Abstract
Levofloxacin showed comparable in vitro susceptibility to ciprofloxacin among Enterobacteriaceae, Pseudomonas aeruginosa, enterococci, and Staphylococcus aureus, while greater susceptibility was observed in Stenotrophomonas maltophilia and Staphylococcus epidermidis, mainly when oxacillin resistant. The susceptibility of Streptococcus pneumoniae to levofloxacin reached 99%.
The recent introduction of levofloxacin, a new fluoroquinolone (FQ), into the Italian clinical scenario led to the need to locally confirm its in vitro activity. Nowadays, only qualitative studies on selected bacterial species have been reported with levofloxacin in Italy (1, 3, 4, 5, 6, 10, 13) but larger studies on clinical isolates are missing. We report here the major findings of a survey carried out on the bacterial species most frequently encountered in the routine work of 24 Italian laboratories distributed Italywide (see Acknowledgments).
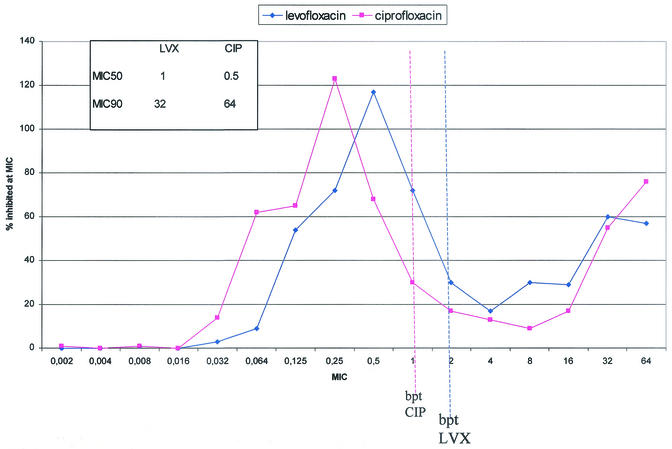
Each center was requested to collect 194 consecutive, no-copy clinical isolates belonging to a predefined list of bacterial species without any limitation on the ward and/or sample of isolation. All the isolates had to be identified according to the laboratory method used to obtain them. Participants were asked to limit the number of collected Escherichia coli, Staphylococcus aureus, and Staphylococcus epidermidis strains to 30 each per center. The MICs of levofloxacin and ciprofloxacin were determined locally by the Etest (AbBiodisk) method according to the manufacturer's instructions. All the assays were performed on Mueller-Hinton agar except for Streptococcus pneumoniae and Moraxella catarrhalis, which were tested by using Mueller-Hinton agar plus 5% sheep blood, and Haemophilus influenzae, which was tested by using Haemophilus test medium (all media were from the same batch). Susceptibility breakpoints were ≤2 μg/ml for levofloxacin and ≤1 μg/ml for ciprofloxacin according to NCCLS (11). To contain intralaboratory variability, quality controls with E. coli strain ATCC 25922, S. pneumoniae strain ATCC 49619, and H. influenzae strain ATCC 49247 were requested of each center before, during, and after the study's conclusion. The Etest method was selected because of its reliability in testing FQs (9), its ease of use, and its definition of the MIC. Between January and November 2000, case report forms for a total of 4,003 clinical isolates were collected. The quality control results confirmed that the Etest assays were carried out properly during the study, and any center results had to be discharged. As reported by others (9), a slight overestimation (1.6%) of the ciprofloxacin MIC for E. coli ATCC 25922 occurred, but the increase never determined a change in the category. Detailed figures on the isolation wards, samples, and the samples' FQ susceptibilities will be published elsewhere. The isolates and their susceptibilities to levofloxacin and ciprofloxacin are illustrated in Table 1. The Enterobacteriaceae were unhomogeneously susceptible to the study drugs, with a percentage of susceptible strains ranging from 75.2 to 93.9% for both ciprofloxacin and levofloxacin. The Pseudomonas aeruginosa isolates exhibited the expected susceptibilities to both FQs. The resulting nonsusceptibility values related mainly to the isolates coming from the intensive care unit, which represented almost 20% of the isolates. Stenotrophomonas maltophilia, an emerging pathogen, was primarily isolated among the strains from the intensive care unit (48.6%) and internal medicine wards (36.2%), respiratory (62.8%) and blood (16.8%) samples being the more frequent sources. Among the gram-positive isolates, a bimodal FQ MIC distribution was observed for both S. aureus and S. epidermidis (data not shown). Interestingly, while the S. aureus population could be divided into oxacillin-resistant (oxa-R) FQ-resistant and oxacillin-susceptible (oxa-S) FQ-susceptible strains, among S. epidermidis the same subgrouping revealed 47.2% of oxa-R strains still susceptible to levofloxacin (Table 1). According to the NCCLS breakpoints, the clinical susceptibility of S. pneumoniae to levofloxacin was 99% whereas ciprofloxacin could not be categorized, and data were expressed by means of only the MIC at which 50% of the isolates tested were inhibited (MIC50) and MIC90 (Table 1). As far as the enterococcal species are concerned, both study drugs exerted comparable moderate activities (Table 1). Significant levels of FQ resistance in E. coli, Proteus mirabilis, and Enterobacter cloacae were detected compared to European (8) or recent American (12) data. In our study, a close correlation between FQ resistance and β-lactamase production in P. mirabilis was confirmed (data not shown). The 33.8% P. aeruginosa nonsusceptibility to ciprofloxacin we found was very close to the 31.9% obtained in a survey carried out in Italy in 1995 (2). The susceptibility and MIC distribution of P. aeruginosa to levofloxacin were comparable to those to ciprofloxacin (Fig. 1). Figure 1 is quite similar to figures recently published by Sahm et al. (12) from the U.S. and to those from other studies carried out in Europe (7) and in Italy in particular (1, 13). It can be concluded that, despite a ciprofloxacin mean MIC 1 or 2 dilutions lower, there is no different in the impact on the in vitro clinical susceptibility of P. aeruginosa to the study drugs due to the favorable pharmacokinetic properties of levofloxacin that allow a breakpoint of 2 μg/ml. Levofloxacin has been reported to exert good in vitro activity on S. maltophilia (3, 15); nevertheless, the observed percentages of ciprofloxacin-susceptible (58.9%) and intermediate (18.5%) strains were high between the two studies (3, 15). Such a result is probably due to assay variations (14, 16). S. epidermidis isolates were more susceptible to levofloxacin than ciprofloxacin, mainly when they were oxa-R, as described by Cafiso et al. (4), who found levofloxacin to exert 30% more activity than ciprofloxacin on oxa-R coagulase-negative staphylococci. The susceptibility of S. pneumoniae to levofloxacin was very high, reflecting a previous work (G. P. Gesu, C. G. Gechtman, C. Bonato, A. Cavallero, R. Ieri, and F. Marchetti, Abstr. 9th Int. Congr. Infect. Dis., abstr. 74.003, 2000). To our knowledge, this is the largest Italian study carried out on enterococci and FQs. Both study drugs exerted moderate activities on enterococci, and FQ high-level resistance was associated with resistance to glycopeptides (data not shown). In conclusion, the findings of the present study suggest that resistance surveillance should be intensified for select Enterobacteriaceae and P. aeruginosa strains. Levofloxacin turned out to exert good in vitro activity on either gram-positive or gram-negative clinical isolates.
TABLE 1.
Clinical isolates and their susceptibilities to levofloxacin and ciprofloxacin
| Strain (n) | Antimicrobial agent | % Susceptible | % Intermediate | % Resistant | MIC50 | MIC90 |
|---|---|---|---|---|---|---|
| Staphylococcus aureus (615) | Levofloxacin | 71.2 | 4.9 | 23.9 | 0.25 | 32 |
| Ciprofloxacin | 68.5 | 1.8 | 29.8 | 0.5 | 64 | |
| Staphylococcus aureus oxa-R (198) | Levofloxacin | 20.7 | 13.1 | 66.2 | 8 | 32 |
| Ciprofloxacin | 17.2 | 1.5 | 81.3 | 32 | 64 | |
| Staphylococcus aureus oxa-S (417) | Levofloxacin | 95.2 | 1.0 | 3.8 | 0.25 | 0.5 |
| Ciprofloxacin | 92.8 | 1.9 | 5.3 | 0.25 | 1 | |
| Escherichia coli (596) | Levofloxacin | 86.2 | 1.8 | 11.9 | 0.064 | 16 |
| Ciprofloxacin | 82.0 | 1.3 | 16.6 | 0.064 | 32 | |
| Pseudomonas aeruginosa (551) | Levofloxacin | 64.8 | 3.1 | 32.1 | 1 | 32 |
| Ciprofloxacin | 66.2 | 3.1 | 30.7 | 0.5 | 64 | |
| Enterococcus faecalis (485) | Levofloxacin | 72.4 | 0.8 | 26.8 | 1 | 64 |
| Ciprofloxacin | 67.4 | 5.4 | 27.2 | 1 | 64 | |
| Klebsiella pneumoniae (295) | Levofloxacin | 94.6 | 0.7 | 4.7 | 0.064 | 1 |
| Ciprofloxacin | 93.9 | 1.0 | 5.1 | 0.064 | 0.5 | |
| Proteus mirabilis (285) | Levofloxacin | 85.3 | 0.7 | 14.0 | 0.064 | 16 |
| Ciprofloxacin | 80.4 | 4.6 | 15.1 | 0.064 | 32 | |
| Staphylococcus epidermidis (267) | Levofloxacin | 64.4 | 8.6 | 27.0 | 1 | 32 |
| Ciprofloxacin | 51.3 | 8.2 | 40.4 | 1 | 64 | |
| Staphylococcus epidermidis oxa-R (163) | Levofloxacin | 47.2 | 12.9 | 39.9 | 4 | 64 |
| Ciprofloxacin | 30.7 | 11.0 | 58.3 | 4 | 64 | |
| Staphylococcus epidermidis oxa-S (104) | Levofloxacin | 91.3 | 1.9 | 6.7 | 0.125 | 2 |
| Ciprofloxacin | 83.7 | 3.8 | 12.5 | 0.125 | 8 | |
| Streptococcus pneumoniae (218) | Levofloxacin | 99.1 | 0 | 0.9 | 0.5 | 1 |
| Ciprofloxacin | 0.5 | 2 | ||||
| Haemophilus influenzae (210) | Levofloxacin | 100 | 0 | 0 | 0.064 | 0.25 |
| Ciprofloxacin | 100 | 0 | 0 | 0.064 | 0.25 | |
| Enterobacter cloacae (157) | Levofloxacin | 79.0 | 1.9 | 19.1 | 0.064 | 64 |
| Ciprofloxacin | 75.2 | 3.8 | 21.0 | 0.064 | 64 | |
| Stenotrophomonas maltophilia (124) | Levofloxacin | 85.5 | 4.0 | 10.5 | 0.5 | 8 |
| Ciprofloxacin | 58.9 | 18.5 | 22.6 | 1 | 8 | |
| Serratia marcescens (81) | Levofloxacin | 92.6 | 0 | 7.4 | 0.125 | 2 |
| Ciprofloxacin | 86.4 | 6.2 | 7.4 | 0.064 | 2 | |
| Citrobacter freundii (80) | Levofloxacin | 87.5 | 1.3 | 11.3 | 0.064 | 8 |
| Ciprofloxacin | 85 | 2.5 | 12.5 | 0.064 | 8 | |
| Moraxella catarrhalis (39) | Levofloxacin | 100 | 0 | 0 | 0.064 | 0.125 |
| Ciprofloxacin | 100 | 0 | 0 | 0.064 | 1 |
FIG. 1.
Distribution of the study drug MICs for the 551 isolates of P. aeruginosa collected in this study. LVX, levofloxacin; CIP, ciprofloxacin; bpt, breakpoint.
Acknowledgments
We thank the following investigators for contributing to this study: R. Antonetti, Foggia; L. Avallone, Salerno; M. Bolletti Censi, Naples; G. Buonopane, Avellino; A. Chiarini, Palermo; G. Chierchi, Sassari; P. Cipriani, Rome; E. Costa, Milan; A. De Toffoli, Venice; G. Fortina, Novara; R. Fregoso, La Spezia; G. Gasperi, Bologna; L. M. Greco, Cosenza; A. La Vitola, Naples; M. Lemmi, Genoa; E. Manso, Ancona; R. Milano, Turin; C. Monti Bragadin, Trieste; P. Panetta, Taranto; A. Piceni, Potenza; G. Riario Sforza, Pescara; F. Rumpianesi, Modena; A. Sala, Lecco; and G. C. Schito, Genoa, Italy.
This work was financially supported by GlaxoSmithKline S.p.A, Verona, Italy.
REFERENCES
- 1.Bonfiglio, G. 2001. Is levofloxacin as active as ciprofloxacin against Pseudomonas aeruginosa? Chemotherapy 47:239-242. [DOI] [PubMed] [Google Scholar]
- 2.Bonfiglio, G., V. Carciotto, G. Russo, S. Stefani, G. C. Schito, E. Debbia, and G. Nicoletti. 1998. Antibiotic resistance in Pseudomonas aeruginosa: an Italian survey. J. Antimicrob. Chemother. 41:307-310. [DOI] [PubMed]
- 3.Bonfiglio, G., C. Cascone, C. Azzarelli, V. Cafiso, F. Marchetti, and S. Stefani. 2000. Levofloxacin in vitro activity and time-kill evaluation on Stenotrophomonas maltophilia clinical isolates. J. Antimicrob. Chemother. 45:115-117. [DOI] [PubMed] [Google Scholar]
- 4.Cafiso, V., C. Messina, M. Santagati, F. Campanile, G. Bonfiglio, and S. Stefani. 2001. In vitro activity of levofloxacin against coagulase positive and negative staphylococci. Drugs 27:107-111. [PubMed] [Google Scholar]
- 5.Donati, M., F. Rumpianesi, F. Marchetti, V. Sambri, and R. Cevenini. 1998. Comparative in vitro activity of levofloxacin against Chlamydia spp. J. Antimicrob. Chemother. 48:670-671. [DOI] [PubMed] [Google Scholar]
- 6.Drago, L., E. De Vecchi, B. Mombelli, L. Nicola, M. Valli, and M. R. Gismondo. 2001. Activity of levofloxacin and ciprofloxacin against urinary pathogens. J. Antimicrob. Chemother. 48:37-45. [DOI] [PubMed] [Google Scholar]
- 7.Gales, A. C., R. N. Jones, J. Turnidge, R. Rennie, and R. Ramphal. 2001. Characterization of Pseudomonas aeruginosa isolates: occurrence rates, antimicrobial suceptibility patterns, and molecular typing in the global SENTRY antimicrobial surveillance program, 1997-1999. Clin. Infect. Dis. 32(Suppl. 2):S146-S155. [DOI] [PubMed] [Google Scholar]
- 8.Hanberger, H., J. A. Garcia-Rodriguez, M. Gobernado, H. Goossens, L. E. Nilsson, M. J. Struelens, and the French and Portuguese ICU Study Groups. 1999. Antibiotic susceptibility among aerobic gram-negative bacilli in intensive care units in 5 European countries. JAMA 281:67-71. [DOI] [PubMed] [Google Scholar]
- 9.Jones, R. N., M. E. Erwin, and J. L. Croco. 1996. Critical appraisal of E-test for the detection of fluoroquinolone resistance. J. Antimicrob. Chemother. 38:21-25. [DOI] [PubMed] [Google Scholar]
- 10.Montanari, M. P., M. Mingoia, F. Marchetti, and P. E. Varaldo. 1999. In vitro activity of levofloxacin against gram-positive bacteria. Chemotherapy 45:411-417. [DOI] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 3rd ed. Approved standard M100-S7. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 12.Sahm, D. F., I. A. Critchley, L. J. Kelly, J. A. Karlowsky, D. C. Mayfield, C. Thornsberry, Y. R. Mauriz, and J. Kahn. 2001. Evaluation of current activities of fluoroquinolones against gram-negative bacilli using centralized in vitro testing and electronic surveillance. Antimicrob. Agents Chemother. 45:267-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segatore, B., D. Setacci, M. Perilli, N. Franceschini, A. De Sanctis, F. Marchetti, and G. Amicosante. 1999. Italian survey on the comparative levofloxacin susceptibility in 334 clinical isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:428-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traub, W. H., B. Leonhard, and D. Bauer. 1998. Antibiotic susceptibility of Stenotrophomonas (Xanthomonas) maltophilia: comparative (NCCLS criteria) evaluation of antimicrobial drugs with the agar dilution and the agar disk diffusion (Bauer-Kirby) tests. Chemotherapy 44:164-173. [DOI] [PubMed] [Google Scholar]
- 15.Visalli, M. A., M. R. Jacobs, and P. C. Appelbaum. 1998. Activities of three quinolones, alone and in combination with extended-spectrum cephalosporins or gentamicin, against Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 42:2002-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao, J. D. C., M. Louie, L. Louie, J. Goodfellow, and A. E. Simor. 1995. Comparison of E test and agar dilution for antimicrobial susceptibility testing of Stenotrophomonas (Xanthomonas) maltophilia. J. Clin. Microbiol. 33:1428-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]