Abstract
The serum- and glucocorticoid-regulated kinase (sgk1) is induced by mineralocorticoids and, in turn, upregulates heterologously expressed renal epithelial Na+ channel (ENaC) activity in Xenopus oocytes. Accordingly, Sgk1 is considered to mediate the mineralocorticoid stimulation of renal ENaC activity and antinatriuresis. Here we show that at standard NaCl intake, renal water and electrolyte excretion is indistinguishable in sgk1-knockout (sgk1–/–) mice and wild-type (sgk1+/+) mice. In contrast, dietary NaCl restriction reveals an impaired ability of sgk1–/– mice to adequately decrease Na+ excretion despite increases in plasma aldosterone levels and proximal-tubular Na+ and fluid reabsorption, as well as decreases in blood pressure and glomerular filtration rate.
Introduction
Mineralocorticoid regulation of the renal epithelial Na+ channel (ENaC) is of paramount importance for the maintenance of extracellular fluid volume and blood pressure (1). Targeted disruption of αENaC (2, 3), βENaC (4), γENaC (5), or the mineralocorticoid receptor (6) in mice is either not compatible with survival or leads to severe salt wasting. Similarly, loss-of-function mutations of ENaC subunits (7, 8) or the mineralocorticoid receptor (9) in humans cause pseudohypoaldosteronism type 1, characterized by salt wasting and hypotension, despite elevated levels of aldosterone. Conversely, gain-of-function mutations of ENaC (Liddle syndrome) (10) or of the mineralocorticoid receptor (11) lead to severe hypertension. Low dietary Na+ intake (12, 13) or exogenous application of aldosterone (14) increases the apical abundance of ENaC in the renal collecting system, the renal target for mineralocorticoid action (15). Recently, compelling evidence has been gathered pointing to an important role for the serum- and glucocorticoid-regulated kinase (Sgk1) in the regulation of ENaC by mineralocorticoids (16). The sgk1 gene was originally cloned as a glucocorticoid-sensitive (17, 18) or a cell volume–regulated (19) gene and was subsequently shown to be strongly upregulated by mineralocorticoids (20–23). In kidney, Sgk1 is highly expressed in the collecting system (14, 20, 24), where aldosterone-induced apical translocation of ENaC is preceded by an upregulation of Sgk1 in ENaC-positive epithelial cells (14). In Xenopus oocytes expressing the α, β, and γ subunits of ENaC, coexpression of Sgk1 results in a marked upregulation of Na+ channel activity (20, 21, 23–26), presumably by increasing ENaC abundance in the cell membrane (14, 23, 26). Although highly suggestive, these observations do not allow one to estimate the contribution of Sgk1 to renal Na+ reabsorption and extracellular fluid homeostasis. To address the functional significance of Sgk1 expression in vivo, we generated sgk1-deficient mice. We show that Sgk1 indeed participates in, but does not fully account for, the mineralocorticoid regulation of distal-tubular Na+ reabsorption.
Methods
Generation of sgk1–/– mice.
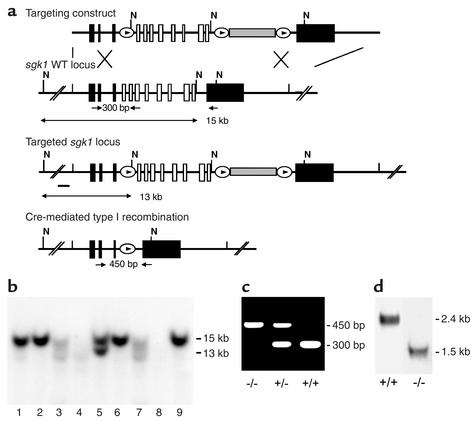
Genomic fragments of sgk1 were isolated from a λ phage genomic library (AB-1) prepared from 129/Sv(ev) embryonic stem (ES) cells. A 7-kb fragment encompassing the entire transcribed region on 12 exons was subcloned into pBluescript (Stratagene, La Jolla, California, USA). This plasmid was used for generation of a conditional targeting vector (Figure 1a). The vector was linearized at a unique NotI site and electroporated into R1 ES cells. Positive clones were identified by Southern blot analysis. A targeted ES cell clone was transiently transfected with Cre recombinase. The resulting recombination types were identified by Southern blot analysis. One clone with a recombination between the first and third loxP site (type I recombination) was injected into C57BL/6 blastocytes. Male chimeras were bred to C57BL/6 and 129/SvJ females. Heterozygous sgk1-deficient mice were either inbred or backcrossed to 129/SvJ wild-type mice for two generations and then intercrossed to generate homozygous sgk1–/– and sgk1+/+ mice. Male and female sgk1–/– mice were used in the studies and compared with littermate sgk1+/+ mice of the respective gender. Mice were genotyped by PCR.
Figure 1.
Generation of sgk1–/– mice. (a) Targeting strategy. The neomycin resistance cassette (gray box) was flanked by two loxP sites (ovals) and inserted into intron 11. Exons 4–11, which code for the Sgk1 kinase domain (open boxes), were “floxed” by inserting a third loxP site into intron 3. N indicates NheI restriction sites, and the small black bar indicates the external 5′ probe used for Southern blot analysis. Expected fragment sizes of the wild-type and targeted sgk1 locus are also indicated. One homologously recombined ES cell clone was transiently transfected with Cre recombinase, and a clone that had undergone recombination between the first and the third loxP site (type I recombination) was chosen for injection. Arrows below the gene indicate PCR primers used for genotyping. Numbers between the arrows indicate the size of the amplified fragments. Crossed bars below a indicate homologous recombination. (b) Southern blot of NheI-digested genomic DNA from ES cell clones after gene targeting hybridized with a 5′ external probe (black bar in a). Lane 5 shows a targeted ES cell line. (c) Genotyping by PCR of genomic tail DNA of homozygous (–/–) and heterozygous (–/+) sgk1-deficient mice and wild-type mice (+/+) using a mix of three specific primers (arrows in a). (d) Autoradiograph of Northern blot analysis of Sgk1-specific transcripts in +/+ and –/– mice. The deletion of the kinase domain from the genome results in a size reduction of 0.9 kb at the mRNA level in sgk1–/– mice.
Northern blot analysis.
Total RNA was isolated from 8-week-old mice using the RNeasy kit (QIAGEN Inc., Valencia, California, USA) and 5 μg was loaded per lane. The probe specific for Sgk1 was labeled with [α-32P]dCTP (Rediprime kit; Amersham Biosciences UKLimited, Buckinghamshire, United Kingdom).
Metabolic cages.
Mice were placed in metabolic cages (Tecniplast Deutschland GmbH, Hohenpeissenberg, Germany) to assess urinary excretion of fluid and Na+ as well as body weight. To assure quantitative urine collection, metabolic cages were siliconized and urine was collected under water-saturated oil. Mice had free access to tap water and the indicated diets; 24-hour urine collections were performed during an adaptation period of 3 days on a standard NaCl diet (2.5 g Na+/kg) and subsequently in response to dietary NaCl restriction for 7 days.
Low-NaCl diets.
In metabolic cage experiments, the response to dietary NaCl restriction was assessed in two series of experiments by application of a diet containing either 0.15 g Na+/kg or 0 g Na+/kg. For measurements under anesthesia such as blood pressure, glomerular filtration rate (GFR), and micropuncture experiments (see below), only the response to the low-NaCl diet containing 0.15 g Na+/kg could be assessed because initial experiments with the 0 g Na+/kg diet revealed that sgk1–/– mice did not survive the required surgical preparation. Plasma aldosterone measurements, immunohistochemistry, and isolated cortical collecting duct perfusion (see below) do not require extensive surgical preparation and were determined in response to the 0 g Na+/kg diet.
Blood pressure measurements and GFR.
Mice were anesthetized with 100 mg/kg intraperitoneal Inactin (Research Biomedicals International, Natick, Massachusetts, USA) and 100 mg/kg intramuscular ketamine. The femoral artery was cannulated for blood pressure measurement and blood sample withdrawal. The jugular vein was cannulated for continuous maintenance infusion of 2.25 g/dl BSA in 0.9% NaCl at a rate of 0.4 ml/h. For assessment of GFR, [3H]inulin was added to deliver 20 μCi/h. Urine collections were performed using a bladder catheter.
Micropuncture experiments.
Mice were prepared as described above. In addition, the left kidney was approached through a flank incision and prepared for micropuncture (27). The last proximal-tubular or first distal-tubular loop on the kidney surface was identified and fluid collections were made. Tubular fluid volumes were determined from column length in a constant-bore capillary. Concentrations of [3H]inulin were measured by liquid-phase scintillation counting. The concentration of Na+ in tubular fluid was determined using a micro-flame-emission photometer (ELEX 6361; Eppendorf AG, Hamburg, Germany) (27).
Determination of plasma and urine concentrations.
Plasma was separated from EDTA-blood collected immediately after anesthesia by heart puncture. The plasma and urine concentrations of Na+ and K+ were measured by micro-flame-emission photometry. Plasma concentrations of aldosterone were measured by radioimmunoassay (Immunotech, Marseille, France). Urine concentrations of chloride were measured by electrometric titration (Chloridometer 6610; Eppendorf AG), and urine osmolarity was determined by freezing point depression (Osmomat 030; Gonotec GmbH, Berlin, Germany).
Immunohistochemistry of the renal collecting system.
Kidney fixation and immunohistochemistry for ENaC were performed as described (14).
Results
On a standard NaCl diet, sgk1-deficient mice display only subtle changes in renal physiology.
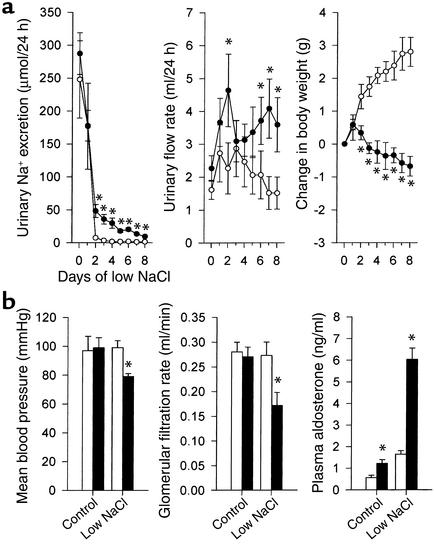
We generated sgk1-deficient mice by conditional gene targeting in murine R1 ES cells (Figure 1). Surprisingly, sgk1–/– mice did not display any gross functional abnormalities. Moreover, histology was normal in all organs analyzed, including skin, brain, skeletal muscle, heart, lung, liver, spleen, pancreas, intestine, colon, ovary, uterus, urinary bladder, and kidney. On a normal diet (2.5 g Na+/kg) with free access to water, there was no significant difference in growth, blood pressure, GFR, or urinary excretion of fluid, Na+, chloride, or potassium between the sgk1–/– and the respective wild-type mice (Figure 2b). However, there were increased aldosterone levels in the sgk1–/– mice (Figure 2b), suggesting that conservation of Na+ homeostasis was achieved only by enhanced aldosterone release. Despite hyperaldosteronism, the plasma K+ concentration was slightly but significantly (P < 0.05) higher in sgk1–/– mice (4.7 ± 0.2 mmol/l, n = 14) than in sgk1+/+ mice (4.1 ± 0.2 mmol/l, n = 13). Decreased activity of the renal ENaC is known to favor the development of hyperkalemia (28).
Figure 2.
Deficient adaptive response to low Na+ intake in sgk1–/– mice. (a) Urinary excretion of Na+ and water, and change in body weight in response to a low-NaCl diet (0 g Na+/kg) assessed in awake mice in metabolic cages (n = 6 each group). (b) Shown are mean arterial blood pressure and GFR in anesthetized mice on the control diet and at day 3 after initiating the low-NaCl diet (0.15 g Na+/kg) (n = 5–6 each group); and plasma aldosterone concentration in mice on control diet and at day 3 after initiating low-NaCl diet (0 g Na+/kg) (n = 8–9 each group). Filled circles and bars indicate sgk1–/– mice, open circles and bars indicate sgk1+/+ mice. *P < 0.05 versus sgk1+/+ mice.
The urine-concentrating ability of sgk1–/– mice is unaltered.
After 36 hours of water deprivation, urine osmolarity increased from 1,082 ± 130 to 3,978 ± 611 milliosmoles (mosmol)/l in sgk1–/– mice (n = 6). These values were similar to those measured in sgk1+/+ mice (683 ± 93 and 3,348 ± 410 mosmol/l, n = 6). Also, weight loss during water deprivation was similar (sgk1–/–, 8.0% ± 1.0%, n = 6; sgk1+/+, 8.6% ± 0.8%, n = 6), indicating that the urine-concentrating ability in the thick ascending limb and the collecting duct of sgk1–/– mice is normal.
Dietary NaCl restriction discloses an impaired ability of sgk1–/– mice to adequately upregulate distal-tubular Na+ reabsorption.
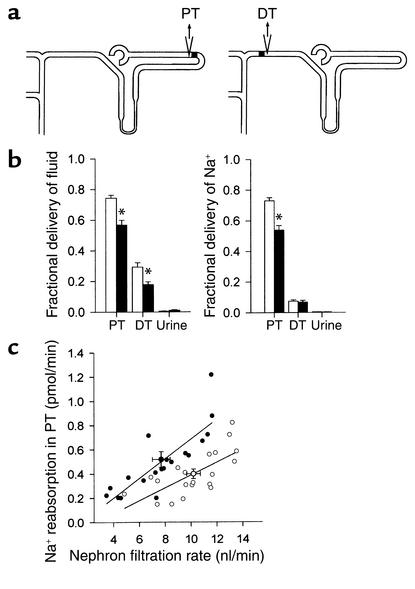
The above results suggest that under normal dietary conditions, renal physiology in sgk1–/– mice is not severely affected. In sharp contrast, dietary NaCl restriction disclosed an impaired ability of sgk1–/– mice to retain Na+ (Figure 2a). Although upon reduced NaCl intake (0 g Na+/kg), sgk1–/– mice decreased renal NaCl excretion, the extent of this decrease was significantly smaller than in sgk1+/+ mice (Figure 2a). Moreover, while sgk1+/+ mice underwent an increase in body weight, sgk1–/– mice showed a net Na+ loss that was accompanied by weight loss under these conditions (Figure 2a). Similar results were obtained with a diet of 0.15 g Na+/kg. Three days and 7 days after initiating this diet, Na+ excretion was significantly higher in sgk1–/– mice than in sgk1+/+ mice (71 ± 16 vs. 24 ± 9 and 37 ± 9 vs. 15 ± 3 μmol/24 h for day 3 and day 7, respectively; P < 0.05). These experiments were conducted for better comparability to experiments in anesthetized animals that survived surgical preparations only upon infusion of saline and pretreatment with the 0.15g Na+/kg diet but not the 0g Na+/kg diet. In anesthetized, saline-infused sgk1–/– mice but not sgk1+/+ mice, a 0.15g Na+/kg diet leads to a significant decrease in blood pressure and GFR (Figure 2b). Micropuncture experiments confirmed a reduction in single-nephron GFR in sgk1–/– mice in tubular fluid collections from distal tubules (8.4 ± 0.7, n = 13 vs. 10.3 ± 0.5 nl/min, n = 23 nephrons, P < 0.05) as well as from proximal tubules (Figure 3b) and revealed a marked increase of fractional Na+ reabsorption in the proximal tubule (Figure 3b). Accordingly, the delivery of fluid and Na+ to the last proximal-tubular loop and the delivery of fluid to the first distal-tubular loop on the kidney surface was strongly reduced (Figure 3).
Figure 3.
Single-nephron GFR and proximal-tubular Na+ reabsorption are altered in sgk1–/– mice on a low-NaCl diet. (a) Schematic diagram of free-flow collection of tubular fluid from the last loop of the proximal tubule (PT) and the first loop of the distal tubule (DT) on the kidney surface in micropuncture experiments in anesthetized mice at day 3 after initiating a low-NaCl diet (0.15 g Na+/kg). (b) Fractional delivery of fluid and Na+ to proximal tubule and distal tubule as well as urine (n = 13–23 nephrons in 5–6 mice). *P < 0.05 versus sgk1+/+ mice. (c) Correlation of Na+ reabsorption in proximal tubule with single-nephron filtration rate. Note that nephron filtration rate is reduced in sgk1–/– mice and that for any given nephron filtration rate the Na+ reabsorption is higher in sgk1–/– mice than in sgk1+/+ mice, indicating a primary increase in proximal reabsorption in sgk1–/– mice. Symbols are as given in Figure 2 legend.
The renal collecting duct system in sgk1–/– mice on a low NaCl diet expresses αENaC in the apical membrane, but exhibits a decreased amiloride-sensitive transepithelial potential difference.
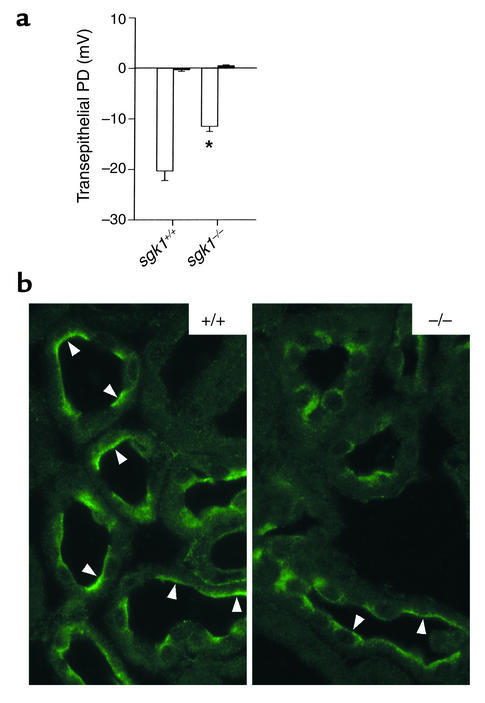
Electrophysiological analysis of isolated perfused collecting duct (29) under conditions of a low-NaCl diet (0 g Na+/kg) revealed an amiloride-sensitive transepithelial potential difference in sgk1–/– mice that was smaller than that in sgk1+/+ mice (Figure 4a). The electrophysiological finding of an amiloride-sensitive transepithelial potential difference is buttressed by immunohistochemical detection of the αENaC subunit in the early portions of the renal collecting system (i.e., the connecting tubule) in both sgk1+/+ and sgk1–/– mice 3 days after initiating the 0 g Na+/kg diet (Figure 4b).
Figure 4.
Amiloride-sensitive transepithelial potential difference is reduced and αENaC is present in the luminal cell membrane of the renal collecting system of sgk1–/– mice on a low-NaCl diet. (a) Transepithelial potential difference (PD) in isolated perfused collecting ducts at day 3 after initiating a low-NaCl diet (0 g Na+/kg) in the absence (n = 12–16 ducts in 3–5 mice) (white bar) and presence (n = 4–5 ducts in 3–5 mice) (black bar) of 10 μM amiloride. *P < 0.05 versus sgk1+/+ mice. (b) Immunohistochemical localization of αENaC in renal connecting tubule profiles at day 3 after initiating a low-NaCl diet (0 g Na+/kg). Arrowheads indicate apical localization of αENaC.
Discussion
The present study demonstrates that Sgk1 participates in, but does not fully account for, the mineralocorticoid regulation of ENaC. On a standard NaCl diet, sgk1–/– mice show unaltered renal Na+ excretion, suggesting that the constitutively present isoforms sgk2 and sgk3 might be able to compensate for the loss of Sgk1 expression (30). However, Sgk1 expression is strongly induced by aldosterone in response to low NaCl intake in the renal collecting duct (16, 20, 22, 23). In accord with this observation, we find that NaCl deprivation unmasks the impaired ability of sgk1–/– mice to adequately upregulate distal-tubular Na+ reabsorption. Our observation that the phenotype of sgk1–/– mice is silent and becomes overt only under challenging conditions highlights the importance of Sgk1 for the adaptational response to altered physiological or pathological conditions. Accordingly, the lack of Sgk1 did not interfere appreciably with the viability of the mice during unrestricted access to NaCl, but clearly compromised survival of anesthesia and surgery during NaCl depletion.
Recent studies have shown that Sgk1 phosphorylates (and presumably thereby inactivates) the ubiquitin ligase Nedd4-2 (31, 32). Consequently, Sgk1 retards ubiquitination and subsequent degradation of ENaC (31, 32), resulting in an increase in ENaC abundance within the cell membrane. Our studies cannot determine whether the reduced ENaC function observed in sgk1–/– mice is a result of accelerated retrieval of the channel protein due to disinhibition of Nedd4-2, or another mechanism. It may be that Sgk1 phosphorylates a Nedd4 other than Nedd4-2, and this may be important for channel function. However, we have shown that Sgk1 is not required for the insertion of ENaC into the apical membrane of distal tubules and collecting ducts. Owing to the residual abundance of ENaC in the cell membrane of sgk1–/– mice, the defect in these mice is relatively mild compared with that in mice with a knockout mutation of αENaC (2, 3), βENaC (4), γENaC (5), or mineralocorticoid receptor (6).
Nevertheless, lack of Sgk1 clearly impedes the upregulation of distal-tubular Na+ reabsorption during Na+ restriction, leading to Na+ depletion despite the presence of increased plasma aldosterone levels and compensatory increases in proximal renal tubular Na+ reabsorption. The Na+ depletion leads to a dramatic decline in mean arterial blood pressure, a severe decrease in glomerular filtration rate, and eventually even the death of the animal.
Acknowledgments
We thank B. Rossier for ENaC antibodies and S. Berger for valuable discussions. This work was supported by grants from Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung (to D. Kuhl, F. Lang, and V. Vallon), and Schweizer Nationalfonds (to J. Loffing).
Footnotes
See the related Commentary beginning on page 1233.
Peer Wulff’s present address is: Department of Clinical Neurobiology, University Hospital of Neurology, Heidelberg, Germany.
Gunther Kauselmann’s present address is: Artemis Pharmaceuticals GmbH, Cologne, Germany.
Conflict of interest: No conflict of interest has been declared.
Nonstandard abbreviations used: epithelial Na+ channel (ENaC); serum- and glucocorticoid-regulated kinase (Sgk1); embryonic stem (ES); glomerular filtration rate (GFR).
References
- 1.Lifton RP. Molecular genetics of human blood pressure variation. Science. 1996;272:676–680. doi: 10.1126/science.272.5262.676. [DOI] [PubMed] [Google Scholar]
- 2.Hummler E, et al. Early death due to defective neonatal lung liquid clearance in alpha-ENaC-deficient mice. Nat Genet. 1996;12:325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- 3.Hummler E, et al. A mouse model for the renal salt-wasting syndrome pseudohypoaldosteronism. Proc Natl Acad Sci USA. 1997;94:11710–11715. doi: 10.1073/pnas.94.21.11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald FJ, et al. Disruption of the beta subunit of the epithelial Na+ channel in mice: hyperkalemia and neonatal death associated with a pseudohypoaldosteronism phenotype. Proc Natl Acad Sci USA. 1999;96:1727–1731. doi: 10.1073/pnas.96.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker PM, et al. Role of γENaC subunit in lung liquid clearance and electrolyte balance in newborn mice. Insights into perinatal adaptation and pseudohypoaldosteronism. J Clin Invest. 1998;102:1634–1640. doi: 10.1172/JCI3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger S, et al. Mineralocorticoid receptor knockout mice: pathophysiology of Na+ metabolism. Proc Natl Acad Sci USA. 1998;95:9424–9429. doi: 10.1073/pnas.95.16.9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang SS, et al. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet. 1996;12:248–253. doi: 10.1038/ng0396-248. [DOI] [PubMed] [Google Scholar]
- 8.Strautnieks SS, Thompson RJ, Gardiner RM, Chung E. A novel splice-site mutation in the gamma subunit of the epithelial sodium channel gene in three pseudohypoaldosteronism type 1 families. Nat Genet. 1996;13:248–250. doi: 10.1038/ng0696-248. [DOI] [PubMed] [Google Scholar]
- 9.Geller DS, et al. Mutations in the mineralocorticoid receptor gene cause autosomal dominant pseudohypoaldosteronism type I. Nat Genet. 1998;19:279–281. doi: 10.1038/966. [DOI] [PubMed] [Google Scholar]
- 10.Shimkets RA, et al. Liddle’s syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell. 1994;79:407–414. doi: 10.1016/0092-8674(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 11.Geller DS, et al. Activating mineralocorticoid receptor mutation in hypertension exacerbated by pregnancy. Science. 2000;289:119–123. doi: 10.1126/science.289.5476.119. [DOI] [PubMed] [Google Scholar]
- 12.Loffing J, et al. Differential subcellular localization of ENaC subunits in mouse kidney in response to high- and low-Na diets. Am J Physiol. 2000;279:F252–F258. doi: 10.1152/ajprenal.2000.279.2.F252. [DOI] [PubMed] [Google Scholar]
- 13.Masilamani S, Kim G-H, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC α, β, and γ subunit proteins in rat kidney. J Clin Invest. 1999;104:19–23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loffing J, et al. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol. 2001;280:F675–F682. doi: 10.1152/ajprenal.2001.280.4.F675. [DOI] [PubMed] [Google Scholar]
- 15.Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242:583–585. doi: 10.1126/science.2845584. [DOI] [PubMed] [Google Scholar]
- 16.Lang F, Cohen P. Regulation and physiological roles of serum- and glucocorticoid-induced protein kinase isoforms. Sci STKE. 2001;108:RE17. doi: 10.1126/stke.2001.108.re17. [DOI] [PubMed] [Google Scholar]
- 17.Webster MK, Goya L, Firestone GL. Immediate-early transcriptional regulation and rapid mRNA turnover of a putative serine/threonine protein kinase. J Biol Chem. 1993;268:11482–11485. [PubMed] [Google Scholar]
- 18.Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol. 1993;13:2031–2040. doi: 10.1128/mcb.13.4.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waldegger S, Barth P, Raber G, Lang F. Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc Natl Acad Sci USA. 1997;94:4440–4445. doi: 10.1073/pnas.94.9.4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen SY, et al. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci USA. 1999;96:2514–2519. doi: 10.1073/pnas.96.5.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Náray-Fejes-Tóth A, Canessa C, Cleaveland ES, Aldrich G, Fejes-Tóth G. Sgk is an aldosterone-induced kinase in the renal collecting duct. Effects on epithelial Na+ channels. J Biol Chem. 1999;274:16973–16978. doi: 10.1074/jbc.274.24.16973. [DOI] [PubMed] [Google Scholar]
- 22.Shigaev A, Asher C, Latter H, Garty H, Reuveny E. Regulation of sgk by aldosterone and its effects on the epithelial Na(+) channel. Am J Physiol. 2000;278:F613–F619. doi: 10.1152/ajprenal.2000.278.4.F613. [DOI] [PubMed] [Google Scholar]
- 23.Wagner CA, et al. Effects of the serine/threonine kinase SGK1 on the epithelial Na(+) channel (ENaC) and CFTR: implications for cystic fibrosis. Cell Physiol Biochem. 2001;11:209–218. doi: 10.1159/000051935. [DOI] [PubMed] [Google Scholar]
- 24.Lang F, et al. Deranged transcriptional regulation of cell-volume-sensitive kinase hSGK in diabetic nephropathy. Proc Natl Acad Sci USA. 2000;97:8157–8162. doi: 10.1073/pnas.97.14.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bohmer C, et al. The shrinkage-activated Na(+) conductance of rat hepatocytes and its possible correlation to ENaC. Cell Physiol Biochem. 2000;10:187–194. doi: 10.1159/000016349. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez de la Rosa D, Zhang P, Náray-Fejes-Tóth A, Fejes-Tóth G, Canessa CM. The serum and glucocorticoid kinase sgk increases the abundance of epithelial sodium channels in the plasma membrane of Xenopus oocytes. J Biol Chem. 1999;274:37834–37839. doi: 10.1074/jbc.274.53.37834. [DOI] [PubMed] [Google Scholar]
- 27.Vallon V, et al. Role of KCNE1-dependent K+ fluxes in mouse proximal tubule. J Am Soc Nephrol. 2001;12:2003–2011. doi: 10.1681/ASN.V12102003. [DOI] [PubMed] [Google Scholar]
- 28.Wright, F.S., and Giebisch, G. 1992. Regulation of potassium excretion. In The kidney. D.W. Seldin and G. Giebisch, editors. Raven Press. New York, New York, USA. 2209–2247.
- 29.Völkl H, Lang F. Electrophysiology of betaine transport in isolated perfused straight proximal tubule. Pflugers Arch. 2001;442:136–140. doi: 10.1007/s004240000503. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi T, Deak M, Morrice N, Cohen P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J. 1999;344:189–197. [PMC free article] [PubMed] [Google Scholar]
- 31.Debonneville C, et al. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na(+) channel cell surface expression. EMBO J. 2001;20:7052–7059. doi: 10.1093/emboj/20.24.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder PM, Olson DR, Thomas BC. SGK modulates Nedd4-2-mediated inhibition of ENaC. J Biol Chem. 2002;277:5–8. doi: 10.1074/jbc.C100623200. [DOI] [PubMed] [Google Scholar]