Urbanek, and I. Eichler. 1997. Cytokine concentrations in sputum from patients with cystic fibrosis and their relation to eosinophil activity. Am. J. Respir. Crit. Care Med. 155:1050-1054. [DOI] [PubMed] [Google Scholar]
- 11.Kube, D., U. Sontich, D. Fletcher, and P. B. Davis. 2001. Proinflammatory cytokine responses to P. aeruginosa infection in human airway epithelial cell lines. Am. J. Physiol. 280:L493-L502. [DOI] [PubMed] [Google Scholar]
- 12.Lillard, J. W., N. B. Prosper, D. D. Taub, and J. R. McGhee. 2001. RANTES potentiates antigen-specific mucosal immune responses. J. Immunol. 166:162-169. [DOI] [PubMed] [Google Scholar]
- 13.Logan, J., D. Hiestand, P. Daram, Z. Huang, D. D. Muccio, J. Hartman, B. Haley, W. J. Cook, and E. J. Sorscher. 1994. Cystic fibrosis transmembrane conductance regulator mutations that disrupt nucleotide binding. J. Clin. Investig. 94:228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mickle, J. E., and G. Cutting. 2000. Genotype-phenotype relationships in cystic fibrosis. Med. Clin. N. Am. 84:597-607. [DOI] [PubMed] [Google Scholar]
- 15.Mohler, P. J., S. M. Kreda, R. C. Boucher, M. Sudol, M. J. Stutts, and S. L. Milgram. 1999. Yes-associated protein 65 localizes p62c-Yes to the apical compartment of airway epithelia by association with EBP50. J. Cell Biol. 147:879-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moriuchi, H., M. Moriuchi, and A. S. Fauci. 1997. Nuclear factor-κB potently up-regulates the promoter activity of RANTES, a chemokine that blocks HIV infection. J. Immunol. 158:3483-3491. [PubMed] [Google Scholar]
- 17.Moyer, B. D., J. Denton, K. H. Karlson, D. Reynolds, S. Wang, J. E. Mickle, M. Milewski, G. R. Cutting, W. B. Guggino, M. Li, and B. A. Stanton. 1999. A PDZ-interacting domain in CFTR is an apical membrane polarization signal. J. Clin. Investig. 104:1353-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moyer, B. D., K. H. Karlson, and B. A. Stanton. 1998. Membrane trafficking of the cystic fibrosis gene product, cystic fibrosis transmembrane conductance regulator, tagged with green fluorescent protein in Madin-Darby canine kidney cells. J. Biol. Chem. 273:21759-21768. [DOI] [PubMed] [Google Scholar]
- 19.Moyer, B. D., D. Loffing-Cueni, J. Loffing, D. Reynolds, and B. A. Stanton. 1999. Butyrate increases apical membrane CFTR but reduces chloride secretion in MDCK cells. Am. J. Physiol. 277:F271-F276. [DOI] [PubMed] [Google Scholar]
- 20.Murray, C. M., S. Chu, and P. L. Zeitlin. 1996. Gestational and tissue-specific regulation of ClC-2 chloride channel expression. Am. J. Physiol. 271:L829-L837. [DOI] [PubMed] [Google Scholar]
- 21.Negulescu, P. A., N. Shastri, and M. D. Cahalan. 1994. Intracellular calcium dependence of gene expression in single T lymphocytes. Proc. Natl. Acad. Sci. USA 91:2873-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasyk, E. A., and J. K. Foskett. 1995. Mutant (ΔF508) cystic fibrosis transmembrane conductance regulator Cl− channel is functional when retained in endoplasmic reticulum of mammalian cells. J. Biol. Chem. 270:12347-12350. [DOI] [PubMed] [Google Scholar]
- 23.Pasyk, E. A., and J. K. Foskett. 1997. Cystic fibrosis transmembrane conductance regulator-associated ATP and adenosine 3′-phosphate 5′-phosphosulfate channels in endoplasmic reticulum and plasma membranes. J. Biol. Chem. 272:7746-7751. [DOI] [PubMed] [Google Scholar]
- 24.Prince, L. S., K. Peter, S. R. Hatton, L. Zaliauskiene, L. F. Cotlin, J. P. Clancy, R. B. Marchase, and J. F. Collawn. 1999. Efficient endocytosis of the cystic fibrosis transmembrane conductance regulator requires a tyrosine-based signal. J. Biol. Chem. 274:3602-3609. [DOI] [PubMed] [Google Scholar]
- 25.Riordan, J. R., J. M. Rommens, B. Kerem, N. Alon, R. Rozmahel, Z. Grzelczak, J. Zielenski, S. Lok, N. Plavsic, and J. L. Chou. 1989. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245:1066-1073. [DOI] [PubMed] [Google Scholar]
- 26.Rubenstein, R. C., M. E. Egan, and P. L. Zeitlin. 1997. In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing ΔF508-CFTR. J. Clin. Investig. 100:2457-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwiebert, E. M., D. J. Benos, M. E. Egan, M. J. Stutts, and W. B. Guggino. 1999. CFTR is a conductance regulator as well as a chloride channel. Physiol. Rev. 79:S145-S166. [DOI] [PubMed] [Google Scholar]
- 28.Schwiebert, E. M., L. P. Cid-Soto, M. Carter, D. Stafford, C. M. Murray, W. B. Guggino, and G. R. Cutting. 1998. Functional analysis of an alternative chloride channel (CLC-2) in cystic fibrosis airway epithelial cells. Proc. Natl. Acad. Sci. USA 95:3879-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwiebert, E. M., M. E. Egan, T. H. Hwang, S. B. Fulmer, S. S. Allen, G. Cutting, and W. B. Guggino. 1995. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell 81:1063-1073. [DOI] [PubMed] [Google Scholar]
- 30.Schwiebert, E. M., and W. B. Guggino. 1997. Defective chloride and sodium transport in CF, p. 2555-2571. In R. G. Crystal, J. B. West, E. R. Weibel, and P. J. Barnes (ed.), The lung. Lippincott-Raven Publishers, Philadelphia, Pa.
- 31.Schwiebert, E. M., M. M. Morales, S. Devidas, M. E. Egan, and W. B. Guggino. 1998. Chloride channel and chloride conductance regulator domains of CFTR. Proc. Natl. Acad. Sci. USA 95:2674-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwiebert, L. M., K. Estell, and S. M. Propst. 1999. Chemokine expression in CF epithelia: implications for the role of CFTR in RANTES expression. Am. J. Physiol. 276:700-710. [DOI] [PubMed] [Google Scholar]
- 33.Sheng, M., G. McFadden, and M. E. Greenberg. 1990. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron 4:571-582. [DOI] [PubMed] [Google Scholar]
- 34.Short, D. B., K. W. Trotter, D. Reczek, S. M. Kreda, A. Bretscher, R. C. Boucher, M. J. Stutts, and S. L. Milgram. 1999. An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J. Biol. Chem. 273:19797-19801. [DOI] [PubMed] [Google Scholar]
- 35.Steagall, W. K., H. L. Elmer, K. G. Brady, and T. J. Kelley. 2000. Cystic fibrosis transmembrane conductance regulator-dependent regulation of epithelial inducible nitric oxide synthase expression. Am. J. Respir. Cell Mol. Biol. 22:45-50. [DOI] [PubMed] [Google Scholar]
- 36.Strieter, R. M., and S. L. Kunkel. 1997. Chemokines, p. 155-186. In R. G. Crystal, J. B. West, E. R. Weibel, and P. J. Barnes (ed.), The lung. Lippincott-Raven Publishers, Philadelphia, Pa.
- 37.Sun, F., M. J. Hug, C. M. Lewarchik, C. Yun, N. A. Bradbury, and R. A. Frizzell. 2000. E3KARP mediates the association of ezrin and PKA with CFTR in airway cells. J. Biol. Chem. 275:29539-29546. [DOI] [PubMed] [Google Scholar]
- 38.Tabary, O., S. Escotte, J. P. Couetil, D. Hubert, D. Dusser, E. Puchelle, and J. Jacquot. 1999. Genistein inhibits constitutive and inducible NF-κB activation and decreases IL-8 production by human cystic fibrosis bronchial gland cells. Am. J. Pathol. 155:473-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tousson, A., B. A. Van Tine, A. P. Naren, G. M. Shaw, and L. M. Schwiebert. 1998. Characterization of CFTR expression and chloride channel activity in human endothelia. Am. J. Physiol. 275:C1555-C1564. [DOI] [PubMed] [Google Scholar]
- 40.Venkatakrishnan, A., A. A. Stecenko, G. King, T. R. Blackwell, K. L. Brigham, J. W. Christman, and T. S. Blackwell. 2000. Exaggerated activation of nuclear factor-κB and altered IκB-β processing in cystic fibrosis bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 23:396-403. [DOI] [PubMed] [Google Scholar]
- 41.Wang, S., H. Yue, R. B. Derin, W. B. Guggino, and M. Li. 2000. Accessory protein facilitated CFTR-CFTR interaction, a molecular mechanism to potentiate the chloride channel activity. Cell 103:169-179. [DOI] [PubMed] [Google Scholar]
- 42.Weber, A. J., G. Soong, R. Bryan, S. Saba, and A. Prince. 2001. Activation of NF-κB in airway epithelial cells is dependent on CFTR trafficking and Cl− channel function. Am. J. Physiol. 281:L71-L78. [DOI] [PubMed] [Google Scholar]
- 43.Welsh, M. J., and A. E. Smith. 1993. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell 73:1251-1254. [DOI] [PubMed] [Google Scholar]
- 44.Zeitlin, P. L., L. Lu, J. Rhim, G. Cutting, G. Stretten, K. A. Keiffer, R. Craig, and W. B. Guggino. 1991. A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-12-SV40 infection. Am. J. Respir. Cell Mol. Biol. 4:313-319. [DOI] [PubMed] [Google Scholar]