Abstract
Mice homozygous for an allele encoding the selenocysteine (Sec) tRNA[Ser]Sec gene (Trsp) flanked by loxP sites were generated. Cre recombinase-dependent removal of Trsp in these mice was lethal to embryos. To investigate the role of Trsp in mouse mammary epithelium, we deleted this gene by using transgenic mice carrying the Cre recombinase gene under control of the mouse mammary tumor virus (MMTV) long terminal repeat or the whey acidic protein promoter. While both promoters target Cre gene expression to mammary epithelium, MMTV-Cre is also expressed in spleen and skin. Sec tRNA[Ser]Sec amounts were reduced by more than 70% in mammary tissue with either transgene, while in skin and spleen, levels were reduced only with MMTV-Cre. The selenoprotein population was selectively affected with MMTV-Cre in breast and skin but not in the control tissue, kidney. Moreover, within affected tissues, expression of specific selenoproteins was regulated differently and often in a contrasting manner, with levels of Sep15 and the glutathione peroxidases GPx1 and GPx4 being substantially reduced. Expression of the tumor suppressor genes BRCA1 and p53 was also altered in a contrasting manner in MMTV-Cre mice, suggesting greater susceptibility to cancer and/or increased cell apoptosis. Thus, the conditional Trsp knockout mouse allows tissue-specific manipulation of Sec tRNA and selenoprotein expression, suggesting that this approach will provide a useful tool for studying the role of selenoproteins in health.
Selenium is an essential micronutrient in the diet of mammals and numerous other life forms (see reference 26 for a review). Many health benefits have been attributed to this element, including a role in the prevention of cancer (10) and heart disease and other cardiovascular and muscle disorders (11), in delaying the aging process (33) and the onset of AIDS in human immunodeficiency virus-positive patients (1), in male reproduction (17), in mammalian development (5), in immune function (33), and as an antiviral agent (2). Selenium is incorporated into protein in the form of selenocysteine (Sec), and Sec has its own tRNA (designated Sec tRNA[Ser]Sec) and its own code word, UGA (26). Sec is indeed the 21st naturally occurring amino acid in the genetic code. Most certainly, the health benefits of selenium are due in large part to its presence in protein (26).
Sec tRNA[Ser]Sec is the only known tRNA that governs the expression of an entire class of proteins, the selenoproteins (26). This provides a unique opportunity to study the expression of selenoproteins by manipulating the levels and characteristics of Sec tRNA[Ser]Sec. For example, the levels of numerous selenoproteins were reduced in a protein- and tissue-specific manner in transgenic mice carrying mutant Sec tRNA[Ser]Sec transgenes lacking the highly modified base isopentenyladenosine in its anticodon (37). Glutathione peroxidase 1 (GPx1) and thioredoxin reductases 1 (TR1) and 3 (TR3) were the most and least affected selenoproteins, respectively, and selenoprotein expression was most and least affected in liver and testes, respectively. Increasing the level of Sec tRNA[Ser]Sec expression by severalfold (36, 37) or decreasing the level of expression by as much as one-half (5, 7) had no effect on selenoprotein expression. Removal of the Sec tRNA[Ser]Sec gene (Trsp), however, is embryonic lethal (5). One means of examining the role of genes that are embryonic lethal is gene silencing on a conditional basis by using Cre/loxP technology (see recent reviews in references 3 and 28). Furthermore, this approach affords us an opportunity to study the role of selenoproteins in specific tissues and organs as well as their relevance to health.
We therefore generated a conditional knockout of Trsp by inserting a fragment of mouse DNA encoding this gene into a vector containing the neomycin (Neo) and thymidine kinase (TK) genes. The newly prepared construct has three loxP sites flanking Neo and Trsp. We generated a mouse that selectively replaced the Trsp wild-type allele with the loxP-Neo-loxP-Trsp-loxP construct. We also selectively removed Neo in recombinant embryonic stem (ES) cells by transfection with a construct encoding the Cre recombinase that generated a mouse encoding floxed Trsp (designated Trspfl). The Trspfl/+ mouse became the parental line for studying conditional knockout of Trsp in the Cre-loxP system.
Breast is an ideal tissue for studying the role of selenoproteins either in its development or in the formation of breast cancer because several model systems that target the mammary gland have been developed in mice (12, 29, 42, 43). Furthermore, this tissue is a major focus of cancer occurrence in women. In inherited breast and ovarian cancer, the BRCA1 tumor suppressor gene frequently appears to be altered (34). Germ line mutations in BRCA1 are associated with approximately two-thirds of all familial breast cancers (18). BRCA1 plays an essential role in several cellular pathways, including transcriptional control, DNA repair, and transcription-coupled repair of oxidative DNA damage (13, 41). p53 is another tumor suppressor gene which normally plays a central role in maintaining the genetic integrity of the cell by preventing cells with damaged DNA from further proliferation. Mutation and deletion of p53 are the most common genetic defects seen in clinical cancer (15). BRCA1 and p53 are known to physically interact, with BRCA1 acting as a coactivator of p53 (45).
Transgenic mice carrying Cre recombinase under the control of two different regulatory elements, the promoter for the whey acidic protein gene (WAP) and that for the mouse mammary tumor virus (MMTV) long terminal repeat, have shown a high specificity for expression in mammary epithelium (42, 44). However, the two promoters have different temporal and tissue specificities. WAP is expressed in alveolar cells in midpregnancy and lactation, while MMTV is already expressed in ductal epithelial cells of newborns and also in skin and spleen (42, 43).
In the present study, we crossed Trspfl/fl mice with Trspfl/+ WAP-Cre or MMTV-Cre mice to generate offspring carrying two floxed alleles and Cre recombinase. The effect of the Cre recombinase-driven promoters on the expression of Sec tRNA[Ser]Sec and, consequently, on the expression of the selenoprotein population are reported herein. These studies establish a new animal model to assess the role of selenoproteins in health in a selenoprotein-specific and organ-specific manner.
MATERIALS AND METHODS
Materials.
The pPNT and the pLMJ58 plox/Neo vectors were from two of our laboratories (those of B. J. Lee and L. Tessarollo, respectively), the pBS246 vector was from Gibco-BRL, and the pBSKS+ vector was from Stratagene. The 129/SV mouse genomic library was obtained from Incyte Genomics, [75Se]selenious acid (specific activity, 1,000 Ci/mmol) was from the Research Reactor Facility, University of Missouri, Columbia, Mo., and [3H]serine (specific activity, 36 Ci/mmol) was from Amersham. Restriction endonucleases and agarose were obtained from New England Biolabs and/or Gibco-BRL. Vent DNA polymerase, Taq DNA polymerase, and deoxynucleoside triphosphates were from New England Biolabs, and spermidine trihydrochloride used in the digestion of genomic DNA was from Sigma.
Nylon membranes (Hybond N+) and 32P-labeled nucleotides were from Amersham, hybridization solution, QuickHyb, was from Stratagene, and Dulbecco's modified Eagle's medium with high glucose and fetal calf serum were used for culturing mouse embryo fibroblasts and embryonic stem (ES) cells from Gibco-BRL and HyClone, respectively. Penicillin-streptomycin, l-Glutamine, minimal essential medium, nonessential amino acids, trypsin-EDTA, geneticin, and leukemia inhibitory factor or ESGRO were from Gibco-BRL. Gelatin and dimethyl sulfoxide were from Sigma, and ganciclovir was from Cytovene Syntex. C-20 polyclonal anti-BRCA1 antibody was from Santa Cruz, and monoclonal anti-p53 antibody was from Oncogene. All other reagents were commercial products of the highest grade available.
C57BL/6 mice used for generating a Trsp conditional knockout carrying Neo-Trsp flanked by loxP sites were from the stock of one of us (L. Tessarollo), transgenic FVB/N mice carrying EIIa Cre were obtained from Heiner Westphal (National Institutes of Health), and transgenic C57BL/6 × 129 mice carrying the Cre recombinase driven by the MMTV long terminal repeat promoter or the WAP promoter were generated previously (42). The care of animals was in accordance with the National Institutes of Health institutional guidelines under the expert direction of Grace Lidl and D. L. Sly (National Cancer Institute, National Institutes of Health, Bethesda, Md.) and Mark St. Clair (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md.).
Construction of targeting vector and generation of cells and mice carrying target alleles.
The methods for preparing the targeting vector and cells and mice encoding the target alleles are described below in Materials and Methods, while the identification of each of these components is given in the Results. The primers used in identifying the various regions inserted during vector construction and in generating the different regions cloned into the targeted vector are shown in Fig. 1B and Table 1, footnote a.
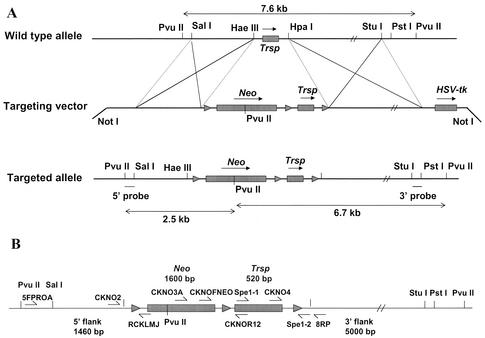
FIG. 1.
Targeting vector, targeted allele, and screening strategy used in generating the conditional knockout of Trsp. (A) Map showing the wild-type allele (upper portion of panel A) encoding Trsp and the restriction sites used in generating the targeting vector (center portion of panel A) that encodes Neo (with its PvuII restriction site, which is critical in distinguishing between the wild-type and target alleles [see below and text]), and Trsp flanked by loxP sites (designated by ▸), herpes simplex virus thymidine kinase (HSV-tk), and the NotI restriction sites used in constructing this vector, and the targeted allele (lower portion of panel A) encoding Trsp and Neo flanked by loxP sites (designated by ▸) and the restriction sites used in constructing this allele, in identifying homologous recombination and in distinguishing wild-type and targeted alleles (see text) are presented. Sizes of fragments generated from the wild-type allele (see top of panel A) and targeted allele (see bottom of panel A) by PvuII digestion are also shown. (B) Primers used in identifying the insertion or generation of different regions during construction of the targeting vector and in determining the presence of different regions involved in generating the targeted allele and floxed Trsp and/or Neo are shown along with the sizes of each region cloned into the targeting vector. Maps in panels A and B are not drawn to scale.
TABLE 1.
Summary of PCR screening techniques used for recombinationa
| Forward primer | Reverse primer | Expected product | Product length | Sequencing primer | Identification |
|---|---|---|---|---|---|
| CKNO5FPROA | RCKLMJ | 5′ flank and 1st loxP | 1.7 kb | CKNOF11 | Recombinant only |
| CKNO2 | RCKLMJ | 1st loxP | 100 bp | CKNO2 | Recombinant only |
| CKNOFNeo | CKNO8RP | 2nd loxP, gene and 3rd loxP | 800 bp | CKNO4 & CKNO8RP | Recombinant only |
| Spe1 | CKNO8RP | Gene only and gene and 3rd loxP | 700 bp and 800 bp | Wild type & recombinant (two products) | |
| Spe1 | Spe2 | Gene and 3rd loxP | 700 bp | Spe1 | Recombinant only |
| CKNO3A | CKNOR12 | 2nd loxP | 700 bp | CKNOR12 | Recombinant only |
Primer designations and sequences are as follows: CKNO2, GCA ACG GCA GGT GTC GCT CTG CG; CKNO3A, GAC GGC GAT GAT CTC GTC GTG; CKNO4, GCG ACC AGC GCG CAG TTA ACC; CKNO8RP, CGT GCT CTC TCC ACT GGC TCA; CKNOF11, CCA TCA CCT AGG GAC TCA G; CKNOR12, AGT GCC TGT CTC CCT AAC T; CKNOFNEO, CGC CGC TCC CGA TTC GCA GCG; RCKLMJ, GGC TGG ACG TAA ACT CCT C; Spe1, CTA GAC TAG TGG CCG CGT GAG AAG TTT TTC; Spe2, GGC CAG TAC TAG TGA ACC TCT TC.
Targeting vector construction and generation of Trspfl-Neofl cells.
The targeting vector was constructed with Neo and Trsp flanked by loxP sites (designated Trspfl-Neofl) and the regions upstream and downstream of the tRNA gene as shown in Fig. 1. The targeting vector was linearized with NotI, and ES cells were electroporated with 20 μl of DNA (1 μg/μl) with a Bio-Rad Gene Pulser set at 250 V with a capacitance of 500 μF in a 0.4-cm electrode gap electroporation cuvette (Bio-Rad). The electroporated cells were transferred to plates containing ES cell medium and incubated for 18 to 24 h, and the transfected cell population was positively and negatively selected for 8 to 9 days with 250 μg of G418 per ml and 2 μM ganciclovir, respectively.
Candidate ES cell clones were screened for homologous recombination by Southern hybridization of PvuII-digested genomic DNA, and the resulting cells carrying a Trspfl-Neofl allele were used to generate chimeric mice.
Generation of Trspfl-Neofl mice.
Homologous recombinant ES cell clones carrying a Trspfl-Neofl allele were injected into C57BL/6 blastocysts and transferred to pseudopregnant females. The resulting high-percentage chimeras (90% or greater based on coat color) were mated to wild-type C57BL/6 mice, and tail DNA samples from the F1 offspring were analyzed for germ line transmission.
Removal of Neo and Trsp.
Mice carrying a Trspfl allele lacking Neo were generated from recombinant ES cells carrying the Trspfl-Neofl allele (see Fig. 1) by transfection with a vector carrying the Cre recombinase gene under the control of the β-actin promoter (23). Clones were obtained by G418 selection, and those cells carrying a Trspfl allele without Neo were identified by Southern hybridization of PvuII-digested genomic DNA. An ES cell clone that had lost Neo but retained Trspfl was injected into C57BL/6 blastocysts and transferred to pseudopregnant females. Resulting chimeras were mated with wild-type C57BL/6 mice, and tail DNA from F1 offspring were analyzed for germ line transmission by Southern hybridization and PCR.
Mice carrying Trspfl/fl were mated with transgenic mice carrying EIIa Cre to obtain a standard knockout of Trsp. Tail DNA of the F1 offspring was analyzed for the loss of the tRNA gene by PCR as given in the Results.
Conditional removal of Trsp.
The MMTV-Cre transgene was identified by PCR analysis of tail DNA with primers corresponding to the MMTV long terminal repeat (5′-GGT TCT GAT CTG AGC TCT GAG TG-3′) and to the Cre gene (5′-CAT CAC TCG TTG CAT CGA CCG G-3′), resulting in a 280-bp PCR product. The WAP-Cre transgene was identified by PCR analysis of tail DNA with primers corresponding to the WAP promoter (5′-TAG AGC TGT GCC AGC CTC TTC C-3′) and to the Cre primer described above, resulting in a PCR product 240 bp in size. Trspfl/+-WAP-Cre or Trspfl/+-MMTV-Cre transgenic mice were generated by mating WAP-Cre and MMTV-Cre transgenic mice with Trspfl/fl mice. The presence of Trspfl in Cre transgenic mice was detected. The offspring were mated with Trspfl/fl mice, and the resulting Trspfl/fl-WAP-Cre, Trspfl/fl-MMTV-Cre, and Trspfl/fl pups were used for Sec tRNA[Ser]Sec and selenoprotein analysis.
Isolation and aminoacylation of tRNA, RPC-5 chromatography, and Northern hybridization.
Total tRNA was isolated from tissues and aminoacylated with [3H]serine under limiting tRNA conditions (24), and the resulting labeled seryl-tRNA was chromatographed twice on an RPC-5 column (27), first in buffer without Mg2+ and then in buffer with Mg2+ (14, 25, 36, 37). Seryl-tRNASer is more hydrophobic than seryl-tRNA[Ser]Sec in the absence of Mg2+, and therefore elutes later on the RPC-5 column, and is less hydrophobic in the presence of Mg2+, and therefore elutes earlier. The tRNASer and tRNA[Ser]Sec populations can therefore be chromatographically resolved from each other and quantitated following labeling with [3H]serine and chromatography as described previously (14, 25, 36, 37).
The level of the Sec tRNA[Ser]Sec population was also determined by Northern blot analysis by standardizing the signal obtained with a 32P-5′-end-labeled oligonucleotide that was complementary to the 20 nucleotides at the 3′ end of tRNA[Ser]Sec (not including the CCA terminus) against that obtained with a 32P-5′-end-labeled oligonucleotide that was complementary to the 20 nucleotides at the 3′ end of serine tRNASer1 (not including the CCA terminus), which was used as an internal control. This technique was a modification of that of Bosl et al. (5), where 0.01 A260 unit of total tRNA was electrophoresed on a 15% TBE (Tris-borate-EDTA)-urea-polyacrylamide gel for 2 h at 180 v, the tRNA was transblotted onto a nylon membrane for 1.5 h at 30 V, and the membrane was cross-linked as for Southern blot analysis (37). The membrane was then hybridized with the tRNA[Ser]Sec probe and washed, the signal level was determined, and the membrane was stripped of this probe and rehybridized with the serine tRNASer1 probe. 5′-End labeling of probes was carried out with [γ-32P]ATP in the presence of T4 polynucleotide kinase following the vendor's procedures (Life Technologies), and hybridization assays, washing of filters, and autoradiograms were carried out as for Southern blot analysis (37).
Labeling of selenoproteins and GPx1 and TR1 assays.
Lactating mice with the genotypes Trspfl/fl-WAP-Cre, Trspfl/fl-MMTV-Cre, and Trspfl/fl were injected intraperitoneally with 50 μCi of 75Se/g and sacrificed 48 h after injection. Tissues and organs were excised and immediately placed into liquid nitrogen and stored at −80°C until ready for use. Tissues were homogenized in 40 mM Tris-HCl (pH 7.4)-1 mM EDTA-0.5 mM 4-(2-aminoethyl)benzenesulfonyl fluoride, sonicated for 2 min, and centrifuged at 4°C for 20 min. Supernatants were electrophoresed on sodium dodecyl sulfate (SDS)-polyacrylamide gels, separated proteins were transferred to polyvinylidene difluoride nylon membranes, and transblots were exposed to a PhosphorImager as described previously (21, 37). Gels were stained with Coomassie blue.
GPx1 activity was assayed directly in extracts, while TR1 activity was assayed after enrichment on ADP-Sepharose as described previously (37).
Tissues.
The tissues used for tRNA[Ser]Sec and selenoprotein analyses from Trspfl/fl-WAP-Cre, Trspfl/fl-MMTV-Cre, and Trspfl/fl mice were mammary (entire mammary gland), skin (ears only), spleen, and kidney. The same tissues and, in addition, liver tissues from heterozygous standard knockout (Trsp+/−) mice and their wild-type siblings were used. Tissues were excised from labeled mice and used immediately for selenoprotein analysis (see above), while those used for tRNA analysis were immediately placed into liquid nitrogen and stored at −80°C until ready for use.
Western blot analysis.
Protein samples were prepared from kidney and mammary tissues as described above, and ≈25 μg was electrophoresed on SDS-4 to 12% polyacrylamide gels, transferred to polyvinylidene difluoride membranes, and immunoblotted with anti-BRCA1, anti-p53, and antiactin antibodies, and the resulting band intensities were quantitated with NIH Image.
RESULTS
Targeting vector.
The targeting vector was constructed from fragments encoding Trsp and the surrounding DNA that were isolated from a 129/Sv mouse genomic library and cloned into pPNT, which provided the backbone for the conditional knockout vector (Fig. 1A). A 520-bp HaeIII-HpaI fragment encoding Trsp was cloned into the SmaI sites of pBS246, which contained two loxP sites. The gene and the second loxP site were PCR amplified with primers Spe1 and Spe2 (see Table 1) containing SpeI sites at both ends and then subcloned into the SpeI site of pBSKS+. A 1.46-kb SalI-NotI fragment encoding the region just upstream of the 520-bp Trsp-containing fragment was blunt ended and cloned into the EcoRI site (also blunt ended) immediately 5′ to the floxed phosphoglycerate kinase (PGK) I promoter/Neo (Neo) cassette of the pLMJ58 plox/Neo vector. The 4,113-bp pLMJ58 plox/Neo vector encodes a 1.6-kb EcoRI-XhoI pGKNeobpA fragment, which was blunt ended and inserted into the blunt-ended BamHI site of the plox plasmid pBS246. This fragment, which contains the 5′-flanking region of Trsp along with Neofl, was released from the vector as a 3.3-kb NotI-ScaI fragment and cloned into the NotI and XbaI sites upstream of Trspfl in pBSKS+.
A 5.0-kb HpaI-StuI fragment encoding the region immediately downstream of the Trsp-containing fragment was prepared by initially cloning a 5.1-kb, blunt-ended HpaII-StuI fragment upstream of the PGK-herpes simplex virus thymidine kinase-bpA cassette (used for negative selection) into the blunt-ended XhoI and XbaI sites of pPNT, replacing the Neo gene. The 3′-flanking region or the long arm is upstream of the PGK- herpes simplex virus thymidine kinase-bpA cassette and was used for negative selection. The 3.8-kb fragment containing the 5′-flanking sequence and the floxed Neo-Trsp fragment was released from pBSKS+ with NotI and SmaI and cloned into the NotI and HpaI sites of the pPNT vector upstream of the region containing the 3′-flanking fragment of Trsp to yield the targeting vector (bottom map in Fig. 1A).
Gene targeting and homologous recombination.
Following electroporation of the targeting vector into ES cells and selection of recombinant cells (see Materials and Methods), candidate ES cell clones were screened for homologous recombination by isolating genomic DNA, digesting with PvuII, and probing the digests by Southern hybridization. A 270-bp PvuII-SalI fragment located upstream of the Neo-Trsp-containing 520-bp fragment (designated the 5′ probe) was used to monitor insertion of the 5′-flanking region. A 250-bp StuI-PstI fragment located downstream of the Neo-Trsp-containing fragment (designated the 3′ probe) was used to monitor insertion of the 3′-flanking region.
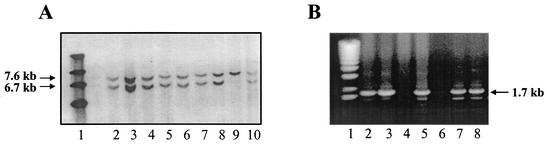
PvuII-digested, wild-type genomic DNA yielded a 7.6-kb signal with both the 5′ and 3′ probes, while recombinants yielded a 6.7-kb signal with the 3′ probe and a 2.5-kb signal with the 5′ probe, as this endonuclease cuts once inside the Neo gene (see Fig. 1A). One hundred sixty selected clones were screened as potential homologous recombinants. Eight were identified as homologous recombinants by screening with the 3′-end probe (Fig. 2A). Five of these eight were verified as true homologous recombinants by PCR (Fig. 2B) and sequencing with the primers shown in Fig. 1B and Table 1 (see also legend to Fig. 2B) to have three intact loxP sites flanking Trsp and Neo.
FIG. 2.
Screening of ES cell clones for recombinant, targeted vector inserted DNA. In A, DNA that was isolated from 160 clones, digested with PuvII, electrophoresed on agarose gels, transblotted as given in Materials and Methods, and hybridized with the 3′-end probe yielded nine potential positives that were rescreened on the same blot, as shown in lanes 2 to 10. Seven of these clones (from lanes 2 to 8) yielded a 2.5-kb signal with the 5′-end probe (data not shown). In B, PCR analysis of these clones with CKNO5FPROA and RCKLMJ as primers yielded a 1.7-kb fragment (lanes 2 to 8). Subsequent sequencing of the PCR-generated fragments demonstrated the presence of Neofl-Trspfl (see text). Lane 1 in panels A and B contains molecular size markers.
Trspfl-Neofl mice and Sec tRNA[Ser]Sec analysis.
Homologous recombinant ES cell clones were used to generate a chimeric mouse carrying a Trspfl-Neofl allele in its germ line as described in Materials and Methods. All F1 offspring of parental chimeric and C57BL/6 mice yielded a 9.2-kb signal which represented the wild-type allele and either the wild-type 129/Sv 7.6-kb allele or the recombinant 6.7-kb allele as identified by Southern hybridization with the 3′ probe (data not shown). It should be noted that C57BL/6 mice encode a polymorphism in the region where the PvuII site occurs in ES cells that accounts for the 9.2-kb PvuII fragment in this mouse line. About 50% of the offspring were identified as heterozygous for Trspfl and Neofl. Offspring from this parental mouse that carried a Trspfl-Neofl allele were intercrossed to yield mice homozygous for the targeted allele.
Sec tRNA[Ser]Sec levels and isoform distributions in liver, kidney, heart, lung, brain, spleen, and muscle were examined in these homozygous mice to determine whether the presence of Neo had any effect on the expression of Trsp. No differences were observed in the level of the Sec tRNA population and the distributions of the isoforms in these tissues of wild-type mice and mice carrying the homozygous targeted allele (data not shown), suggesting that the presence of Neo had no influence on Sec tRNA[Ser]Sec expression.
Generation of Trspfl mice.
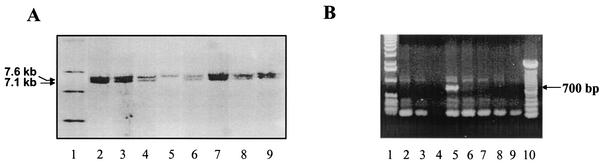
Neo was removed from recombinant ES cells carrying the Trspfl-Neofl allele by transfecting these cells with a vector expressing the Cre recombinase under the control of the β-actin promoter. DNA was isolated from these recombinant cells and digested with PvuII. A fragment about 100 bp larger than the corresponding wild-type fragment due to the presence of vector sequences was observed by Southern hybridization with the 3′ probe (Fig. 3A, lane 5). This fragment was distinguished from the allele lacking Trsp-Neo by being 500 bp shorter than the corresponding wild-type fragment (Fig. 3A, see lanes 2 to 4 and 6 to 9). The presence of Trsp and the flanking loxP sites (i.e., Trspfl) was partially confirmed by PCR with primers CKNO2 and Spe2, which yielded a 700-bp product (Fig. 3B, lane 5). Other primer combinations, Spe1 and Spe2, CKNO2 and CKNO8RP, and CKNO2 and CKNOR12 and sequencing of the PCR-generated products further confirmed the presence of Trspfl.
FIG. 3.
Screening of Cre-transfected cells. In A, DNA was digested with PvuII, electrophoresed on agarose gels, transblotted as given in Materials and Methods, and hybridized with the 3′ probe. Lanes 2 to 4 and 6 to 9 yielded a 7.6-kb wild-type signal and a 7.1-kb signal, indicating they have lost both Neo and Trsp. Lane 5 yielded a signal with a slight upward shift of ≈100 bp, indicating loss of Neo. In B, PCR analysis of the fragments in lanes 2 to 9 of panel A with CKNO2 and Spe2 as primers showed a 700-bp fragment in lane 5, confirming the loss of Neo and presence of Trsp only in the DNA of cells shown in lane 5 of panel A. Lane 1 in panel A and lanes 1 and 10 in panel B contain molecular size markers.
It should be noted that due to the small size difference between the wild-type and targeted alleles generated by PvuII digestion, screening was also carried out by PCR with the primer combinations CKNO2 and Spe2, Spe1 and Spe2, CKNO2 and CKNO8RP, and CKNO2 and CKNOR12.
Chimeric mice carrying only Trspfl were generated as described in Materials and Methods. Analysis of the DNA, as described above, from the offspring of one of the chimeric mice confirmed that about 40% of them carried Trspfl without Neo in the germ line (data now shown).
Intercrosses between mice carrying the Trspfl allele generated Trspfl/fl offspring, and these mice were used in matings to generate selective deletion of Trsp in specific tissues and the standard Trsp knockout mice (see below).
Conditional knockout of Trsp.
To induce a tissue-specific deletion of Trsp, we generated Trspfl/fl-WAP-Cre or Trspfl/fl-MMTV-Cre mice and used them for Sec tRNA[Ser]Sec and selenoprotein expression analyses. Trspfl/fl mice were used as the control. The presence of Trspfl/fl was detected by PCR analysis with the CKNO2 and CKNO8RP primers as described in Table 1, footnote a, and the product was a 1.1-kb fragment.
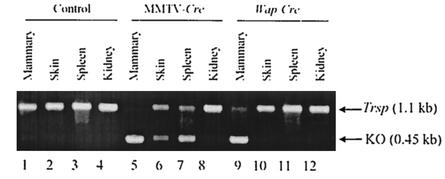
The deletion of Trsp from genomic DNA in mammary epithelium, skin, spleen, and kidney was determined by PCR analysis (Fig. 4). Trsp was virtually absent in DNA from mammary tissue of Trspfl/fl-WAP-Cre and Trspfl/fl-MMTV-Cre mice and only partially lost in DNA from skin and spleen of Trspfl/fl-MMTV-Cre mice. Interestingly, a longer film exposure of the gel in Fig. 4 revealed a partial loss of Trsp in DNA from the control tissue, kidney, of Trspfl/fl-MMTV-Cre mice, indicating low levels of MMTV promoter activity in the control tissue. As expected, there was no loss of Trsp from DNA of these tissues in Trspfl/fl, nontransgenic mice.
FIG. 4.
Selective removal of Trsp in various tissues. DNA was isolated from breast, skin, spleen, and kidney, and products were generated by PCR with the CKNO2 and CKNO8RP primers with DNA from Trspfl/fl (lanes 1 to 4; designated control), Trspfl/fl-MMTV-Cre (lanes 5 to 8; designated MMTV-Cre), and Trspfl/fl-WAP-Cre (lanes 9 to 12; designated WAP-Cre) mice. PCR with this DNA encoding floxed Trsp yielded a 1.1-kb fragment, and without floxed Trsp yielded a 0.45-kb fragment.
Phenotypic changes in Trspfl/fl-WAP-Cre, Trspfl/fl-MMTV-Cre and Trspfl/fl mice.
Primiparous females were able to nurse their litters, and no differences in pup growth were observed. Whole-mount and histological analyses of tissues harvested after lactation was fully established (day 10 of lactation) revealed normal mammary development with expanded lumina that contained secreted milk and lipid droplets (data not shown) (42, 43).
Sec tRNA[Ser]Sec analysis in tissues of conditional knockout mice.
tRNA was prepared from various tissues of Trspfl/fl-WAP-Cre, Trspfl/fl-MMTV-Cre, and Trspfl/fl mice; tRNA from Trspfl/fl mice was used as a control. The tissues selected for analysis were those reported previously to be affected by the WAP gene (mammary tissue [42]) or the MMTV gene (mammary tissue, spleen, and skin [42]). Kidney was used as the control tissue. Isolated tRNA from each tissue was aminoacylated with [3H]serine, which labeled the serine and Sec tRNA populations. These two aminoacylated tRNA populations were separated from each other by RPC-5 chromatography, and the amounts of the two major Sec tRNA[Ser]Sec isoforms relative to the total seryl-tRNA were determined as described previously (14, 25, 36, 37).
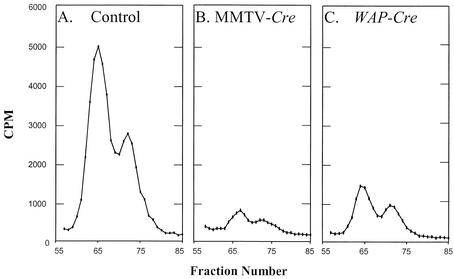
The Sec tRNA[Ser]Sec isoforms differed from each other by a single 2′-O-methylribosyl moiety in the wobble position of the anticodon and were designated 5′-methylcarboxymethyluridine (mcm5U) and 5′-methylcarboxymethyluridine-2′-O-methylribose (mcm5Um). The mcm5U isoform eluted earlier from the RPC-5 column than the mcm5Um isoform. The levels and distributions of the Sec tRNA[Ser]Sec isoforms were initially examined in mammary tissue of Trspfl/fl-WAP-Cre, Trspfl/fl-MMTV-Cre and Trspfl/fl, mice as shown in Fig. 5A, B, and C, respectively. The levels of the Sec tRNA[Ser]Sec isoforms dropped dramatically in mammary tissue harboring the Cre recombinase, and the MMTV promoter-driven Cre was more efficient in reducing the tRNA[Ser]Sec population than the WAP promoter-driven Cre. The amounts of the Sec tRNA[Ser]Sec population relative to the seryl-tRNA population and the distributions of the isoforms were determined for each of the other tissues, skin, spleen, and kidney, in the same manner as for mammary tissue. The data for each of the four tissues examined are summarized in Table 2.
FIG. 5.
Relative amounts of Sec tRNA[Ser]Sec in mammary tissue of conditional Trsp knockout mice. Total tRNA was isolated from mammary tissue of Trspfl/fl, Trspfl/fl-WAP-Cre, and Trspfl/fl-MMTV-Cre mice, the tRNA was aminoacylated with [3H]serine and fractionated by RPC-5 chromatography, and the amount of [3H]seryl-tRNA[Ser]Sec in Trspfl/fl-WAP-Cre and Trspfl/fl-MMTV-Cre mammary tissue was standardized to that found in control mammary tissue, with the total [3H]seryl-tRNASer serving as an internal control, as described in Materials and Methods.
TABLE 2.
Levels and distributions of Sec tRNA[Ser]Sec isoacceptors in tissues of Trspfl/fl, Trspfl/fl-MMTV-Cre, and Trspfl/fl-WAP-Cre micea
| Tissue | Genotype | Sec tRNA[Ser]Sec
|
||||
|---|---|---|---|---|---|---|
| % (total)b | Relative amtc | Distributiond (%)
|
Ratio, mcm5U/ mcm5Ume | |||
| mcm5U | mcm5Um | |||||
| Mammary | Trspfl/fl | 2.32 | 1.00 | 65.3 | 34.7 | 1.88 |
| Trspfl/fl-MMTV-Cre | 0.42 | 0.18 | 59.0 | 41.0 | 1.44 | |
| Trspfl/fl-WAP-Cre | 0.68 | 0.29 | 60.7 | 39.3 | 1.54 | |
| Skin | Trspfl/fl | 3.34 | 1.00 | 41.9 | 58.1 | 0.72 |
| Trspfl/fl-MMTV-Cre | 2.03 | 0.61 | 58.3 | 41.7 | 1.40 | |
| Trspfl/fl-WAP-Cre | 3.17 | 0.95 | 49.8 | 50.2 | 0.99 | |
| Spleen | Trspfl/fl | 4.00 | 1.00 | 52.9 | 47.1 | 1.12 |
| Trspfl/fl-MMTV-Cre | 1.85 | 0.46 | 50.9 | 49.1 | 1.04 | |
| Trspfl/fl-WAP-Cre | 3.84 | 0.96 | 53.8 | 46.2 | 1.16 | |
| Kidney | Trspfl/fl | 4.20 | 1.00 | 36.4 | 63.6 | 0.57 |
| Trspfl/fl-MMTV-Cre | 4.90 | 1.17 | 38.3 | 61.7 | 0.62 | |
| Trspfl/fl-WAP-Cre | 4.62 | 1.10 | 32.1 | 67.9 | 0.47 | |
Total tRNA was isolated from tissues of offspring from matings of the corresponding parents, and the tRNA was fractionated. The amounts of total Sec tRNA[Ser]Sec and the distributions of mcm5U and mcm5Um were determined as given in Materials and Methods.
Percentage of tRNA[Ser]Sec within the seryl-tRNA population.
Amount of tRNA[Ser]Sec relative to that in the wild type, which was assigned a value of 1.00.
Percentage of mcm5U and mcm5Um in the seryl-tRNA population, resolved by RPC-5 chromatography (see Materials and Methods).
Amount of mcm5U divided by the amount of mcm5Um.
As shown in Table 2, the level of Sec tRNA[Ser]Sec was reduced by ≈80% in mice harboring Cre recombinase driven by the MMTV promoter and ≈70% in mice harboring this enzyme driven by the WAP promoter. The MMTV promoter-driven Cre also affected the Sec tRNA[Ser]Sec population in spleen and skin, but the decrease was less than in mammary tissue, being 54% and 39%, respectively. The distributions of mcm5U and mcm5Um were only slightly altered in the presence of WAP-Cre and MMTV-Cre in each tissue compared to those in the corresponding control with the exception of the values in skin. Interestingly, the distribution of the two isoforms shifted most dramatically in the presence of MMTV-Cre, while the distribution in the presence of Wap-Cre was intermediate between that of the control and MMTV-Cre. Similar levels and distributions of the Sec tRNA[Ser]Sec isoforms were observed in the kidney of Trspfl/fl-WAP-Cre, Trspfl/fl-MMTV-Cre, and Trspfl/fl mice.
Selenoprotein synthesis in tissues of conditional knockout mice.
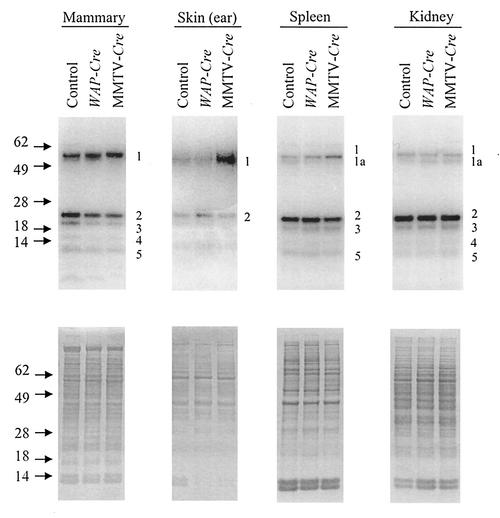
The biosynthesis of selenoproteins in each of the four tissues from Trspfl/fl-WAP-Cre, Trspfl/fl-MMTV-Cre, and Trspfl/fl mice was assessed by labeling the selenoprotein population with 75Se. Proteins from mammary, skin, spleen, and kidney tissues of labeled mice were extracted and examined by gel electrophoresis. Coomassie blue-stained gels of total proteins from these tissues were very similar (lower panel of Fig. 6), suggesting that the presence of Trspfl/fl or Trspfl/fl coupled with the Cre-driven promoters had little or no effect on protein synthesis as a whole. However, analysis of the selenoproteins detected by 75Se labeling in a representative gel showed variations in protein expression (upper panel of Fig. 6). Major selenoprotein bands are designated 1, 1a, 2, 3, 4, and 5. As reported previously, bands 1 and 1a are likely TR1 (21, 37, 39, 40), while bands 2 and 3, which have been purified and identified (21, 37), are GPx1 and GPx4, respectively. Band 4 is unknown, and band 5, which has been purified and identified (19, 21, 37), is Sep15. Bands 2 to 5 appear to be present in smaller amounts in mammary tissue carrying the Cre-driven promoters, whereas band 1 is increased in MMTV-Cre mice.
FIG. 6.
Protein and selenoprotein analysis in tissues of mice carrying floxed Trsp and Cre promoter-specific transgenes. Mice were labeled with 75Se; protein was extracted from tissues, electrophoresed, and stained with Coomassie blue, and 75Se-labeled proteins were detected with a PhosphorImager (see Fig. 5) as given in Materials and Methods. 75Se-labeled proteins from mammary, skin, spleen, and kidney tissues of Trspfl/fl (control), Trspfl/fl-WAP-Cre (WAP-Cre), and Trspfl/fl-MMTV-Cre (MMTV-Cre) mice are shown in the upper panels, and total protein analysis is shown in the lower panels.
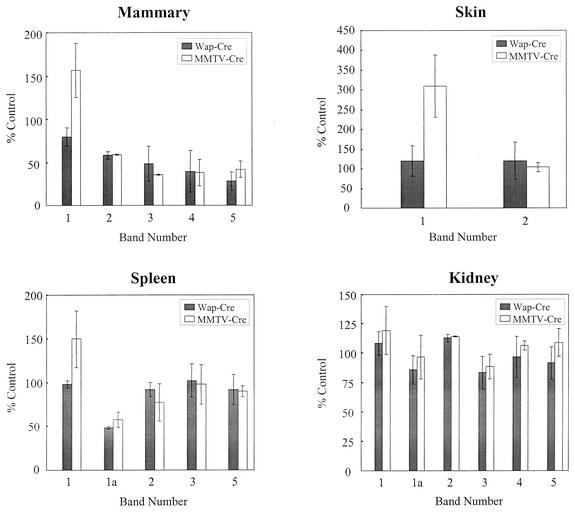
Each 75Se-labeled band shown in Fig. 6 was quantitated on a PhosphorImager along with those from two other gels of protein extracts from mice that were independently labeled with 75Se. The averages of these values relative to the controls are shown in Fig. 7. The most significant reductions in selenoprotein levels occurred in GPx1, GPx4, Sep15, and band 4 in mammary tissue of MMTV-Cre and WAP-Cre mice. The only other apparent significant variation occurred in the skin of MMTV-Cre mice, where TR1 was approximately 2.5 times higher than in the corresponding control tissue, and this was confirmed by direct determination of total TR activity (data not shown).
FIG. 7.
Quantitation of 75Se-labeled selenoproteins in tissues of mice carrying floxed Trsp and promoter-specific transgenes. 75Se-labeled bands of selenoproteins numbered 1, 1a (in spleen and kidney only), and 2 to 5, shown in Fig. 6, and the same labeled bands from two additional experiments from different mice of the same genotype were quantitated with a PhosporImager as given in Materials and Methods. The results are reported in the figure as mean values ± standard deviation (n = 3). Relative amounts of each band, indicated as a bar, from mammary gland, skin, spleen, and kidney of Trspfl/fl (control), Trspfl/fl-WAP-Cre (WAP-Cre), and Trspfl/fl-MMTV-Cre (MMTV-Cre) mice are shown.
In contrast, selenoprotein expression was not affected in the kidney, as assessed both by 75Se labeling (Fig. 6) and by measuring GPx1 activity (Table 3) and TR activity (data not shown). Thus, MMTV-Cre and WAP-Cre mice provide an animal model in which selenoprotein expression can be manipulated in both a selenoprotein-specific and tissue-specific manner.
TABLE 3.
GPx1 levels in various tissues of Trspfl/fl, Trspfl/fl-MMTV-Cre, and Trspfl/fl-WAP-Cre mice
| Tissue | Genotype | Mean GPx1 activitya (nmol of NADPH oxidized/min) ± SD | % of control |
|---|---|---|---|
| Mammary | Trspfl/fl (control) | 47.9 ± 3.3 | |
| Trspfl/fl-MMTV-Cre | 14.1 ± 6.6 | 29.4 | |
| Trspfl/fl-WAP-Cre | 18.0 ± 6.8 | 37.6 | |
| Skin (ear) | Trspfl/fl | 70.5 ± 9.1 | |
| Trspfl/fl-MMTV-Cre | 54.2 ± 4.8 | 76.9 | |
| Trspfl/fl-WAP-Cre | 79.6 ± 15.8 | 112.9 | |
| Spleen | Trspfl/fl | 50.8 ± 10.5 | |
| Trspfl/fl-MMTV-Cre | 34.0 ± 7.2 | 66.9 | |
| Trspfl/fl-WAP-Cre | 56.9 ± 9.5 | 112.0 | |
| Kidney | Trspfl/fl | 164.9 ± 22.2 | |
| Trspfl/fl-MMTV-Cre | 158.0 ± 18.2 | 95.8 | |
| Trspfl/fl-WAP-Cre | 155.3 ± 16.8 | 94.2 |
Biochemical assays of GPx1 were carried out in triplicate as given previously (37).
As GPx1 has been shown to be highly affected during alterations of the Sec tRNA[Ser]Sec population (37) (Fig. 6 and 7), we used this selenoprotein as a marker to measure its activity directly to further verify the 75Se labeling results and the effects of reducing the Sec tRNA[Ser]Sec levels in Trspfl/fl-WAP-Cre and Trspfl/fl-MMTV-Cre mice on selenoprotein synthesis (Table 3). The results of biochemical assay of GPx1 activities were similar to those observed with 75Se labeling with the exception of that in skin, which showed a slight reduction in activity.
Brca1 and p53 cancer marker genes.
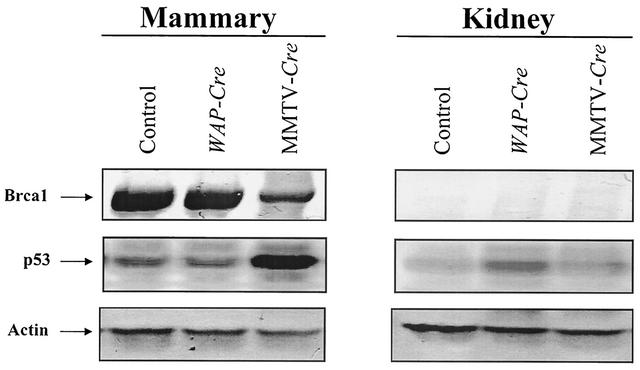
As BRCA1 (the mouse homolog is designated Brca1) and p53 are significant cancer marker genes (see the introduction), we examined the levels of the corresponding proteins in mammary tissue of Trspfl/fl-MMTV-Cre and Trspfl/fl-Wap-Cre mice. Immunoblots with anti-BRCA1 antibodies showed that Brca1 expression was decreased significantly only in mammary tissue of MMTV-Cre mice, as shown in Fig. 8. p53 expression was significantly upregulated in MMTV-Cre mice. Quantitation of these blots confirmed that Brca1 expression was reduced by ≈3-fold and p53 expression was enhanced by ≈4-fold (see the legend to Fig. 8).
FIG. 8.
Western blot analysis of protein fractions from kidney and mammary tissues of floxed Trsp and promoter-specific transgenes. Protein samples were separated from Trspfl/fl (control), Trspfl/fl-WAP-Cre (WAP-Cre), and Trspfl/fl-MMTV-Cre (MMTV-Cre) mice by SDS-PAGE and blotted with antibodies against Brca1, p53, and actin (as control), and band intensities were quantitated as described in Materials and Methods. Representative Western blots are given in the figure from triplicate experiments with tissues from two different sets of animals. Brca1 was reduced by 67% ± 8%, and p53 was increased by 400% ± 20% (mean values ± standard deviation).
Standard Trsp knockout mice and Sec tRNA[Ser]Sec and selenoprotein analysis.
Mice heterozygous for the loss of Trsp were obtained by crosses between parents carrying Trspfl/fl and the EIIa Cre transgene as given in Materials and Methods. Tail DNA of the F1 offspring was analyzed for loss of the tRNA gene by PCR with the CKNO2 and CKNO8RP primers. The mutant allele lacking Trsp yielded a ≈450-bp fragment, and the wild-type allele yielded a ≈900-bp fragment.
Multiple matings between mice that were heterozygous for the loss of Trsp yielded only wild-type or heterozygous offspring (total of 37 wild-type and 39 heterozygous were obtained from four litters). Thus, the loss of Trsp in our floxed mice was embryonic lethal (see also reference 5).
The levels of the Sec tRNA[Ser]Sec population in various tissues of Trsp+/− and Trsp+/+ sibling mice were examined by RPC-5 chromatography and Northern blot hybridization (Table 4). The distributions of mcm5U and mcm5Um were examined only in kidney and liver, as shown in experiment A of the table. The distributions did not appear to vary significantly in these tissues with reduced Sec tRNA[Ser]Sec population from Trsp+/− mice compared to the corresponding control tissues from Trsp+/+ mice. The levels of the Sec tRNA[Ser]Sec population were reduced only about 30% in liver, kidney, and spleen, about 20% in mammary tissue, and not at all in skin of Trsp+/− mice, as determined by Northern blot hybridization (experiment B, Table 4).
TABLE 4.
Sec tRNA[Ser]Sec levels in Trsp+/+ and Trsp+/− micea
| Expt | Tissue | Genotype of offspring | Sec tRNA[Ser]Sec
|
||||
|---|---|---|---|---|---|---|---|
| % of total or of control | Relative amt | Distribution (%)
|
Ratio, mcm5U/mcm5Um | ||||
| mcm5U | mcm5Um | ||||||
| A | Liver | Trsp+/+ | 3.19 | 1.00 | 43.1 | 56.9 | 0.76 |
| Trsp+/− | 1.96 | 0.61 | 45.9 | 54.1 | 0.85 | ||
| Kidney | Trsp+/+ | 4.18 | 1.00 | 44.2 | 55.8 | 0.79 | |
| Trsp+/− | 2.89 | 0.69 | 41.0 | 59.0 | 0.69 | ||
| B | Liver | Trsp+/− | 70.1 | ||||
| Kidney | Trsp+/− | 71.4 | |||||
| Spleen | Trsp+/− | 67.8 | |||||
| Mammary | Trsp+/− | 78.9 | |||||
| Skin | Trsp+/− | 102.7 | |||||
Sec tRNA[Ser]Sec levels and distributions were determined by RPC-5 chromatography as described in Materials and Methods and in Table 2, footnotes a to e. The values for Sec tRNA[Ser]Sec levels in experiment B were determined by Northern blot analysis as described in Materials and Methods.
Labeling of Trsp−/+ and Trsp+/+ sibling mice with 75Se and examination of the resulting selenoproteins in liver, kidney, spleen, mammary tissue, and skin showed no apparent changes in the selenoprotein population in these animals (data not shown). These data suggest that Sec tRNA[Ser]Sec levels are not limiting in selenoprotein biosynthesis (see also Discussion).
DISCUSSION
The present study describes the generation of mice in which the Sec tRNA[Ser]Sec gene was inactivated in specific tissues. The targeted allele encodes Trspfl, which can be used widely in the loxP/Cre system to remove the gene during development (4) or in a variety of tissues. Since the tRNA product of this gene governs the expression of an entire class of proteins, the selenoproteins, the ability to selectively remove Trsp provides us with an important tool with which to perturb selenoprotein synthesis during development or in a tissue-specific manner.
Trsp was selectively removed from mammary epithelium with Cre recombinase that was under the control of the MMTV long terminal repeat or the WAP gene promoter. The two promoters target mammary epithelial cells at different times in development, with MMTV expression occurring even prior to birth, while high levels of the WAP-driven transgene are found only in the second half of pregnancy and lactation (42, 43). Sec tRNA[Ser]Sec levels were reduced by ≈70% in mammary tissue with WAP-Cre and ≈80% in that with MMTV-Cre. In turn, the selenoprotein levels were selectively reduced in mammary tissue; labeling of the selenoprotein population with 75Se suggested that the amounts of GPx1, GPx4, Sep15, and an 18-kDa selenoprotein were substantially decreased, but TR1 levels were less affected. This hierarchy with respect to differential selenoprotein expression is discussed further below.
MMTV has been reported to be expressed in skin and spleen (42, 43), and indeed, the levels of the Sec tRNA[Ser]Sec population were substantially reduced in these tissues of Trspfl/fl-MMTV-Cre mice. Even though the Sec tRNA[Ser]Sec population was reduced less in skin than in spleen in these mice, TR1 activity was increased over twofold only in skin. This increase in activity was accompanied by a dramatic change in the distributions of the two isoforms in skin which were virtually unchanged in the spleen (Table 2). This observation suggests that the distribution of the two major Sec tRNA[Ser]Sec plays an important role in the expression of different selenoproteins (8, 37).
In a previous study, the amount of the wild-type Sec tRNA[Ser]Sec population was unaltered by the introduction of a Sec tRNA[Ser]Sec mutant that lacked the highly modified isopentenyladenosine base in its anticodon loop (37). The distribution of the two isoforms, however, changed dramatically in this tissue; mcm5U increased and mcm5Um decreased. The alteration in isoform distribution was accompanied by a substantial reduction in GPx1 in the liver, but TR1 was less affected, while TR3 was significantly enhanced. It was not clear if the alteration in selenoprotein expression was due to misreading by the isopentenyladenosine mutant Sec tRNA[Ser]Sec or due to a reduction in the amount of the mcm5Um isoform.
During selenium deficiency in mammals, the most dramatic change in the Sec tRNA[Ser]Sec population involves the distribution of the two Sec isoforms: mcm5U becomes more abundant and mcm5Um less abundant in cells (25) and numerous tissues (8, 14). The reverse is true in the same cells and tissues when they are selenium sufficient. These observations support the possibility that GPx1 expression is linked to a Sec tRNA[Ser]Sec population in which mcm5Um is enriched (8), while thioredoxin reductase expression is associated with enriched mcm5U (37).
A hierarchy in selenoprotein expression exists in mammals, in which some selenium-containing proteins are preferentially expressed during selenium deficiency (reviewed in reference 26) or during alterations in the Sec tRNA[Ser]Sec population (37). The observations in the present study in which a reduction in the Sec tRNA[Ser]Sec population and/or an alteration in the distribution of isoforms results in an alteration of selenoprotein expression (see Table 3 and Fig. 6 and 7) provide further support that this hierarchy is directly linked to the status of the tRNA governing selenoprotein synthesis. An essential element involved in the expression of selenoproteins in mammals is a stem-loop structure in the 3′ untranslated region of their mRNAs designated the SECIS element (30). Interestingly, there are contrasting viewpoints whether the class of SECIS elements is involved in the hierarchy of selenoprotein expression (16, 31), but it would seem that the expression of certain selenoproteins may be directly linked to distributions and levels of the two Sec tRNA[Ser]Sec isoforms.
The effect of MMTV-Cre- and WAP-Cre-mediated recombination on Sec tRNA[Ser]Sec and selenoprotein levels was examined in the entire mammary gland and not specifically in the target cells, the mammary epithelium. We investigated selenoprotein levels during lactation, when approximately 90% of the mammary gland is composed of epithelium. It is therefore possible that the Sec tRNA[Ser]Sec could be completely removed in the epithelium of this gland and the low levels of Sec tRNA[Ser]Sec and altered selenoprotein synthesis observed may be contributed from other cell types in the gland, including fat, fibroblasts, and myoepithelium. Isolation of pure secretory epithelium would no doubt result in degradation of tRNA and proteins while the specific isolation of these cells was taking place. There were no phenotypic changes observed in the mammary gland with respect to generating milk or to the transfer of milk to the suckling pups, suggesting that altered selenoprotein synthesis does not play a role in these processes.
Apoptosis is a frequent phenomenon in breast cancer. It has been shown that BRCA1 may interact closely with p53 in determining whether a cell should undergo apoptosis or reversible growth arrest (32, 45). Cells with functional p53 die by apoptosis, while similar cells lacking p53 function continue to proliferate (32). Our studies showing a decrease in Brca1 expression and an increase in p53 expression in MMTV-Cre mice suggest that the mice might be more susceptible to breast cancer if p53 expression was inactivated. Brca1, which is the mouse homolog of BRCA1, is crucial for the growth and development of the mouse embryo (6). It has been reported that Brca1 homozygous deletions (−/−) are lethal early in embryonic development due to the failure of the affected cells to proliferate (22). Recent studies show that Wap-Cre- or MMTV-Cre-mediated excision of Brca1 exon 11 in mouse mammary epithelial cells resulted in increased apoptosis (44), with a low frequency of mammary tumor formation which accelerated in the background of p53 null alleles, suggesting that genetic instability due to disruption of Brca1 may inactivate p53 leading to tumor formation.
The relationship between selenium and p53 has been observed recently (35, 38, and references therein). Expression of p53 regulates affected selenoprotein expression in a colon cancer cell line (20), whereas one of the selenoproteins, TR1, is thought to regulate p53 activity through the redox state of thioredoxin (35). Supplementation of cells with selenium or selenomethionine activates p53 through upregulation of TR1 (38), while TR1 inhibitors repress p53 (35). However, because of contrasting patterns of expression of TR1 and GPx1 in various cancer model systems and in response to p53 expression (20), the precise relationship between p53 and selenium is not fully understood. The alteration in p53 and Brca1 levels following introduction of MMTV-Cre transgenes into Trspfl/fl mice that was observed in the present study would appear to add another level of regulation in the relationship between selenoproteins and p53.
We also generated a standard knockout of Trsp. Consistent with previous findings (5), no homozygous offspring from heterozygous Trsp knockout parents was born, indicating that Sec tRNA[Ser]Sec and selenoprotein expression are essential for development. We examined Trspfl/+ mice in greater detail to provide a better understanding of the consequences of removing one gene copy on the Sec tRNA[Ser]Sec population and on selenoprotein synthesis. Despite a reduction in the Sec tRNA[Ser]Sec population in liver and kidney, there appeared to be little or no effect on the distribution of the two major isoforms in these tissues or on selenoprotein expression. The Sec tRNA[Ser]Sec population was also reduced in a number of other tissues without apparent effect on selenoprotein synthesis suggesting that the levels of this tRNA is not limiting in selenoprotein expression (5, 7, 37).
Selenium has been shown to provide enormous health benefits to mammals, such as decreasing the incidence of cancer and heart disease and delaying the aging process, as discussed in the introduction. Most certainly, the conditional knockout of Trsp will provide an invaluable tool with which to study the role of selenoproteins as a chemopreventive agent in a number of these diseases and under various physiological and pathological conditions. The National Cancer Institute, based on an earlier human clinical trial demonstrating a dramatic reduction in prostate cancer in males supplemented with 200 μg of selenium per day (9), recently undertook a new trial of examining the effects of selenium and/or vitamin E on prostate cancer in 32,000 men (NCI Selenium and Vitamin E Cancer Prevention Trial [SELECT], http://cancer.gov/select). This study was undertaken without knowledge of the mechanism(s) of how selenium acts at the molecular level or if selenoproteins are involved in preventing this disease. The selective reduction or loss of selenoproteins in specific tissues with Cre-mediated recombination undoubtedly will help elucidate the role of selenium and selenoproteins in cancer prevention.
Acknowledgments
This work was supported in part by NIH grants GM065204 and CA080946 to V.N.G. and 21C Frontier Functional Human Genome Project from the Ministry of Science and Technology of Korea to B.J.L.
REFERENCES
- 1.Baum, M. K., A. Campa, M. J. Miguez-Burbano, X. Burbano, and G. Shor-Posner. 2001. Role of selenium in HIV/AIDS, p. 247-255. In D. L. Hatfield (ed.), Selenium: its molecular biology and role in human health. Kluwer Academic Publishers, Norwell, Mass.
- 2.Beck, M. A. 2001. Selenium as an antiviral agent, p. 235-245. In D. L. Hatfield (ed.), Selenium: its molecular biology and role in human health. Kluwer Academic Publishers, Norwell, Mass.
- 3.Bedell, M. A., N. A. Jenkins, and N. G. Copeland. 1997. Mouse models of human disease. I. Techniques and resources for genetic analysis in mice. Genes Dev. 11:1-10. [DOI] [PubMed] [Google Scholar]
- 4.Betz, U. A., C. A. Vosshenrich, K. Rajewsky, and W. Muller. 1996. Bypass of lethality with mosaic mice generated by Cre-LoxP-mediated recombination. Curr. Biol. 6:1307-1316. [DOI] [PubMed] [Google Scholar]
- 5.Bosl, M. R., K. Takadu, M. Oshima, S. Nishimura, and M. M. Taketo. 1997. Early embryonic lethality caused by targeted disruption of the mouse selenocysteine tRNA gene (Trsp). Proc. Natl. Acad. Sci. USA 94:5531-5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, Y., W. H. Lee, and H. K. Chew. 1999. Emerging roles of BRCA1 in transcriptional regulation and DNA repair. J. Cell Physiol. 181:385-392. [DOI] [PubMed] [Google Scholar]
- 7.Chittum, H. S., H. J. Baek, A. M. Diamond, P. Fernandez-Salguero, F. Gonzalez, T. Ohama, D. L. Hatfield, M. Kuehn, and B. J. Lee. 1997. Selenocysteine tRNA[Ser]Sec levels and selenium-dependent glutathione peroxidase activity in mouse embryonic stem cells heterozygous for a targeted mutation in the tRNA[Ser]Sec gene. Biochemistry 36:8634-8639. [DOI] [PubMed] [Google Scholar]
- 8.Chittum, H. S., K. E. Hill, B. A. Carlson, B. J. Lee, R. F. Burk, and D. L. Hatfield. 1997. Replenishment of selenium deficient rats with selenium results in redistribution of the selenocysteine tRNA population in a tissue specific manner. Biochim. Biophys. Acta 1359:25-34. [DOI] [PubMed] [Google Scholar]
- 9.Clark, L. C., G. F. Combs Jr., B. W. Turnbull, E. H. Slate, D. K. Chalker, J. Chow, L. S. Davis, R. A. Glover, G. F. Graham, E. G. Gross, A. Krongrad, J. L. Lesher, Jr., H. K. Park, B. B. Sanders, Jr., C. L. Smith, and J. R. Taylor. 1996. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 276:1957-1963. [PubMed] [Google Scholar]
- 10.Combs, G. R., and L. Liu. 2001. Selenium as a cancer preventative agent, p. 205-217. In D. L. Hatfield (ed.), Selenium: its molecular biology and role in human health. Kluwer Academic Publishers, Norwell, Mass.
- 11.Coppinger, R. J., and A. M. Diamond. 2001. Selenium deficiency and human disease, p. 219-233. In D. L. Hatfield (ed.), Selenium: its molecular biology and role in human health. Kluwer Academic Publishers, Norwell, Mass.
- 12.Cui, Y., K. M, Miyoshi, E. Claudio, U. K. Siebenlist, F. J. Gonzalez, J. Flaws, K.-U. Wagner, and L. Hennighausen. 2002. Loss of the peroxisome proliferation-activated receptor gamma (PPARγ) does not affect mammary development and propensity for tumor formation but leads to reduced fertility. J. Biol. Chem. 277:17830-17835. [DOI] [PubMed] [Google Scholar]
- 13.Deng, C.-X., and S. G. Brodie. 2000. Roles of BRCA1 and its interacting proteins. BioEssays 22:728-737. [DOI] [PubMed] [Google Scholar]
- 14.Diamond, A. M., I. S. Choi, P. F. Crain, T. Hashizume, S. C. Pomerantz, R. Cruz, C. Steer, K. E. Hill, R. F. Burk, J. A. McCloskey, and D. L. Hatfield. 1993. Dietary selenium affects methylation of the wobble nucleoside in the anticodon of selenocysteine tRNA[Ser]Sec. J. Biol. Chem. 268:14215-14223. [PubMed] [Google Scholar]
- 15.Evan, G., and T. Littlewood. 1998. A matter of life and cell death. Science 281:1317-1322. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher, J. E., P. R. Copeland, D. M. Driscoll, and A. Krol. 2001. The selenocysteine incorporation machinery: interactions between the SECIS RNA and the SECIS-binding protein SBP2. RNA 7:1442-1453. [PMC free article] [PubMed] [Google Scholar]
- 17.Flohe, L., R. Brigelius-Flohe, M. Maiorino, A. Roveri, J. Wissing, and F. Ursini. 2001. Selenium and male reproduction, p. 273-281. In D. L. Hatfield (ed.), Selenium: its molecular biology and role in human health. Kluwer Academic Publishers, Norwell, Mass.
- 18.Futreal, P. A., Q. Liu, D. Shattuck-Eidens, C. Cochran, K. Harshman, S. Tavtigian, L. M. Bennett, A. Haugen-Strano, J. Swensen, Y. Miki, et al. 1994. BRCA1 mutations in primary breast and ovarian carcinomas. Science 266:120-122. [DOI] [PubMed] [Google Scholar]
- 19.Gladyshev, V. N., K.-T. Jeang, J. C. Wootton, and D. L. Hatfield. 1998. A new human selenium-containing protein. J. Biol. Chem. 273:8910-8915. [DOI] [PubMed] [Google Scholar]
- 20.Gladyshev, V. N., V. M. Factor, F. Housseau, and D. L. Hatfield. 1998. Contrasting patterns of regulation of the antioxidant selenoproteins, thioredoxin reductase, and glutathione peroxidase, in cancer cells. Biochem. Biophys. Res. Commun. 251:488-493. [DOI] [PubMed] [Google Scholar]
- 21.Gladyshev, V. N., T. C. Stadtman, D. L. Hatfield, and K.-T. Jeang. 1999. Levels of major selenoproteins in T cells decrease during HIV infection and low-molecular-mass selenium compounds increase. Proc. Natl. Acad. Sci. USA 96:835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gowen, L. C., B. L. Johnson, A. M. Latour, K. K. Sulik, and B. H. Koller. 1996. Brca1 deficiency results in early embryonic lethality characterized by neuroepithelial abnormalities. Nat. Genet. 12:191-194. [DOI] [PubMed] [Google Scholar]
- 23.Gunning, P., T. H. Leavitt, G. Muscat, S. Y. Ng, and L. Kedes. 1987. A human β-actin expression vector system directs high-level accumulation of antisense transcripts. Proc. Natl. Acad. Sci. USA 84:4831-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatfield, D. L., C. R. Mathews, and M. Rice. 1979. Aminoacyl-transfer RNA populations in mammalian cells: chromatographic profiles and patterns of codon recognition. Biochim. Biophys. Acta 564:414-423. [DOI] [PubMed] [Google Scholar]
- 25.Hatfield, D. L., B. J. Lee, L. Hampton, and A. M. Diamond. 1991. Selenium induces changes in the selenocysteine tRNA[Ser]Sec population in mammalian cells. Nucleic acids Res. 19:939-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatfield, D. L., and V. N. Gladyshev. 2002. How selenium has altered our understanding of the genetic code. Mol. Cell. Biol. 22:3565-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelmers, A. D., and D. E. Heatherly. 1971. Columns for rapid chromatographic separation of small amounts of tracer-labeled transfer ribonucleic acids. Anal. Biochem. 44:486-495. [DOI] [PubMed] [Google Scholar]
- 28.Kwan, K.-M. 2002. Conditional alleles in mice: practical considerations for tissue-specific knockouts. Genesis 32:49-62. [DOI] [PubMed] [Google Scholar]
- 29.Le Provost, F., G. Riedlinger, S. H. Yim, J. Benedict, F. J. Gonzalez, J. Flaws, and L. Hennighausen. 2002. The aryl hydrocarbon receptor (AhR) and its nuclear translocator (Arnt) are dispensable for normal mammary gland development but are required for fertility. Genesis 32:231-239. [DOI] [PubMed] [Google Scholar]
- 30.Low, S. C., and M. J. Berry. 1996. Knowing when not to stop: selenocysteine incorporation in eukaryotes. Trends Biochem. Sci. 21:203-208. [PubMed] [Google Scholar]
- 31.Low, S. C., E. Grundner-Culemann, J. W. Harney, and M. J. Berry. 2000. SECIS-SBP2 interactions dictate selenocysteine incorporation efficiency and selenoprotein hierarchy. EMBO J. 19:6882-6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacLachlan, T. K., K. Somasundaram, M. Sgagias, Y. Shifman, R. J. Muschel, K. H. Cowan, and W. S. El-Deiry. 2000. BRCA1 effects on the cell cycle and the DNA damage response are linked to altered gene expression. J. Biol. Chem. 275:2777-2785. [DOI] [PubMed] [Google Scholar]
- 33.McKenzie, R. C., T. S. Rafferty, G. J. Beckett, and J. R. Arthur. 2001. Effects of selenium on immunity and aging, p. 257-272. In D. L. Hatfield (ed.), Selenium: its molecular biology and role in human health. Kluwer Academic Publishers, Norwell, Mass.
- 34.Miki, Y., J. Swensen, D. Shattuck-Eidens, P. A. Futreal, K. Harshman, S. Tavtigian, Q. Liu, C. Cochran, L. M. Bennett, W. Ding, et al. 1994. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266:66-71. [DOI] [PubMed] [Google Scholar]
- 35.Moos, P. J., K. Edes, P. Cassidy, E. Massuda, and F. A. Fitzpatrick. 2003. Electrophilic prostaglandins and lipid aldehydes repress redox sensitive transcription factors p53 and HIF by impairing the selenoprotein thioredoxin reductase. J. Biol. Chem. 278:745-750. [DOI] [PubMed]
- 36.Moustafa, M. E., M. A. El-Saadani, K. M. Kandeel, D. B. Mansur, B. J. Lee, D. L. Hatfield, and A. M. Diamond. 1998. Overproduction of selenocysteine tRNA in Chinese hamster ovary cells following transfection of the mouse tRNA[Ser]Sec gene. RNA 4:1436-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moustafa, M., B. A. Carlson, M. A. El-Saadani, G. V. Kryukov, Q.-I. Sun, J. W. Harney, K. E. Hill, G. F. Combs, L. Feigenbaum, D. B. Mansur, R. F. Burk, M. J. Berry, A. M. Diamond, B. J. Lee, V. N. Gladyshev, and D. L. Hatfield. 2001. Selective inhibition of selenocysteine tRNA maturation and selenoprotein synthesis in transgenic mice expressing isopentenyladenosine-deficient selenocysteine tRNA. Mol. Cell. Biol. 21:3840-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seo, Y. R., M. R. Kelley, and M. L. Smith. Selenomethionine regulation of p53 by a ref1-dependent redox mechanism. Proc. Natl. Acad. Sci. USA 99:14548-14553. [DOI] [PMC free article] [PubMed]
- 39.Sun, Q.-A., Y. Wu, F. Zappacosta, K.-T. Jeang, B. J. Lee, D. L. Hatfield, and V. N. Gladyshev. 1999. Redox regulation of cell signaling by selenocysteine in mammalian thioredoxin reductases. J. Biol. Chem. 274:24522-24530. [DOI] [PubMed] [Google Scholar]
- 40.Sun, Q.-A., F. Zappacosta, V. M. Factor, P. J. Wirth, D. L. Hatfield, and V. N. Gladyshev. 2001. Heterogeneity within animal thioredoxin reductases. Evidence for alternative first exon splicing. J. Biol. Chem. 276:3106-3114. [DOI] [PubMed] [Google Scholar]
- 41.Venkitaraman, A. R. 2002. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108:171-182. [DOI] [PubMed] [Google Scholar]
- 42.Wagner, K.-U., R. J. Wall, L. St-Onge, P. Gruss, A. Wynshaw-Boris, L. Garrett, M. Li, P. A. Furth, and L. Hennighausen. 1997. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 25:4323-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner, K.-U., K. McAllister, T. Ward, B. Davis, R. Wiseman, and L. Hennighausen. 2001. Spatial and temporal expression of the Cre gene under the control of the MMTV-long terminal repeat in different lines of transgenic mice. Transgenic Res. 10:545-553. [DOI] [PubMed] [Google Scholar]
- 44.Xu, X., K. U. Wagner, D. Larson, Z. Weaver, C. Li, T. Ried, L. Hennighausen, A. Wynshaw-Boris, and C. X. Deng. 1999. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat. Genet. 22:37-43. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, H., K. Somasundaram, Y. Peng, H. Tian, H. Zhang, D. Bi, B. L. Weber, and W. S. El-Deiry. 1998. BRCA1 physically associates with p53 and stimulates its transcriptional activity. Oncogene 16:1713-1721. [DOI] [PubMed] [Google Scholar]