Abstract
The survival of Trypanosoma brucei, the causative agent of Sleeping Sickness and Nagana, is facilitated by the expression of a dense surface coat of glycosylphosphatidylinositol (GPI)-anchored proteins in both its mammalian and tsetse fly hosts. We have characterized T. brucei GPI8, the gene encoding the catalytic subunit of the GPI:protein transamidase complex that adds preformed GPI anchors onto nascent polypeptides. Deletion of GPI8 (to give Δgpi8) resulted in the absence of GPI-anchored proteins from the cell surface of procyclic form trypanosomes and accumulation of a pool of non–protein-linked GPI molecules, some of which are surface located. Procyclic Δgpi8, while viable in culture, were unable to establish infections in the tsetse midgut, confirming that GPI-anchored proteins are essential for insect-parasite interactions. Applying specific inducible GPI8 RNAi with bloodstream form parasites resulted in accumulation of unanchored variant surface glycoprotein and cell death with a defined multinuclear, multikinetoplast, and multiflagellar phenotype indicative of a block in cytokinesis. These data show that GPI-anchored proteins are essential for the viability of bloodstream form trypanosomes even in the absence of immune challenge and imply that GPI8 is important for proper cell cycle progression.
INTRODUCTION
Trypanosoma brucei is the heteroxenous, hemoflagellate protozoan parasite responsible for Sleeping Sickness in humans and Nagana in domestic animals in the tsetse belt of sub-Saharan Africa. All life cycle stages of the parasite utilize glycosylphosphatidylinositol (GPI) anchors as the predominant method for attaching proteins to their plasma membrane. In the mammalian host, these proteins include one subunit of the heterodimeric transferrin receptor (Schell et al., 1991), an alanine-rich protein of unknown function (Nolan et al., 2000), and the variant surface glycoprotein (VSG) (Ferguson et al., 1988) essential for evasion of the host's immune system.
The bloodstream forms of T. brucei differentiate into procyclic forms once ingested by the tsetse fly vector. This differentiation involves remodeling of the surface by shedding the VSG coat and replacing it with an invariant coat of GPI-anchored proteins known as procyclins (Roditi et al., 1989). There are four types of procyclin, three bearing between 18 and 30 internal -Glu-Pro- repeats (EP1, EP2, and EP3; see Acosta-Serrano et al., 2000 for alignment), and one with a -Gly-Pro-Glu-Glu-Thr- (GPEET) repeat region (Mowatt et al., 1989). Although EP1 and EP3 both undergo N-glycosylation, EP2 and GPEET do not, although the latter is phosphorylated on the threonine residues of the repeat region (Butikofer et al., 1999). All procyclin isoforms are anchored by a GPI modified with a heterogeneous poly-N-acetyllactosamine side chain (Treumann et al., 1997), widely believed to form a glycocalyx over the cell surface. Displayed above this, both EP and GPEET procyclins are thought to adopt a rod-like conformation (Roditi et al., 1989; Treumann et al., 1997). Although the N-termini of these proteins are proteolytically cleaved during infection of tsetse, their repeat domains are resistant to protease digestion, suggesting that one of the functions of this coat is to act as a mechanical barrier within the proteolytic environment of the tsetse midgut (Acosta-Serrano et al., 2001). A hierarchy of procyclin expression during establishment within the tsetse has recently been discovered (Acosta-Serrano et al., 2001). GPEET is the predominant species at day 3 after the blood meal, but there is a switch to glycosylated EP isoforms by day 7. It is known that there is a dramatic decline in parasite numbers during this initial 3 d, whereupon the parasites remaining rapidly multiply to become an established infection with a maximum density of 2–5 × 105 parasites/mid-gut (Van den Abbeele et al., 1999).
GPI anchors are synthesized in the endoplasmic reticulum (ER) by the sequential addition of sugars and ethanolamine phosphates onto phosphatidylinositol (for a recent reviews see Ferguson, 1999; Kinoshita and Inoue, 2000). Precursor proteins destined to receive a GPI anchor possess an N-terminal, ER-directing signal sequence that is cleaved upon translocation into the ER and a C-terminal domain comprising a GPI anchor addition signal sequence (Moran and Caras, 1991; Gerber et al., 1992; Nuoffer et al., 1993). This C-terminal domain is recognized by a transamidase complex consisting of at least four subunits (Fraering et al., 2001; Ohishi et al., 2001). The complex proteolytically cleaves the protein at the ω-site and in a transamidation reaction adds a preformed GPI en bloc via an amide linkage to the terminal ethanolamine phosphate (Mayor et al., 1991; Maxwell et al., 1995).
Utilization of GPIs for anchoring proteins to the plasma membrane is widespread throughout eukaryotes. GPI anchoring is essential in yeast (Fraering et al., 2001), mammalian embryos (Lin et al., 2000) and bloodstream form T. brucei (Nagamune et al., 2000). However, it is not essential in some mammalian cell lines (Yu et al., 1997; Watanabe et al., 2000), in procyclic T. brucei (Nagamune et al., 2000) or in the related kinetoplastid parasite Leishmania mexicana (Hilley et al., 2000). Although both parasites and their hosts use GPI anchoring, disparity in the substrate specificities of several orthologous GPI biosynthetic enzymes exist (for review, see Ferguson et al., 1999). This raises the possibility that specific inhibitors of parasite GPI biosynthetic enzymes could be designed for use as therapeutic agents in the treatment of disease.
MATERIALS AND METHODS
Identification of T. brucei GPI8
A 1.1-kb fragment containing the open reading frame (ORF) of the L. mexicana GPI8 (Hilley et al., 2000) was used to screen a T. brucei EATRO 795 genomic library under low stringency conditions. A single positive plaque was isolated and DNA was prepared. After restriction endonuclease digestion, an EcoRI fragment of ∼8.5 kb was subcloned into pUC18 to give pGL480. Sequencing identified a 960-base pair ORF encoding TbGPI8.
Southern Blot Analysis
Five micrograms of T. brucei EATRO 795 genomic DNA was digested with restriction endonucleases SacI, BamHI, EcoRI, ApaI, or KpnI, electrophoresed through a 0.7% agarose gel, and blotted onto Hybond C Super (Amersham Pharmacia, Little Chalfant, United Kingdom). The blot was hybridized with a 653-base pair 32P random-primed PCR product (equating to bases 134–787 of the 960-base pair TbGPI8 ORF, Accession number AJ439686) at 65°C overnight. Washes were for 15 min at 65°C with 2× SSC/0.1% SDS and then twice with 0.2× SSC/0.1% SDS.
Culturing and Transfection of Parasites
Procyclic form T. brucei strain EATRO 795 cells were cultured at 27°C in complete SDM79 medium (Brun and Schonenberger, 1979) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum. Five micrograms of linearized DNA was used to transfect 3 × 107 midlog phase procyclics in 0.5 ml Zimmerman PostFusion medium (132 mM NaCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, 0.5 mM Mg acetate, 0.09 mM Ca acetate, pH 7.0) in a 0.4-cm pulse cuvette using a Bio-Rad gene pulser II set at 1.5 kV, 25 μF. After overnight recovery, selection of clones was by limiting dilution in nonadherent tissue culture plates (Falcon) with appropriate antibiotics (15 μg ml−1 G418, 10 μg ml−1 puromycin, 20 μg ml−1 blasticidin). Subsequent culturing was in conventional tissue culture flasks.
Bloodstream form T. brucei strain 427 cells (SMB; Wirtz et al., 1999) were used for RNAi analysis. These trypanosomes express T7 polymerase and the tet repressor protein that facilitate inducible expression of double-stranded RNA. Cells were cultured at 37°C with 5% CO2 in HMI-9 (Hirumi and Hirumi, 1989) supplemented with 5 μg ml−1 hygromycin and 2.5 μg ml−1 G418. Cells (107) resuspended in 400 μl Cytomix (25 mM HEPES, 10 mM K2HPO4, 120 mM KCl, 0.15 mM CaCl2, 5 mM MgCl2, 2 mM EGTA, 1 mM hypoxanthine, 0.5% (wt/vol) glucose, 100 μg ml−1 BSA, pH 7.4) were electroporated with 25 μg NotI linearized pGL654 using a Bio-Rad Gene Pulser II set at 1.5 kV and 25 μF. Clonal populations were derived by limiting dilution with 2.5 μg ml−1 phleomycin. Induction of RNAi expression was with 1 μg ml−1 tetracycline.
Plasmids
For deletion of TbGPI8 the 5′ and 3′ flanks of the gene were amplified by PCR using primer pairs OL674 (CAAGCTTTTCGCCATCACTCTCAGCCG) with OL675 (AACTAGTCGCCTGATCCAACTAATCG) and OL676 (AGGATCCTTACGATTTGTTCTAGTTCC) with OL677 (AAGATCTCAGCTGTAGACAACTCAGCG), respectively, and cloned sequentially into the HindIII/SpeI and BamHI/BglII sites flanking the PURR gene of construct pGL236 (Hilley et al., 2000), giving pGL519. The BSDR gene was excised from pGL437 (Brooks et al., 2000) using SpeI/BamHI and cloned into the same sites of pGL519, replacing the PURR gene and giving pGL610. pGL519 and pGL610 were digested with HindIII/BglII and the PURR/BSDR-containing fragment purified for transfections. Gene deletion was by homologous recombination, replacing the TbGPI8 ORF and utilizing the TbGPI8 5′ and 3′ flanking sequences for correct transcription and processing of drug resistance genes.
For reexpression of TbGPI8, the ORF together with 449 and 579 base pairs of 5′ and 3′ flanking sequence, respectively, was amplified by PCR using primer pair OL840 (CAAAGCTTGATCCTTAGATACATACCCG) with OL841 (TGGGATCCCACCAGTAACAACAGGCAGC) and cloned into the HindIII/BamHI sites of plasmid pXS219 (Bangs et al., 1997), giving pGL620. For transfection, pGL620 was linearized with MluI, allowing integration into the tubulin intergenic region.
For RNAi, the ORF of TbGPI8 was amplified by PCR using primer pair OL765b (ACAAGCCTATGTTGCCCATGTTACTGTGG) with OL766 (CAGGATCCCTAGAACAAATCGAACGTAACTC) and cloned into the BamHI and HindIII sites of p2T7i (LaCount et al., 2000), giving pGL654.
Antisera and Immunoblotting
Antiserum specific to GPI8 was raised by immunization of rabbits with purified recombinant L. mexicana GPI8 (Sharma et al., 2000). TBRP1/247 monoclonal antiserum against EP-procyclin was from Cedar lane Laboratories (Ontario, Canada). K1 antiserum against the GPEET-procyclin (Butikofer et al., 2001) was a kind gift from I. Roditi. Antiserum against the T. brucei aldolase was a kind gift from D. Steverding. Antisera against the T. brucei variant surface glycoprotein (VSG) 221 were kind gifts from M. Boshart and M. Carrington.
Trypanosome cell pellets were resuspended in Laemmli buffer, electrophoresed on 12% SDS-PAGE gels, and transferred onto PVDF membrane (NEN). Blocking was with 5% (wt/vol) skimmed milk in PBS with 0.1% Tween 20. Primary antibodies for GPI8, EP-procyclin, and GPEET-procyclin were used at dilutions of 1:2000, for VSG at 1:5000, and for aldolase at 1:10,000. Secondary antibody (Promega) was conjugated to HRP and was used at 1:2000. Chemiluminescent detection was with the SuperSignal system (Pierce), whereas chemifluorescent detection was with the ECF Western blotting kit and a Typhoon 8600 phosphoimager (Amersham Pharmacia).
Tsetse Fly Infection Study
Procyclic trypanosomes were harvested by centrifugation at 1000 × g for 5 min at 27°C and then resuspended at 106 ml−1 in red blood cells washed with heat-inactivated fetal calf serum. Tsetse flies were fed through a silicon membrane on a 37°C plate and then maintained at 25°C with 65% humidity for 14 d with feeding every 2 d. After this time, they were dissected and their midguts examined for the presence of trypanosomes. Images were captured using a Zeiss Axioplan at ×630 magnification with a Hamamatsu C4742–95 cooled digital CCD camera and processed using Openlab 2.02.
Metabolic Labeling and Extraction of Parasite Material
For metabolic labeling, 108 procyclic parasites were harvested at 1000 × g for 5 min at 27°C and washed twice with PBS. The parasites were resuspended in 10 ml SDM79 containing 250 μCi [1-3H]ethanolamine hydrochloride (Amersham) for 16 h or in 5 ml glucose-free RPMI containing 200 μCi d-[2,6-3H]mannose (Amersham) for 5 h. For periodate treatment, d-[2,6-3H]mannose-labeled procyclics were resuspended in 20 ml SDM79 for 4 h, washed twice with ice-cold PBS and then resuspended in 10 ml ice-cold PBS. Samples were split and NaIO4 added to one of the matched pair to 10 mM final concentration, after which samples were incubated on ice for 30 min in the dark. Cells were washed twice with ice-cold PBS/150 mM glycerol and twice with ice-cold PBS, and pellets were stored at −80°C.
Lipid extracts were prepared as described previously (Field et al., 1991b). After partitioning to remove aqueous-soluble metabolites, organic phases were dried down in a Speedvac (Savant) and resuspended in 20 μl solvent. Ten microliters of each sample was spotted on a Si60 HPTLC plate (Merck) and then developed in chloroform/methanol/water (10:10:3, vol/vol) in a saturating atmosphere. The total migration distance was 16 cm. After development, the plates were air dried, sprayed with EnHance (Perkin Elmer-Cetus), and exposed to autoradiographic film at −85°C. To generate a PP1* standard, PP1 was purified by elution from Si60 TLC plates (Merck) after chromatography in chloroform/methanol/water (10:10:3, vol/vol). After several rounds of extraction in chloroform/methanol/water (10:10:3, vol/vol), samples were dried and repartitioned between butanol/water, and then lipids located by scintillation counting of an aliquot. Periodate treatment was done in 250 μl 40% propan-1-ol, 20% MeOH, 40% PBS, with 10 mM periodate on ice in the dark for 10 min. Reactions were stopped by mixing with an equal volume of 150 mM glycerol in PBS, and samples were chromatographed as described above.
RESULTS
Isolation of T. brucei GPI8
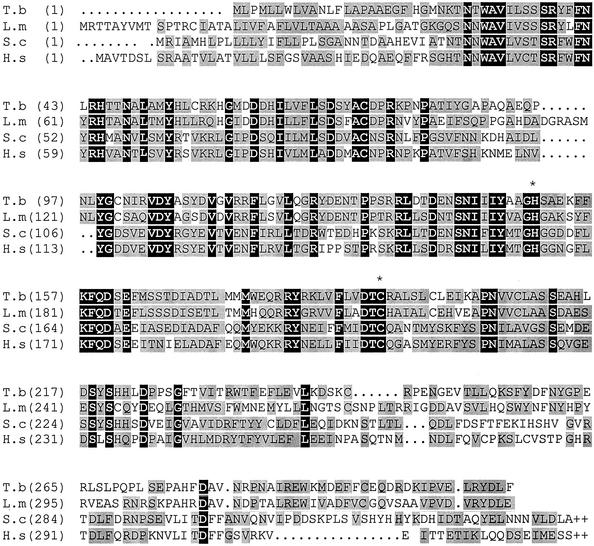
Use of the ORF of the L. mexicana GPI8 to screen a T. brucei λ library resulted in a positive plaque being isolated and 1.6 kb of sequence being obtained. This identified a 960-base pair ORF (TbGPI8) encoding the T. brucei GPI8. TbGPI8 is predicted to be a 37-kDa protein, with a putative signal peptide cleavage site between positions 20 and 21. Comparison of TbGPI8 with the protein from other organisms (Figure 1) reveals 49% identity with L. mexicana and 27% identity with both Saccharomyces cerevisiae and Homo. sapiens. TbGPI8 contains the catalytic histidine and cysteine dyad (marked in Figure 1) that defines the clan CD cysteine proteases (Barrett and Rawlings, 2001). Hydropathy analysis revealed TbGPI8 lacks the C-terminal transmembrane domain reported for S. cerevisiae and H. sapiens (Benghezal et al., 1996). Amplification of TbGPI8 from cDNA using a gene internal and a spliced leader-specific primer pair identified the splice acceptor site 304 base pairs 5′ of the start codon.
Figure 1.
Multiple alignment of GPI8 amino acid sequences from T. brucei (this study), Leishmania mexicana (CAB55340), S. cerevisiae (NP_010618) and Homo sapiens (CAA68871). The alignment was performed using Align X (Vector NTI Suite, Informax Inc.). Amino acids identical in all sequences are highlighted with a black background, and gray blocks denote conservation. Active site cysteine and histidine residues are indicated by an asterisk (∗).
Procyclic Form Δgpi8 Trypanosomes Lack GPI-anchored Proteins
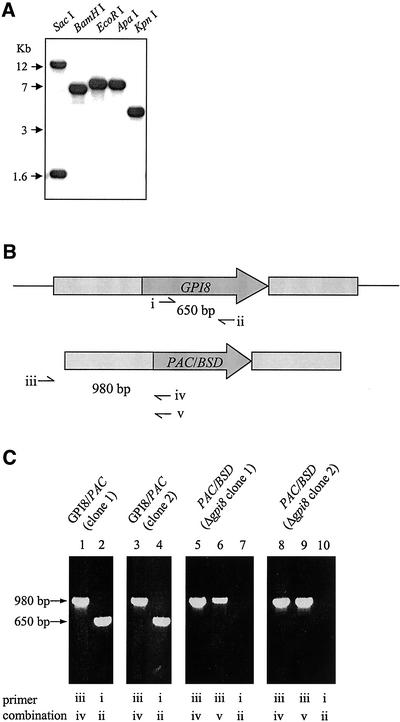
Southern blot analysis of endonuclease-digested genomic DNA (Figure 2A) revealed a single GPI8-hybridizing DNA fragment with KpnI, ApaI, EcoRI, or BamHI. Two DNA fragments were detected with SacI because of the presence of a SacI site near the middle of the gene. These data show that the T. brucei GPI8 is a single-copy gene. To investigate the importance of GPI8 in procyclic T. brucei, sequential rounds of targeted gene replacement with PAC and BSD antibiotic resistance genes flanked by GPI8 5′ and 3′ sequences were performed (Figure 2B). After PCR confirmation of PAC integration at the correct locus, the second allele of GPI8 was deleted from two independent clones using the BSD-containing construct. Clonal populations were derived, and deletion of GPI8 (to give Δgpi8) was confirmed by both PCR (Figure 2C) and Southern blot (unpublished data). The T. brucei GPI8 was targeted into the tubulin locus of the Δgpi8 mutants to generate lines reexpressing GPI8 (designated Δgpi8[GPI8]). Procyclic form wild-type, Δgpi8 and Δgpi8[GPI8] were found to have no discernible difference in either morphology or growth rate in culture.
Figure 2.
Generation of Δgpi8 mutants (A) Southern blot analysis of the T. brucei GPI8 gene. DNA was cut with SacI, BamHI, EcoRI, ApaI, or KpnI, transferred onto nylon membrane and hybridized with the ORF of the gene. (B) Schematic representation of the GPI8 locus (top) and PAC/BSD replacement construct (bottom) with open reading frames (dark gray) and GPI8 flanking regions (light gray) used in the integration construct. Thin lines represent regions not present in the integration construct. Primer sites used to confirm presence of GPI8 (i and ii) and correct integration of PAC/BSD constructs (iii with iv or v) are indicated. (C) PCR of genomic DNA to determine correct integration of markers and deletion of GPI8. GPI8/PAC (clone 1) and (clone 2) are two independent heterozygotes containing a PAC (lanes 1 and 3) and a GPI8 (lanes 2 and 4) gene. Derived from these are two independent Δgpi8 clones, containing PAC (lanes 5 and 8) and BSD (lanes 6 and 9), but lacking GPI8 (lanes 7 and 10). Δgpi8 clone 1 was used for all subsequent studies. Some results were confirmed with clone 2 (unpublished data).
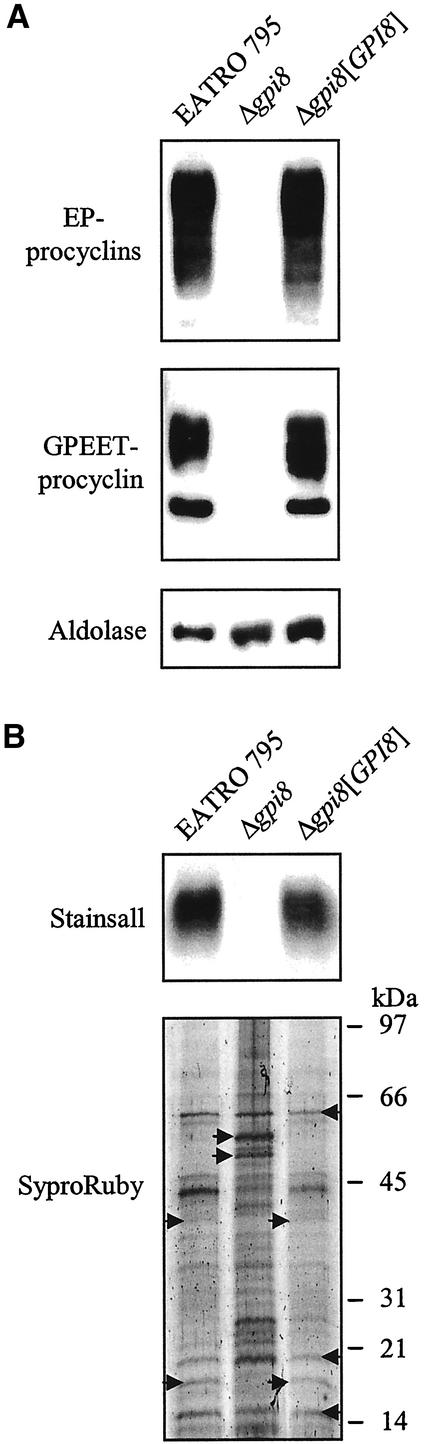
Western blot analysis of whole cell lysates revealed abundant EP and GPEET procyclins in both wild-type and Δgpi8[GPI8] clones, but their apparent absence from Δgpi8 (Figure 3A). This suggested that GPI-anchored proteins were not present in trypanosomes that lack GPI8. However, a recent report has indicated that both of the antibodies against procyclins used in the current study (TBRP1/247 and K1) fail to detect procyclins that no longer possess GPI anchors (Butikofer et al., 2001). Thus it was possible that non-GPI anchored procyclins could still associate with the surface of Δgpi8 cells by means of the hydrophobic C-terminal domain normally cleaved during the GPI:protein transamidation reaction. To investigate this possibility, a previously reported method (Clayton and Mowatt, 1989) was used to hypotonically lyse cells in the presence of protease inhibitors and then extract protein from the membrane fraction using CHAPS. After PAGE, Stainsall was used to detect protein within the gel. Procyclin stained a deep blue in both wild-type and Δgpi8[GPI8] clones, but was not observed in Δgpi8 (Figure 3B). This confirmed that procyclin was not expressed on the cell surface in the absence of GPI anchoring. Sypro Ruby staining of the CHAPS extracts showed that there were a number of proteins expressed in both wild-type and Δgpi8[GPI8] trypanosomes that were apparently absent from Δgpi8 (Figure 3B). Also, a subset of proteins appeared to be expressed at much higher levels in Δgpi8 cells relative to both wild-type and Δgpi8[GPI8].
Figure 3.
Analysis of procyclin expression (A) Western blot analysis of whole cell lysate from wild-type EATRO 795, Δgpi8 and Δgpi8[GPI8] detected with α-EP procyclin, α-GPEET procyclin, or α-aldolase. (B) Wild-type EATRO 795, Δgpi8, and Δgpi8[GPI8] were hypotonically lysed, and the washed pellet was subjected to CHAPS extraction. After SDS-PAGE, CHAPS extracted protein was detected with Stainsall or SyproRuby. Arrows indicate abundant proteins present in all three samples (←) or predominantly in the wild-type EATRO 795 and Δgpi8[GPI8] or Δgpi8 (→).
Effect of ConA on Procyclic Δgpi8
The tetrameric lectin concanavalin A has a strong affinity for mannose residues and binds to the N-linked glycans of EP-procyclins, resulting in procyclic cell death. Cells bearing procyclin isoforms that are either not glycosylated or have altered N-glycans are resistant to ConA-induced death (Pearson et al., 2000). We therefore reasoned that because Δgpi8 cells lack procyclin on the cell surface, then they would be resistant to ConA-induced cell death. To test this hypothesis, wild-type, Δgpi8, or Δgpi8[GPI8] cells were cultured in medium containing 50 μg ml−1 ConA. All three clones showed the same kinetics of cell death, including the characteristic morphology previously described (Welburn et al., 1996; Pearson et al., 2000). This indicates the presence of other mannose-bearing structures that replace procyclin on the surface of procyclic Δgpi8 as ligands to which ConA can bind.
Procyclic Δgpi8 Accumulate GPI Anchor Precursors
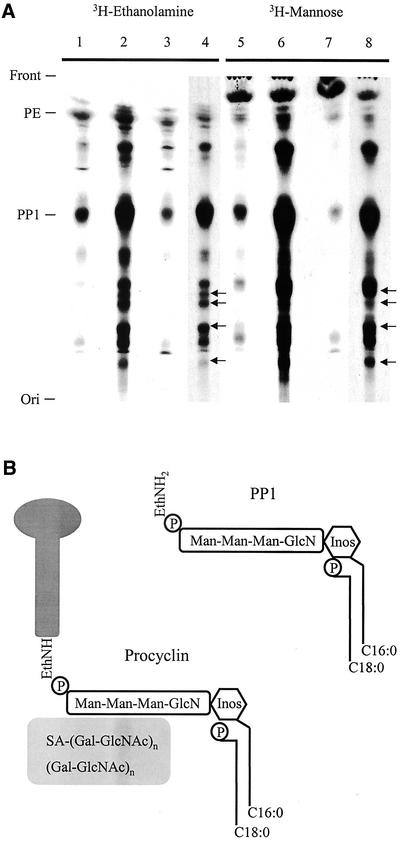
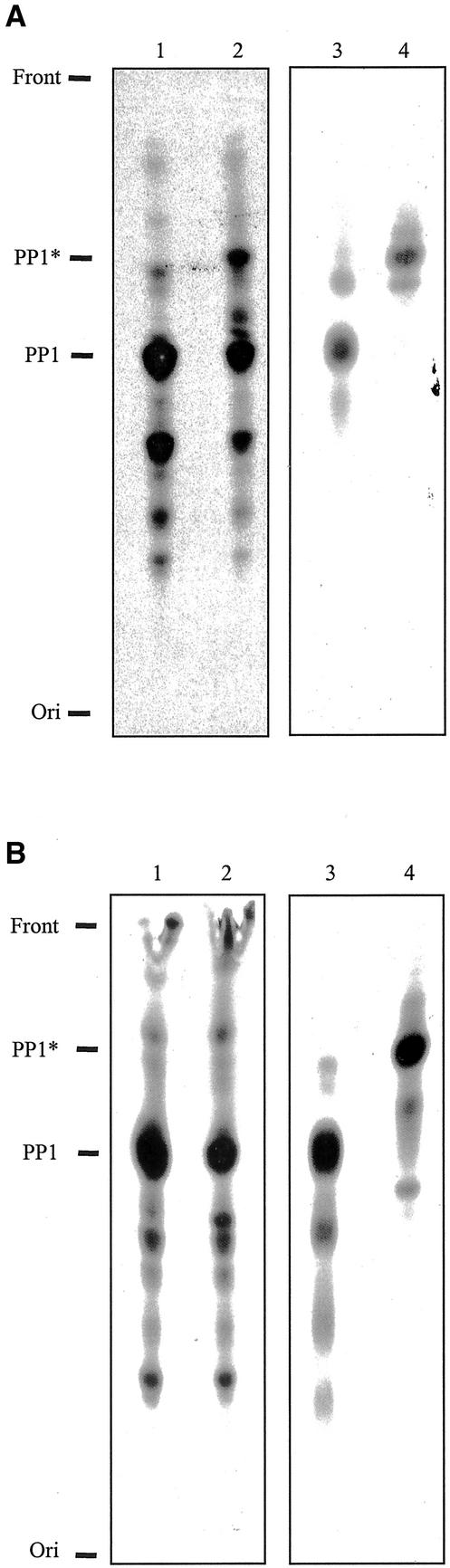
Analysis of chloroform/methanol/water (CMW) extracts from both 3H-ethanolamine– and 3H-mannose–labeled procyclic forms by TLC revealed that although wild-type EATRO 795 and Δgpi8[GPI8] display lipid profiles that are essentially identical, there is an increase in the abundance of ethanolamine- and mannose-containing lipids in Δgpi8 cells (Figure 4A). Designation of lipid species is made based on Rf values from previous experiments (Field et al., 1991b). PP1, the complete preformed GPI substrate of the transamidase complex of procyclic T. brucei (Field et al., 1991a; Mayor et al., 1991), was particularly abundant in cells lacking GPI:transamidase activity. The structure of PP1 and the procyclin GPI anchor are shown (Figure 4B). The accumulation of GPI-precursors was due to the loss of GPI:protein transamidase activity, as reexpression of GPI8 in Δgpi8[GPI8] restored the lipid profile to that of the wild-type parasites. In addition to the build up of previously observed precursors, a number of novel ethanolamine/mannose-containing lipid species also accumulated in Δgpi8 (Figure 4A [←]). This indicates that in the absence of addition to proteins a significant proportion of the anchor pool has undergone further modification. It is feasible that these novel lipid species are present at low levels in wild-type cells, but are difficult to detect because of the relatively low PP1 pool.
Figure 4.
Accumulation of lipids in Δgpi8. (A) Chloroform/methanol/water extracts of 3H-ethanolamine- and 3H-mannose–labeled procyclic forms were chromatographed and visualized by autoradiography. Δgpi8[GPI8] (lanes 1 and 5), Δgpi8 (lanes 2, 4, 6, and 8), and wild-type EATRO 795 (lanes 3 and 7). The positions of the front, origin, phosphatidylethanolamine (PE), and the major insect-stage T. brucei GPI-anchor precursor PP1 (Field et al., 1991b) are indicated at left. A number of novel lipid species were detected in Δgpi8 (indicated by arrows [←]). For clarity, the Δgpi8 extracts are shown after both 2-d (lanes 4 and 8) and 10-d (lanes 2 and 6) exposures. (B) Schematic illustrating the structural features of PP1 and the procyclin GPI anchor. Data are based on studies cited in the text. PP1, top right, contains a cannonical GPI-core glycan, together with an acyl-inositol headgroup (the major fatty acid here is palmitate, although other species are present) and a lyso-glycerolipid backbone (a single fatty acid, predominantly stearate, is attached to the glycerol sn-1 position). The procyclin GPI-anchor structure has the same core as PP1, including an identical fatty acid configuration. Additionally, the procyclin protein C terminus is in amide linkage to the ethanolamine, and there is a large heterogenous glycan of ∼15-kDa molecular weight, which contains sialic acid and is endo-β-galactosidase sensitive (indicating the presence of lactosamine repeat structures). Inositol is represented by a hexagon, the core glycan, and the side chain in the procyclin anchor by rounded rectangles, and the procyclin polypeptide by a lollipop. Diagram is not to scale.
Many eukaryotes express non–protein-linked GPI molecules on their plasma membranes. These include the lipophosphoglycans and GIPLs of Leishmania and ‘free’ GPI anchors in mammalian cells (McConville and Ferguson, 1993; Baumann et al., 2000). We therefore speculated that T. brucei may express free GPI molecules on the extracellular face of the plasma membrane and that the marked accumulation of GPI molecules detected in Δgpi8 cells could be indicative of an amplification of this phenomenon. To determine if this were the case, we utilized a previously published method for identifying expression of free GPIs on the exoplasmic leaflet of the plasma membrane (Baumann et al., 2000). Sodium periodate oxidizes the hydroxyl groups of sugars, but because of its size and negative charge does not diffuse into cells (McConville and Bacic, 1990; Baumann et al., 2000). Δgpi8 were labeled with 3H-mannose and after a chase period incubated in the presence of 10 mM NaIO4. CMW extracts analyzed by TLC revealed that after periodate treatment a significant portion of the PP1 fraction had been oxidized (PP1*), indicating that part of this pool is expressed on the surface of these parasites (Figure 5A, lanes 1 and 2). As a control, PP1 was purified by elution from a TLC plate and treated with periodate to provide an authentic PP1* standard (lanes 3 and 4). To show that periodate did not permeate the plasma membrane and oxidize intracellular PP1, Δgpi8 cells were labeled for 2.5 h with 3H-mannose and then treated with periodate as described above. No substantial change in lipid profile was detected between the treated and untreated samples (compare Figure 5B, lanes 1 and 2). Once extracted from Δgpi8 cells, all PP1 converted to PP1* upon incubation with periodate (lanes 3 and 4). Additionally, a glycerol quench applied before periodate treatment ensured that no oxidation occurred during the experimental procedure (unpublished data). These data provide evidence that a fraction of the PP1 pool of Δgpi8 cells is surface located.
Figure 5.
Surface expression of protein-anchor precursors. (A) Δgpi8 cells labeled with 3H-mannose were chased for 4 h in fresh medium then incubated without (lane 1) or with (lane 2) 10 mM sodium periodate. After quenching of the oxidation reaction, cell pellets were extracted sequentially with chloroform/methanol and chloroform/methanol/water, chromatographed, and visualized by autoradiography. The chloroform/methanol fraction demonstrated even loading of samples as determined by phosphatidylethanolamine abundance. The positions of the front, origin, PP1, and oxidized PP1 (PPI*) in the chloroform/methanol/water fraction are indicated on the left. To provide an authentic standard for PP1*, PP1 was purified by elution from a TLC plate and treated without (lane 3) and with (lane 4) 10 mM periodate for 10 min. Samples were chromatographed and visualized by autoradiography. (B) Δgpi8 cells labeled for 2.5 h with 3H-mannose and then incubated without (lane 1) or with (lane 2) 10 mM sodium periodate. Lipids extracted from Δgpi8 cells were incubated in the absence (lane 3) or presence (lane 4) of 10 mM periodate for 10 min. Samples were chromatographed as in A.
Δgpi8 Do Not Establish a Successful Infection in Tsetse
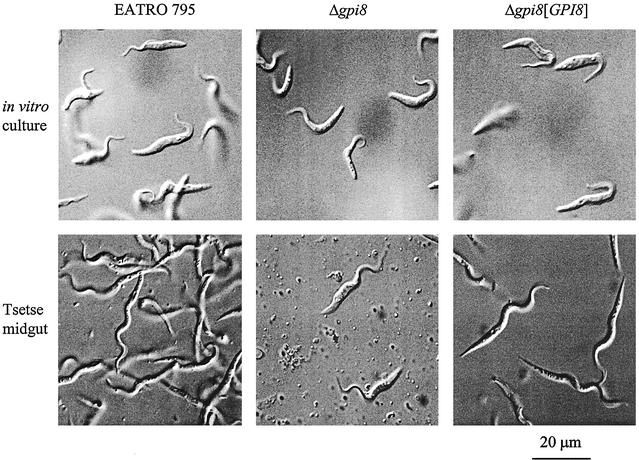
The repeat domains of T. brucei procyclins are known to be resistant to digestion by tsetse proteases (Acosta-Serrano et al., 2001), whereas their GPI anchors are modified with a large poly-N-acetyllactosamine side chain (Treumann et al., 1997). This has led to the hypothesis that one of the major functions of the procyclin coat is to form a glycocalyx, which provides the cell with a protective barrier against the digestive enzymes of the tsetse fly midgut (Ferguson et al., 1993; Ruepp et al., 1997). In the current study, wild-type, Δgpi8 or Δgpi8[GPI8] cells were fed to teneral tsetse flies as part of a blood meal, with midgut dissection of flies 14 d later. Wild-type and Δgpi8[GPI8] procyclics infected 49 and 37% of flies, respectively, whereas Δgpi8 infected <2% (Table 1). Furthermore, both of the flies infected by Δgpi8 harbored very sparse populations of parasites, and these cells resembled cultured procyclic forms rather than the more serpentine procyclic forms that characterize established midgut infections (Figure 6). Because Δgpi8[GPI8] cells behaved in a manner similar to the wild-type parasites, both in rate of infection and midgut morphology, failure of Δgpi8 trypanosomes to establish in tsetse must be due to the lack of GPI:protein transamidase activity.
Table 1.
Wild-type EATRO 795, Δgpi8 and Δgpi8[GPI8] were fed to teneral tsetse flies as part of a blood meal
| Clone | EATRO 795 | Δgpi8 | Δgpi8[GPI8] |
|---|---|---|---|
| Expt 1 | * | 1 /39 | 11 /33 |
| Expt 2 | 24 /49 | 1 /66 | 9 /20 |
| Expt 3 | 29 /59 | * | 24 /65 |
| Collated data | 53 /108 | 2 /105 | 44 /118 |
| % infected | 49 | 2 | 37 |
Each study was carried out at least twice as indicated, dependent on availability of tsetse. Mid-gut dissections were carried out after 14 d. For each experiment, the number of flies harboring parasites/total number of flies dissected is shown. Collated % infection is shown at the bottom.
Figure 6.
Tsetse fly infectivity. Wild-type EATRO 795, Δgpi8, and Δgpi8[GPI8] were fed to teneral tsetse flies as part of a blood meal. After 14 d, midguts were dissected and examined microscopically. Images of living parasites from in vitro cultures and tsetse dissections are displayed.
GPI8 Is Essential for Cell Cycle Progression in Bloodstream form Trypanosomes
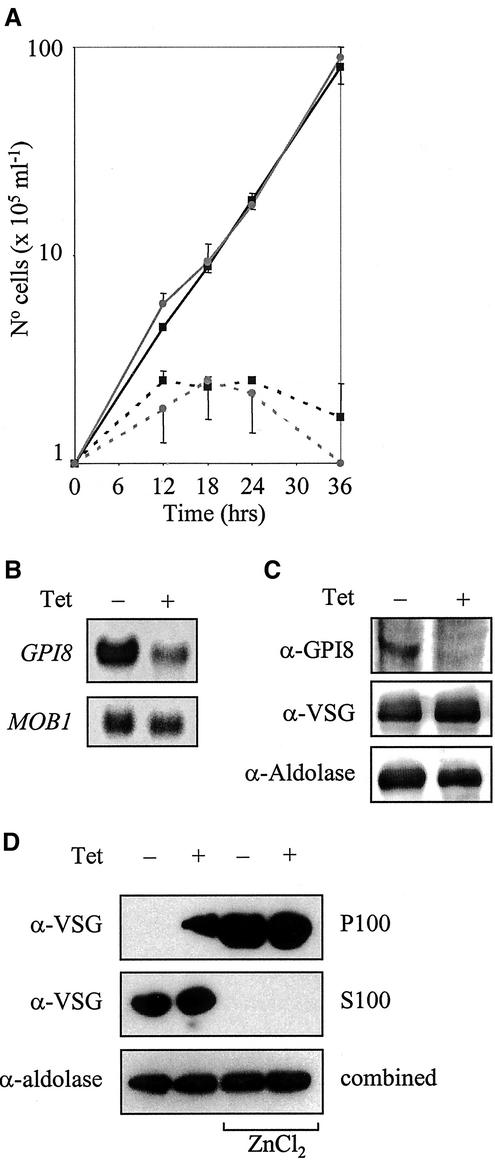
We used an inducible RNAi approach (Ngo et al., 1998; Wang et al., 2000) to investigate the requirement for GPI8 in bloodstream trypanosomes. Culturing cells in the presence of tetracycline-induced expression of double-stranded GPI8 RNA. A severe growth deficit was observed in induced cells when compared with uninduced controls (Figure 7A). Northern blotting confirmed that GPI8 mRNA levels were reduced significantly in induced cells relative to a MOB1 control (Figure 7B), whereas Western blotting demonstrated GPI8 to be apparently absent (Figure 7C). Interestingly, GPI8 RNAi induction resulted in a 10% increase in the abundance of VSG (Figure 7C) in comparison to an adolase control, leading us to speculate that accumulation of unanchored precursor proteins may be occurring as a result of reduced GPI anchoring. To address this question, cells cultured for 24 h −/+ induction were hypotonically lysed and then incubated at 37°C for 30 min. This treatment allowed the endogenous trypanosomal GPI-PLC to hydrolyze the GPI anchor of VSG, converting the glycoprotein from a membrane bound to a water-soluble form (De Almeida and Turner, 1983). As a control, cells were lysed in the presence of 10 mM ZnCl2, a known inhibitor of GPI-PLC. After ultracentrifugation, P100 and S100 fractions were analyzed by Western blotting with α-VSG 221 or with α-aldolase as a loading control. In uninduced cells, GPI-PLC activity resulted in all VSG being found in the S100 fraction as had been predicted. By contrast, RNAi induction resulted in the accumulation of proVSG, which was resistant to GPI-PLC activity and hence remained associated with membranes.
Figure 7.
RNAi of bloodstream form trypanosomes. (A) Growth curve of two independently derived clones in the absence (solid line) or presence (dashed line) of 1 μg ml−1 tetracycline. The experiment was carried out in triplicate and bars denoting SD are shown. (B) Northern blots of total RNA prepared from parasites 12 h −/+ induction, electrophoresed on a 1% agarose gel and transferred onto nylon membrane. Duplicate blots were hybridized with 32P-labeled ORFs of either GPI8 or MOB1. (C) Western blot analysis of whole cell lysate 24 h −/+ induction probed with α-GPI8, α-VSG 221, or α-aldolase. A Typhoon 8600 phosphoimager was used for detection and quantitation. (D) Cells, 1 × 107, cultured for 24 h −/+ induction were lysed on ice in 25 μl 0.05% TX-100 with 1 mg ml−1 pefabloc and 1 μg ml−1 leupeptin and then incubated at 37°C for 30 min. Alternatively cells were lysed as above in the presence of 10 mM ZnCl2 and maintained on ice for 5 min. Lysates were made up to 1 ml, after which they were centrifuged at 100,000 × g for 30 min at 4°C to yield P100 and S100 fractions. Samples were analyzed by Western blotting with α-VSG 221 or α-aldolase.
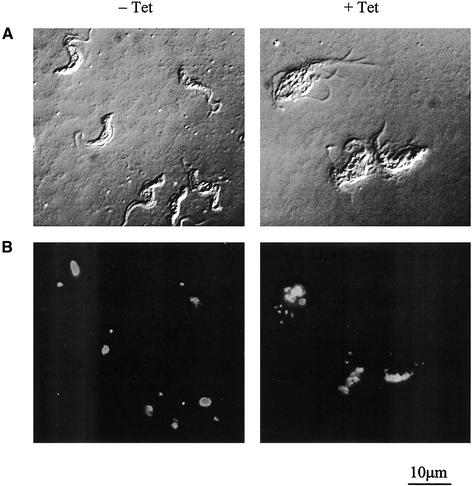
Microscopic analysis revealed that by 12 h post-induction cells had begun to swell and lose motility and that after 18 h the morphology of many was radically altered. DAPI staining revealed that the majority of these cells had karyotype defects (Monsters), with multiple nuclei, kinetoplasts, and flagella (Figure 8). This suggests that lack of GPI:protein transamidase activity results in perturbation of cell cycle control, leading to cell death.
Figure 8.
Microscopic analysis of cultured bloodstream form parasites without (−) or with (+) 18 h tetracycline induction of GPI8 RNAi using (A) phase contrast or (B) DAPI staining of DNA.
DISCUSSION
The GPI8 gene of T. brucei encodes a predicted protein with significant sequence identity to GPI8 from L. mexicana, S. cerevisiae, and H. sapiens. However, although GPI8 is a type I ER membrane protein in both S. cerevisiae and H. sapiens, hydropathy analysis indicates that the trypanosome GPI8 lacks a C-terminal transmembrane domain. This is consistent both with the hydropathy profile of the GPI8 of the closely related trypanosomatid L. mexicana (Hilley et al., 2000; Sharma et al., 2000) and our previous studies that indicated that trypanosomal GPI8 is a soluble ER protein (Sharma et al., 2000). Deletion of GPI8 from procyclic T. brucei resulted in parasites deficient in GPI-anchored proteins (Figure 3) and with an accumulation of precursor GPIs (Figure 4A), confirming that GPI8 is a functional component of the GPI:protein transamidase of T. brucei. The parasite does not have an alternative pathway for GPI anchor addition to proteins, making the GPI8 cysteine protease essential for the production of GPI-anchored proteins.
A previous study demonstrated that disruption of TbGPI10, which encodes the third mannosyltransferase in GPI anchor biosynthesis, resulted in procyclic cells with an in vitro doubling time approximately twice that of their wild-type counterpart and a requirement for nonadherent culture conditions (Nagamune et al., 2000). Surprisingly, despite their reduced fitness in vitro, these mutants retained their ability to establish infection within the midgut of tsetse, albeit with reduced efficiency. As both GPI8 and GPI10 are essential for GPI anchoring of proteins, we expected that deletion of GPI8 would result in a phenotype that mimicked GPI10 null mutants. However, the current study has demonstrated that the GPI8 and GPI10 null mutants differ in several key respects. Although both have been shown to lack GPI-anchored proteins, Δgpi8 did not require nonadherent culture conditions for growth, had a cell cycle of comparable length to their wild-type parent, and yet were unable to establish infection within the tsetse fly midgut. Comparison of metabolically labeled GPI lipids from these two mutants provides an explanation for these differences. Δgpi8 accumulated PP1, the complete preformed GPI substrate of the transamidase complex of procyclic T. brucei (Mayor et al., 1991; Field et al., 1991a). This is in keeping with similar data from L. mexicana (Hilley et al., 2000), S. cerevisiae (Benghezal et al., 1996), and human (Yu et al., 1997), all of which accumulate mature GPI in the absence of GPI8 function. Furthermore, our data indicate that Δgpi8 cells express part of the accumulated PP1 pool on their plasma membrane. Δgpi10, by contrast, did not synthesize complete GPI anchors and accumulated different mannolipids than wild-type cells (Nagamune et al., 2000). They could not express the free surface GPIs we report here, therefore explaining their reduced fitness in vitro.
Expression of free GPIs on the surface of eukaryotic cells is not without precedent (McConville and Ferguson, 1993; Baumann et al., 2000) and has also been discovered recently in “naked” procyclic trypanosomes deficient in procyclin (E. Vassella, personal communication). A number of novel lipid species were found to build up in the Δgpi8 mutant, which were not detected in the wild-type (Figure 4A). These may be a result of the accumulating lipids being acted upon by the normal biosynthetic pathway that decorates lipid anchors in the Golgi and, if this is the case, these lipids are unlikely to be substrates for the GPI:protein transamidase, which is located in the ER. The possibility of free GPIs on the surface explains our observation that Con A was capable of killing Δgpi8 cells. Previous studies have shown that Con A binds to the N-linked glycans of EP procyclins 1 and 3, resulting in a procyclic cell death phenotype that has been likened to apoptosis in metazoan cells (Welburn et al., 1996). Clones that exclusively express the procyclins EP2 and GPEET, both of which lack N-glycosylation sites and are not bound by ConA, are resistant to this form of cell death (Pearson et al., 2000). The plasma membrane of Δgpi8 cells lack all procyclin, but have the tri-mannose GPI, PP1, which is capable of binding ConA (Vidugiriene and Menon, 1994). Although Con A–induced death of wild-type procyclic parasites is mediated through interaction of the lectin with procyclin-borne glycans, the actual mechanism by which this kills the cells is not understood. It is therefore entirely feasible that interaction of Con A with the free GPIs of Δgpi8 cells could elicit the same cellular response. Interestingly, we have noted previously that there are two pools of PP1 in wild-type parasites, one pool that is turned over with rapid kinetics (t1/2 = 8 h) and a more stable pool (M. Field, unpublished). The presence of two pools of PP1 is consistent with the concept that a portion of PP1 is located on the surface of wild-type parasites as well as Δgpi8.
Surface expression of free GPIs by Δgpi8 cells also provides an explanation for our observation that these mutants cannot establish an infection in the tsetse midgut. One of the proposed functions of the GPI-anchored procyclin coat of T. brucei is provision of cells with a protease-resistant glycocalyx, enabling them to survive within the lumen of the tsetse fly midgut. Deletion either of EP procyclin genes (Ruepp et al., 1997) or GPI10 (Nagamune et al., 2000) resulted in reduced ability of procyclic cultures to establish infections within tsetse, supporting this view. However, although reduced infection frequencies were reported in both of these studies, heavy infections were still observed. By contrast, the current study detected Δgpi8 cells in only 2% of flies dissected and with very low parasitaemia and abnormal morphology in both cases. This suggests that tsetse flies are essentially refractory to the successful establishment of Δgpi8. Little is known about the molecular basis for refractoriness of tsetse to trypanosome infection. There is some circumstantial evidence to suggest that tsetse flies possess a trypanocidal midgut lectin (Maudlin and Welburn, 1987; Welburn et al., 1994), although this lectin has never been purified and characterized. Such a lectin could be responsible for the inability of Δgpi8 parasites to establish infection within the midgut of the vector, with the tri-mannose core of their exposed GPI coat being bound by the lectin. Δgpi10 mutants by contrast lack this free GPI on their surface and would not be susceptible to agglutination. An alternative explanation is that the surface architecture of the procyclic form will differ between Δgpi8 and Δgpi10 mutants because of the variation in their free GPIs. Such differences may alter their susceptibility to other tsetse immune responses, such as the antimicrobial peptides attacin, defensin, and diptericin (Hao et al., 2001).
The Δgpi8 cells that were detected in the midguts of the two tsetse were morphologically indistinguishable from counterparts grown in in vitro cultures. By contrast, wild-type and Δgpi8[GPI8] parasites observed in the midguts had all assumed the phenotype characteristic of posterior midgut procyclic trypomastigotes (as described in Van den Abbeele et al., 1999). This shows that the failure to differentiate was a direct result of the GPI8 deletion. However, such a phenotype was not reported with the Δgpi10 mutants, suggesting that it is not simply a consequence of the lack of GPI-anchored proteins.
RNAi was used to show that GPI8 is essential in bloodstream form trypanosomes grown in in vitro culture. Northern and Western blot data demonstrated specific down-regulation of both GPI8 mRNA and protein, with concomitant accumulation of proVSG. Recently it has been shown that proVSG with an altered GPI-anchor signal sequence accumulates within the ER and is then degraded (Bohme and Cross, 2002). Our data provide direct evidence to support more circumstantial data (Nagamune et al., 2000) that expression of GPI-anchored proteins are essential for bloodstream form trypanosomes. Cells with reduced GPI8 rapidly lost viability, displaying obvious dysfunction in cell cycle progression with an accumulation of cells with multiple nuclei, flagella, and kinetoplasts. This phenotype is consistent with a block in cytokinesis. Studies in our laboratory have indicated that trypanosomes lack certain key cell cycle checkpoints that are present in most eukaryotes. In particular, RNAi of the mitotic cyclin CYC6 in bloodstream form trypanosomes led to a block in mitosis but allowed reinitiation of nuclear and kinetoplast S-phase (Hammarton and Mottram, in preparation). For the GPI8 RNAi mutants a block in cytokinesis does not appear to prevent kinetoplast and nuclear replication or subsequent organelle segregation, reinforcing the finding that initiation of S-phase can occur in the absence of completion of cytokinesis. Cell cycle arrest in response to a defect in GPI biosynthesis has been reported previously in S. pombe (Colussi and Orlean, 1997). It has been hypothesized that this phenomenon in yeast could be the result of inefficient delivery of GPI-anchored proteins involved in cell division to their site of action. Depletion of GPI anchors in Trypanosoma cruzi by heterologous expression of T. brucei GPI-PLC lead to an apparent block in mitosis (Garg et al., 1997). In T. brucei, it is possible that reduction or ablation of GPI-anchored receptors (e.g., transferrin) or other GPI-anchored protein such as BARP (Nolan et al., 2000) may render cells incapable of undergoing cytokinesis. Alternatively, it is possible that accumulation of GPI precursors, or intracellular proVSG, results in perturbation of the cell cycle or that VSG occupancy of the plasma membrane is required at a given density. In the later scenario, reduction in the rate at which VSG is anchored and trafficked to the cell surface could negatively impact on the rate at which membrane is synthesized, resulting in cells being unable to undergo cytokinesis at a time when mitosis has been initiated. Whatever the cause, the current work clearly demonstrates that reduced GPI:protein transamidase activity results in a cytokinesis block in bloodstream form trypanosomes.
ACKNOWLEDGMENTS
We thank Alyson Lewis for excellent technical assistance with the tsetse fly infections, Mike Ferguson for suggestions on solvent extractions for the periodate experiment, and Tansy Hammarton for incisive comments on trypanosome cell cycle control. This study was supported by the Medical Research Council (UK). J.C.M. is a MRC Senior Research Fellow. M.C.F. and P.B. acknowledge support from the Wellcome Trust.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–03–0167. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–03–0167.
Corresponding author. E-mail address: j.mottram@udcf.gla.ac.uk.
REFERENCES
- Acosta-Serrano A, Cole RN, Englund PT. Killing of Trypanosoma brucei by concanavalin A: structural basis of resistance in glycosylation mutants. J Mol Biol. 2000;304:633–644. doi: 10.1006/jmbi.2000.4246. [DOI] [PubMed] [Google Scholar]
- Acosta-Serrano A, Vassella E, Liniger M, Renggli CK, Brun R, Roditi I, Englund PT. The surface coat of procyclic Trypanosoma brucei: programmed expression and proteolytic cleavage of procyclin in the tsetse fly. Proc Natl Acad Sci USA. 2001;98:1513–1518. doi: 10.1073/pnas.041611698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangs JD, Ransom DM, McDowell MA, Brouch EM. Expression of bloodstream variant surface glcoproteins in procyclic stage Trypanosoma brucei: role of GPI anchors in secretion. EMBO J. 1997;16:4285–4294. doi: 10.1093/emboj/16.14.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AJ, Rawlings ND. Evolutionary lines of cysteine peptidases. Biol Chem. 2001;382:727–733. doi: 10.1515/BC.2001.088. [DOI] [PubMed] [Google Scholar]
- Baumann NA, Vidugiriene J, Machamer CE, Menon AK. Cell surface display and intracellular trafficking of free glycosylphosphatidylinositols in mammalian cells. J Biol Chem. 2000;275:7378–7389. doi: 10.1074/jbc.275.10.7378. [DOI] [PubMed] [Google Scholar]
- Benghezal M, Benachour A, Rusconi S, Aebi M, Conzelmann A. Yeast Gpi8p is essential for GPI anchor attachment onto proteins. EMBO J. 1996;15:6575–6583. [PMC free article] [PubMed] [Google Scholar]
- Bohme U, Cross GAM. Mutational analysis of the variant surface glycoprotein GPI-anchor signal sequence in Trypanosoma brucei. J Cell Sci. 2002;115:805–816. doi: 10.1242/jcs.115.4.805. [DOI] [PubMed] [Google Scholar]
- Brooks DR, McCulloch R, Coombs GH, Mottram JC. Stable transformation of trypanosomatids through targeted chromosomal integration of the selectable marker gene encoding basticidin S deaminase. FEMS Microbiol Lett. 2000;186:287–291. doi: 10.1111/j.1574-6968.2000.tb09119.x. [DOI] [PubMed] [Google Scholar]
- Brun R, Schonenberger M. Cultivation and in vitro cloning of procyclic forms of Trypanosoma brucei in a semi-defined medium. Acta Trop. 1979;36:289–292. [PubMed] [Google Scholar]
- Butikofer P, Malherbe T, Boschung M, Roditi I. GPI-anchored proteins: now you see 'em, now you don't. FASEB J. 2001;15:545–548. doi: 10.1096/fj.00-0415hyp. [DOI] [PubMed] [Google Scholar]
- Butikofer P, et al. Phosphorylation of a major GPI-anchored surface protein of Trypanosoma brucei during transport to the plasma membrane. J Cell Sci. 1999;112:1785–1795. doi: 10.1242/jcs.112.11.1785. [DOI] [PubMed] [Google Scholar]
- Clayton CE, Mowatt MR. The procyclic acidic repetitive proteins of Trypanosoma brucei—purification and post-translational modification. J Biol Chem. 1989;264:15088–15093. [PubMed] [Google Scholar]
- Colussi PA, Orlean P. The essential Schizosaccharomyces pombe gpi1(+) gene complements a bakers' yeast GPI anchoring mutant and is required for efficient cell separation. Yeast. 1997;13:139–150. doi: 10.1002/(SICI)1097-0061(199702)13:2<139::AID-YEA69>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- De Almeida MLC, Turner MJ. The membrane form of variant surface glycoproteins of Trypanosoma brucei. Nature. 1983;302:349–352. doi: 10.1038/302349a0. [DOI] [PubMed] [Google Scholar]
- Ferguson MAJ. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J Cell Sci. 1999;112:2799–2809. doi: 10.1242/jcs.112.17.2799. [DOI] [PubMed] [Google Scholar]
- Ferguson MAJ, et al. The GPI biosynthetic pathway as a therapeutic target for African sleeping sickness. Biochim Biophys Acta Mol Basis Dis. 1999;1455:327–340. doi: 10.1016/s0925-4439(99)00058-7. [DOI] [PubMed] [Google Scholar]
- Ferguson MAJ, Homans SW, Dwek RA, Rademacher TW. Glycosyl-phosphatidylinositol moiety that anchors Trypanosoma brucei variant surface glycoprotein to the membrane. Science. 1988;239:753–759. doi: 10.1126/science.3340856. [DOI] [PubMed] [Google Scholar]
- Ferguson MAJ, Murray P, Rutherford H, McConville MJ. A simple purification of procyclic acidic repetitive protein and demonstration of a sialylated glycosyl-phosphatidylinositol membrane anchor. Biochem J. 1993;291:51–55. doi: 10.1042/bj2910051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MC, Menon AK, Cross GAM. A glycosylphosphatidylinositol protein anchor from procyclic stage Trypanosoma brucei—lipid structure and biosynthesis. EMBO J. 1991a;10:2731–2739. doi: 10.1002/j.1460-2075.1991.tb07821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MC, Menon AK, Cross GAM. Developmental variation of glycosylphosphatidylinositol membrane anchors in Trypanosoma brucei—identification of a candidate biosynthetic precursor of the glycosylphosphatidylinositol anchor of the major procyclic stage surface glycoprotein. J Biol Chem. 1991b;266:8392–8400. [PubMed] [Google Scholar]
- Fraering P, Imhof I, Meyer U, Strub JM, van Dorsselaer A, Vionnet C, Conzelmann A. The GPI transamidase complex of Saccharomyces cerevisiae contains Gaa1p, Gpi8p, and Gpi16p. Mol Biol Cell. 2001;12:3295–3306. doi: 10.1091/mbc.12.10.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg N, Tarleton RL, MensaWilmot K. Proteins with glycosylphosphatidylinositol (GPI) signal sequences have divergent fates during a GPI deficiency—GPIs are essential for nuclear division in Trypanosoma cruzi. J Biol Chem. 1997;272:12482–12491. doi: 10.1074/jbc.272.19.12482. [DOI] [PubMed] [Google Scholar]
- Gerber LD, Kodukula K, Udenfriend S. Phosphatidylinositol glycan (PI-G) anchored membrane proteins. Amino acid requirements adjacent to the site of cleavage and PI-G attachment in the COOH-terminal signal peptide. J Biol Chem. 1992;267:12168–12173. [PubMed] [Google Scholar]
- Hao Z, Kasumba I, Lehane MJ, Gibson WC, Kwon J, Aksoy S. Tsetse immune responses and trypanosome transmission: Implications for the development of tsetse-based strategies to reduce trypanosomiasis. Proc Natl Acad Sci USA. 2001;98:12648–12653. doi: 10.1073/pnas.221363798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilley JD, Zawadzki J, McConville MJ, Coombs GH, Mottram JC. Leishmania mexicana mutants lacking glycosylphosphatidyl (GPI):protein transamidase provide insights into the biosynthesis and functions of GPI-anchored proteins. Mol Biol Cell. 2000;11:1183–1195. doi: 10.1091/mbc.11.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirumi H, Hirumi K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum-protein without feeder cell-layers. J Parasitol. 1989;75:985–989. [PubMed] [Google Scholar]
- Kinoshita T, Inoue N. Dissecting and manipulating the pathway for glycosylphosphatidylinositol-anchor biosynthesis. Curr Opin Chem Biol. 2000;4:632–638. doi: 10.1016/s1367-5931(00)00151-4. [DOI] [PubMed] [Google Scholar]
- LaCount DJ, Bruse S, Hill KL, Donelson JE. Double-stranded RNA interference in Trypanosoma brucei using head-to-head promoters. Mol Biochem Parasitol. 2000;111:67–76. doi: 10.1016/s0166-6851(00)00300-5. [DOI] [PubMed] [Google Scholar]
- Lin SR, Yu IS, Huang PH, Tsai CW, Lin SW. Chimeric mice with disruption of the gene coding for phosphatidylinositol glycan class A (Pig-a) were defective in embryogenesis and spermatogenesis. Br J Hematol. 2000;110:682–693. doi: 10.1046/j.1365-2141.2000.02209.x. [DOI] [PubMed] [Google Scholar]
- Maudlin I, Welburn SC. Lectin mediated establishment of midgut infections of Trypanosoma congolense and Trypanosoma brucei in Glossina morsitans. Trop Med Parasitol. 1987;38:167–170. [PubMed] [Google Scholar]
- Maxwell SE, Ramalingam S, Gerber LD, Brink L, Udenfriend S. An active carbonyl formed during glycosylphosphatidylinositol addition to a protein is evidence of catalysis by a transamidase. J Biol Chem. 1995;270:19576–19582. doi: 10.1074/jbc.270.33.19576. [DOI] [PubMed] [Google Scholar]
- Mayor S, Menon AK, Cross GAM. Transfer of glycosyl-phosphatidylinositol membrane anchors to polypeptide acceptors in a cell free system. J Cell Biol. 1991;114:61–71. doi: 10.1083/jcb.114.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville MJ, Bacic A. The glycoinositolphos-pholipid profiles of 2 Leishmania major strains that differ in lipophosphoglycan expression. Mol Biochem Parasitol. 1990;38:57–67. doi: 10.1016/0166-6851(90)90205-z. [DOI] [PubMed] [Google Scholar]
- McConville MJ, Ferguson MAJ. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993;294:305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran P, Caras IW. Fusion of sequence elements from nonanchored proteins to generate a fully functional signal for glycophosphatidylinositol membrane anchor attachment. J Cell Biol. 1991;115:1595–1600. doi: 10.1083/jcb.115.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowatt MR, Wisdom GS, Clayton CE. Variation of tandem repeats in the developmentally regulated procyclic acidic repetitive proteins of Trypanosoma brucei. Mol Cell Biol. 1989;9:1332–1335. doi: 10.1128/mcb.9.3.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamune K, et al. Critical roles of glycosylphosphatidylinositol for Trypanosoma brucei. Proc Natl Acad Sci USA. 2000;97:10336–10341. doi: 10.1073/pnas.180230697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo H, Tschudi C, Gull K, Ullu E. Double-stranded RNA induces mRNA degradation in Trypanosoma brucei. Proc Natl Acad Sci USA. 1998;95:14687–14692. doi: 10.1073/pnas.95.25.14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan DP, Jackson DG, Biggs MJ, Brabazon ED, Pays A, Van Laethem F, Paturiaux-Hanocq F, Elliot JF, Voorheis HP, Pays E. Characterization of a novel alanine-rich protein located in surface microdomains in Trypanosoma brucei. J Biol Chem. 2000;275:4072–4080. doi: 10.1074/jbc.275.6.4072. [DOI] [PubMed] [Google Scholar]
- Nuoffer C, Horvath A, Riezman H. Analysis of the sequence requirements for glycosylphosphatidylinositol anchoring of Saccharomyces cerevisiae Gas1 protein. J Biol Chem. 1993;268:10558–10563. [PubMed] [Google Scholar]
- Ohishi K, Inoue N, Kinoshita T. PIG-S, and PIG-T, essential for GPI anchor attachment to proteins, form a complex with GAA1, and GPI8. EMBO J. 2001;20:4088–4098. doi: 10.1093/emboj/20.15.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson TW, Beecroft RP, Welburn SC, Ruepp S, Roditi I, Hwa KY, Englund PT, Wells CW, Murphy NB. The major cell surface glycoprotein procyclin is a receptor for induction of a novel form of cell death in African trypanosomes in vitro. Mol Biochem Parasitol. 2000;111:333–349. doi: 10.1016/s0166-6851(00)00327-3. [DOI] [PubMed] [Google Scholar]
- Roditi I, et al. Procyclin gene-expression and loss of the variant surface glycoprotein during differentiation of Trypanosoma brucei. J Cell Biol. 1989;108:737–746. doi: 10.1083/jcb.108.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruepp S, Furger A, Kurath U, Renggli CK, Hemphill A, Brun R, Roditi I. Survival of Trypanosoma brucei in the tsetse fly is enhanced by the expression of specific forms of procyclin. J Cell Biol. 1997;137:1369–1379. doi: 10.1083/jcb.137.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell D, Evers R, Preis D, Ziegelbauer K, Kiefer H, Lottspeich F, Cornelissen AWCA, Overath P. A transferrin-binding protein of Trypanosoma brucei is encoded by one of the genes in the variant surface glycoprotein gene-expression site. EMBO J. 1991;10:1061–1066. doi: 10.1002/j.1460-2075.1991.tb08045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma DK, Hilley JD, Bangs JD, Coombs GH, Mottram JC, Menon AK. Soluble GPI8 restores glycosylphosphatidylinositol anchoring in a trypanosome cell-free system depleted of lumenal endoplasmic reticulum proteins. Biochem J. 2000;351:717–722. [PMC free article] [PubMed] [Google Scholar]
- Treumann A, Zitzmann N, Hulsmeier A, Prescott AR, Almond A, Sheehan J, Ferguson MJ. Structural characterization of two forms of procyclic acidic repetitive protein expressed by procyclic forms of Trypanosoma brucei. J Mol Biol. 1997;269:529–547. doi: 10.1006/jmbi.1997.1066. [DOI] [PubMed] [Google Scholar]
- Van den Abbeele J, Claes Y, van Bockstaele D, Le Ray D, Coosemans M. Trypanosoma brucei spp. development in the tsetse fly: characterization of the post-mesocyclic stages in the foregut and proboscis. Parasitology. 1999;118:469–478. doi: 10.1017/s0031182099004217. [DOI] [PubMed] [Google Scholar]
- Vidugiriene J, Menon AK. The GPI anchor of cell-surface proteins is synthesized on the cytoplasmic face of the endoplasmic-reticulum. J Cell Biol. 1994;127:333–341. doi: 10.1083/jcb.127.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Morris JC, Drew ME, Englund PT. Inhibition of Trypanosoma brucei gene expression by RNA interference using an integratable vector with opposing T7 promoters. J Biol Chem. 2000;275:40174–40179. doi: 10.1074/jbc.M008405200. [DOI] [PubMed] [Google Scholar]
- Watanabe R, Murakami Y, Marmor MD, Inoue N, Maeda Y, Hino J, Kangawa K, Julius M, Kinoshita T. Initial enzyme for glycosylphosphatidylinositol biosynthesis requires PIG-P and is regulated by DPM2. EMBO J. 2000;19:4402–4411. doi: 10.1093/emboj/19.16.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn SC, Dale C, Ellis D, Beecroft R, Pearson TW. Apoptosis in procyclic Trypanosoma brucei rhodesiense in vitro. Cell Death Differ. 1996;3:229–236. [PubMed] [Google Scholar]
- Welburn SC, Maudlin I, Molyneux DH. Midgut lectin activity and sugar specificity in teneral and fed tsetse. Med Vet Entomol. 1994;8:81–87. doi: 10.1111/j.1365-2915.1994.tb00391.x. [DOI] [PubMed] [Google Scholar]
- Wirtz E, Leal S, Ochatt C, Cross GA. A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol Biochem Parasitol. 1999;99:89–101. doi: 10.1016/s0166-6851(99)00002-x. [DOI] [PubMed] [Google Scholar]
- Yu JL, Nagarajan S, Knez JJ, Udenfriend S, Chen R, Medof ME. The affected gene underlying the class K glycosylphosphatidylinositol (GPI) surface protein defect codes for the GPI transamidase. Proc Natl Acad Sci USA. 1997;94:12580–12585. doi: 10.1073/pnas.94.23.12580. [DOI] [PMC free article] [PubMed] [Google Scholar]