Abstract
Chronic beryllium disease (CBD) is caused by exposure to beryllium in the workplace, and it remains an important public health concern. Evidence suggests that CD4+ T cells play a critical role in the development of this disease. Using intracellular cytokine staining, we found that the frequency of beryllium-specific CD4+ T cells in the lungs (bronchoalveolar lavage) of 12 CBD patients ranged from 1.4% to 29% (mean 17.8%), and these T cells expressed a Th1-type phenotype in response to beryllium sulfate (BeSO4). Few, if any, beryllium-specific CD8+ T cells were identified. In contrast, the frequency of beryllium-responsive CD4+ T cells in the blood of these subjects ranged from undetectable to 1 in 500. No correlation was observed between the frequency of beryllium-responsive bronchoalveolar lavage (BAL) CD4+ T cells as detected by intracellular staining and lymphocyte proliferation in culture after BeSO4 exposure. Staining for surface marker expression showed that nearly all BAL T cells exhibit an effector memory cell phenotype. These results demonstrate a dramatically high frequency and compartmentalization of antigen-specific effector memory CD4+ cells in the lungs of CBD patients. These studies provide insight into the phenotypic and functional characteristics of antigen-specific T cells invading other inaccessible target organs in human disease.
Introduction
Due to its unique chemical and physical properties, beryllium continues to be used in the ceramics, electronics, telecommunications, aerospace, and defense industries (1, 2). It is estimated that more than 800,000 workers in the US have been exposed to beryllium and are therefore at risk for developing chronic beryllium disease (CBD) (1). This disorder develops in 1–16% of exposed workers, depending on the nature of the exposure and the genetic susceptibility of the individual (3–5). Genetic susceptibility and beryllium presentation have been associated with certain HLA-DP alleles, particularly those possessing a glutamate at position 69 (Glu69) of the DP β chain (6–10), and the greatest risk for disease development occurs in those exposed individuals possessing two HLA-DP Glu69-containing molecules (8).
CBD is characterized by the accumulation of CD4+ T cells and the presence of noncaseating granulomatous inflammation in the lung. This disease is clinically and pathologically similar to a more common idiopathic disease, sarcoidosis (11, 12). Considerable evidence suggests that CD4+ T cells are important in the initiation and perpetuation of the beryllium-induced immune response (13–16). The ability of peripheral blood and/or bronchoalveolar lavage (BAL) CD4+ T cells to proliferate in the presence of beryllium salts in vitro forms part of the current disease definition of CBD, and the development of granulomatous inflammation is associated with the accumulation of activated CD4+ T cells in the BAL (16–19). Analysis of T cell receptor (TCR) expression on BAL CD4+ T cells has shown the presence of oligoclonal expansions and TCR motifs specific for beryllium-reactive T cells (20, 21). In addition, recognition of beryllium by CD4+ T cells in CBD patients involves the same HLA-DP alleles associated with disease susceptibility (9, 10). Similar to sarcoidosis (12), CBD appears to be characterized by Th1-type cytokine production in the target organ (22, 23).
In the current study, we used intracellular cytokine staining and proliferative responses to quantitate and characterize beryllium-specific T cells in the lung and blood of CBD patients. The results demonstrate a remarkably large and compartmentalized collection of Th1-secreting antigen-specific effector memory T cells in the target organ. The characteristics of these cells and their paucity in blood have important implications for the study of other CD4+ T cell–dependent diseases.
Methods
Study population.
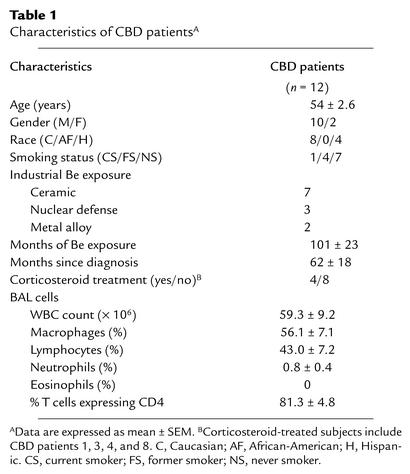
Twelve CBD patients were consecutively enrolled based on the availability of BAL samples. Clinical characteristics are shown in Table 1. The diagnosis of CBD had been previously established using defined criteria (16, 24), including the presence of granulomatous inflammation on lung biopsy, a history of beryllium exposure, and a positive proliferative response of blood and/or BAL T cells to beryllium sulfate (BeSO4) in vitro. Exposure to beryllium occurred in the ceramics, nuclear weapons, and metal alloy industries. All patients had active disease based on elevated BAL white blood cell (WBC), lymphocyte, and CD4 T cell counts compared with normal control subjects evaluated at evaluated at National Jewish Medical and Research Center (15, 20). Four of the 12 patients were chronically treated with corticosteroids. Consistent with the progressive nature of CBD, treated subjects had a longer mean duration since disease diagnosis (116 ± 9.3 vs. 41 ± 22 months). Their disease also appeared to be more active, based on higher BAL WBC counts (80 ± 18 vs. 48 ± 8), higher percentage of lymphocytes (64 ± 10 vs. 33 ± 7), and higher percentage of T cells expressing CD4 (94 ± 6 vs. 75 ± 17). No differences in results were noted for corticosteroid-treated versus untreated patients in the current study, and therefore patients were grouped together for the analyses presented. A group of five sarcoidosis patients and five normal control subjects served as negative controls for the BAL and blood studies, respectively. Informed consent was obtained from each CBD patient and control subject, and the protocol was approved by the Human Subject Institutional Review Boards at the University of Colorado Health Sciences Center and National Jewish Medical and Research Center.
Table 1.
Characteristics of CBD patientsA
Preparation of peripheral blood and BAL cells and cell culture.
PBMCs were isolated from heparinized blood by Ficoll-Hypaque density gradient separation (20, 21). BAL was performed as previously described (25). The yield of WBCs and the percentage of various cell subsets are shown in Table 1.
Proliferation assays were performed as previously described (9, 21). In brief, BAL cells (1 × 105 cells per well) and PBMCs (2.5 × 105 cells/well) were cultured in 96-well flat-bottom microtiter plates in the presence of 5 μg/ml phytohemagglutinin for 48 hours or 1 × 10–5 M BeSO4 for 3 days. The wells were then pulsed with 1 μCi of [3H]thymidine for an additional 18 hours, and incorporation of radioactivity was determined by β-emission spectroscopy. Proliferation assays were performed in quadruplicate.
For stimulation of cytokine secretion, BAL cells (2.5 × 105 to 5 × 105 cells) or PBMCs (1 × 106 cells) were placed in polypropylene tubes (12 × 75 mm; Fisher Scientific Co., Pittsburgh, Pennsylvania, USA) containing 1 ml of RPMI 1640 supplemented with 10% heat-inactivated human serum (Gemini BioProducts, Woodland, California, USA), 20 mM HEPES, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine (all from Life Technologies Inc., Gaithersburg, Maryland, USA) and one of the following experimental conditions: medium alone, 10 ng/ml staphylococcal enterotoxin B (SEB), or 1 × 10–5 M BeSO4. Cells were incubated for a total of 6 hours at 37°C in a humidified 5% CO2 atmosphere with 10 μg/ml brefeldin A added after the first hour of stimulation, as previously described (26–28). In some experiments, the presenting MHC class II molecules were determined by the addition of blocking mAb’s to HLA-DR (hybridoma LB3.1; American Type Culture Collection, Manassas, Virginia, USA), HLA-DP (hybridoma B7.21; a gift from Ian Trowbridge, Salk Institute, La Jolla, California, USA), and HLA-DQ (hybridoma SVLP3; a gift from Francisco Sinigaglia, Roche Milano Ricerche, Milan, Italy) at the beginning of culture as previously described (9). Control experiments using these antibodies have been previously presented (9).
Immunofluorescence staining and analysis for intracellular cytokine expression.
After stimulation, cells were washed and stained with mAb’s directed against CD4 and CD8 (both from Becton Dickinson Immunocytometry Systems, San Jose, California, USA) as described (26–28). Cells were washed with PBS containing 1% BSA and placed in fixation medium (Caltag Laboratories Inc., Burlingame, California, USA) for 15 minutes at room temperature. Following washing with PBS containing 1% BSA, cells were added to permeabilization medium (Caltag Laboratories Inc.), and stained with mAb’s directed against CD69 (Caltag Laboratories Inc.), IFN-γ, TNF-α, and/or IL-2 (all from Becton Dickinson Immunocytometry Systems) for 30 minutes at 4°C. The lymphocyte population was identified using forward and 90° light scatter patterns, and fluorescence intensity was analyzed using a FACSCalibur cytometer (Becton Dickinson Immunocytometry Systems) as previously described (20, 21).
Immunofluorescence staining and analysis for TCR and other cell surface markers.
For analysis of TCR Vβ expression, mAb’s directed against ten different TCR Vβ receptors were used. BAL samples were stained using biotinylated mAb directed against Vβ3.1, Vβ5.1, Vβ6.7, Vβ8.1, Vβ9, Vβ12, Vβ13.1, Vβ13.2, Vβ14, and Vβ17 as previously described (20). Streptavidin-phycoerythrin (Fisher Biotech, Pittsburgh, Pennsylvania, USA) was used as a second-step reagent for TCR staining. In other studies, surface markers were specifically stained with phycoerythrin-labeled mAb’s to CD11a, CD45RO, CD62L, and CCR7 (all from Becton Dickinson Immunocytometry Systems). Cells were also costained with peridinin chlorophyll protein–labeled CD4 and FITC-labeled CD8 (both from Becton Dickinson Immunocytometry Systems). Flow cytometric analysis was performed as described above.
Results
Proliferative responses of BAL cells and PBMCs to BeSO4 in culture.
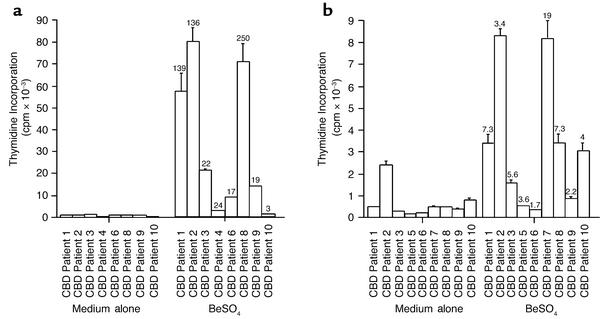
BAL cells from eight CBD patients enrolled in this study were examined for proliferative responses in the presence of 1 × 10–5 M BeSO4 (Figure 1a). All samples demonstrated a positive response (defined as a stimulation index [SI] ≥ 2.5), with SIs ranging from 3 to 250. As shown in Figure 1a, BAL cells from CBD patients 1, 2, and 8 vigorously proliferated in response to beryllium, with peak thymidine incorporation of 52,921, 74,109, and 65,759 cpm, respectively. In contrast, minimal stimulation of BAL cells from CBD patient 10 following exposure to BeSO4 was observed, with peak thymidine incorporation of only 514 cpm compared with background proliferation of 169 cpm.
Figure 1.
Stimulation of BAL cells (a) and PBMCs (b) from the CBD patients. Proliferation of the cells in medium alone and after the addition of 1 × 10–5 M BeSO4 is shown. Proliferation assays were performed with BAL cells from 8 of the 12 CBD patients and with PBMCs from 9 of the 12 patients. The stimulation index (SI) for each proliferative response is shown over the corresponding bar, and an SI indicative of a positive response is currently defined as ≥ 2.5. The data are expressed as the mean cpm ± SEM.
Seven of the nine PBMC samples tested showed a positive proliferative response to BeSO4, with SIs ranging from 3.4 to 19 (Figure 1b). Mononuclear cells from CBD patients 6 and 9 failed to proliferate in response to BeSO4. Overall, the proliferative response of PBMCs to BeSO4 was much decreased compared with that of BAL cells. Only PBMCs from patient 10 showed a greater proliferative response in the presence of BeSO4 compared with BAL cells.
Th1-type cytokine production in BAL T cells after BeSO4 exposure.
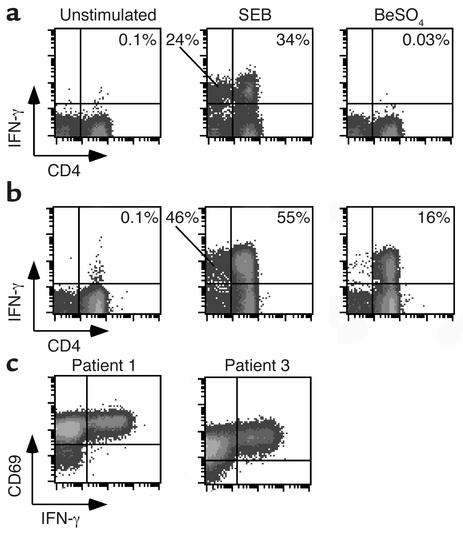
Using intracellular cytokine staining as a measure of response, BAL T cells were stimulated with beryllium or SEB for a total of 6 hours. In Figure 2a, representative examples of intracellular cytokine staining of BAL cells from a sarcoidosis control subject are shown. As expected, we observed minimal background IFN-γ production with medium alone, and lower than background levels were observed following the addition of BeSO4. This finding is consistent with our previous studies, in which we found that exposure of peripheral blood CD4+ T cell clones from normal subjects to BeSO4 inhibited lymphocyte proliferation (21). On the other hand, lymphocytes from the lungs of sarcoidosis patients do possess the ability to secrete Th1-type cytokines. In the example shown (Figure 2a), 34% of CD4+ and 24% of CD8+ T cells expressed IFN-γ after SEB stimulation. Overall, none of the BAL lymphocytes from the sarcoidosis subjects studied demonstrated cytokine production following exposure to BeSO4 (Figures 2a).
Figure 2.
Intracellular staining for IFN-γ in stimulated BAL T cells. Representative experiments are shown for the flow cytometric analysis of BAL cells from a patient with sarcoidosis (a) or CBD (b) stimulated with either medium alone, SEB, or BeSO4 and subsequently stained for surface CD4 and intracellular IFN-γ expression. The number in the upper right quadrant of each density plot indicates the percentage of CD4+ T cells expressing IFN-γ. (c) Representative flow cytometric staining of BAL cells from two CBD patients shows concordance of expression of the activation marker CD69 with IFN-γ after stimulation with BeSO4.
Representative examples of intracellular cytokine staining of BAL cells from a CBD patient (patient 4) are shown in Figure 2b. Minimal IFN-γ production was seen with medium alone, whereas SEB stimulation induced IFN-γ expression in 55% of the CD4+ cells and 46% of the CD8+ cells. Remarkably, 16% of this patient’s CD4+ T cells were beryllium-specific, as determined by BeSO4-induced IFN-γ expression. In contrast to SEB stimulation, few if any CD8+ T cells stained positively for IFN-γ after BeSO4 stimulation. In all of the CBD patients studied, the CD4+ T cells expressing IFN-γ also coexpressed the activation marker CD69 (Figure 2c).
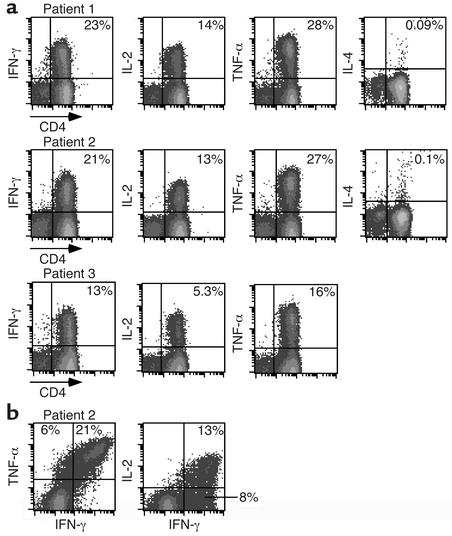
BAL CD4+ T cells from CBD patients demonstrated a striking frequency of beryllium-responsive CD4+ T cells and polarized Th1-type cytokine profile (Figure 3a). For example, studies of cells from patient 1 showed that 23%, 14%, and 28% of BAL CD4+ T cells expressed IFN-γ, IL-2, and TNF-α, respectively, after exposure to beryllium. A similarly high frequency of beryllium-specific BAL CD4+ T cells expressing IFN-γ, IL-2, and TNF-α was present in patients 2 and 3 (Figure 3a). In contrast, IL-4 expression after beryllium stimulation was no greater than background in any of the CBD patients studied (shown for patients 1 and 2 in Figure 3a). Control experiments using PBMCs from normal subjects stimulated with PMA and ionomycin documented IL-4–producing cells and our ability to detect this cytokine if produced (data not shown). Figure 3a also shows that few, if any, CD8+ BAL T cells produce cytokines in response to BeSO4 stimulation.
Figure 3.
Intracellular expression of IFN-γ, IL-2, TNF-α, and IL-4 after stimulation of BAL CD4+ T cells with BeSO4. (a) Staining patterns are shown for BAL cells from CBD patients 1, 2, and 3 after short-term culture with BeSO4. The percentage of CD4+ T cells expressing the various Th1 and Th2 cytokines is shown in the upper right quadrant of each density plot. The expression of IL-4 after BeSO4 exposure was not determined for CBD patient 3. (b) Flow cytometric analyses of TNF-α versus IFN-γ and IL-2 versus IFN-γ are shown for CD4+ T cells from CBD patient 2 following exposure to BeSO4. The percentage of CD4+ T cells expressing both cytokines is shown in the upper right quadrant of the density plot.
Most beryllium-specific CD4+ T cells are capable of secreting both TNF-α and IFN-γ (Figure 3b). In contrast, a much smaller subset of IFN-γ–producing cells also produce IL-2. In the example shown, approximately 40% of the IFN-γ–producing cells were unable to express IL-2.
Cryopreserved BAL samples of CBD patients 3 and 4 from earlier time points were available for analysis. The frequency of beryllium-responsive CD4+ T cells in the earlier sample from patient 3 was comparable to results 24 months later (15% vs. 13%). In patient 4, the frequency of beryllium-responsive CD4 cells also changed little (20% to 16%) over a 2-year period. Both of these individuals were being treated with corticosteroids at both time points. Thus, despite treatment, the high frequency of beryllium-specific CD4+ T cells in the lung persists over time.
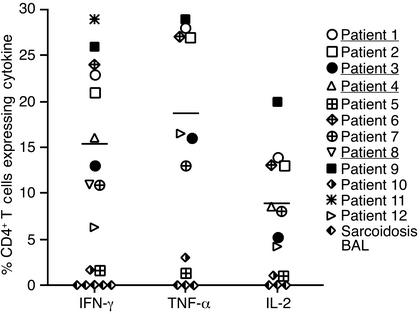
Figure 4 shows the frequency of beryllium-specific CD4+ T cells in BAL for all CBD patients and sarcoidosis subjects evaluated. The percentage of CD4+ T cells staining positively for IFN-γ ranged from 1.7% to 29% (mean 15.3% ± 2.7%), while the percentage of cells expressing TNF-α ranged from 1.4% to 29% (mean 17.8% ± 3.6%). A lower frequency of CBD BAL CD4+ T cells expressed IL-2 (1–20%, mean 8.8% ± 1.9%). Individuals with a high frequency of cells expressing IFN-γ after beryllium stimulation (patients 1, 2, 6, and 9) also expressed higher levels of TNF-α and IL-2. Conversely, subjects with a low frequency of cells expressing IFN-γ after beryllium stimulation (patients 5 and 10) expressed lower levels of TNF-α and IL-2. We observed no association between the frequency of beryllium-specific BAL CD4+ T cells and months of beryllium exposure in the workplace or months since diagnosis (data not shown). The frequency of IFN-γ–expressing CD4+ T cells in the corticosteroid-treated group (patients 1, 3, 4, and 8) and the untreated group was similar (16% ± 5% vs. 15% ± 3%; P = 0.9). None of the CD4+ T cells from sarcoidosis patients expressed cytokines after BeSO4 exposure in culture.
Figure 4.
Percentage of BAL CD4+ T cells that express intracellular IFN-γ, TNF-α, and IL-2 after stimulation with BeSO4. The mean percentage of Th1 cytokines produced by BAL cells from CBD patients is shown as a solid line: mean ± SEM for IFN-γ, 15.3% ± 2.7% (n = 12); TNF-α, 17.9% ± 3.6% (n = 9); and IL-2, 8.8% ± 1.9% (n = 10). Patients being treated with corticosteroids are underlined.
Inhibition of beryllium-induced responses with mAb’s to MHC class II molecules.
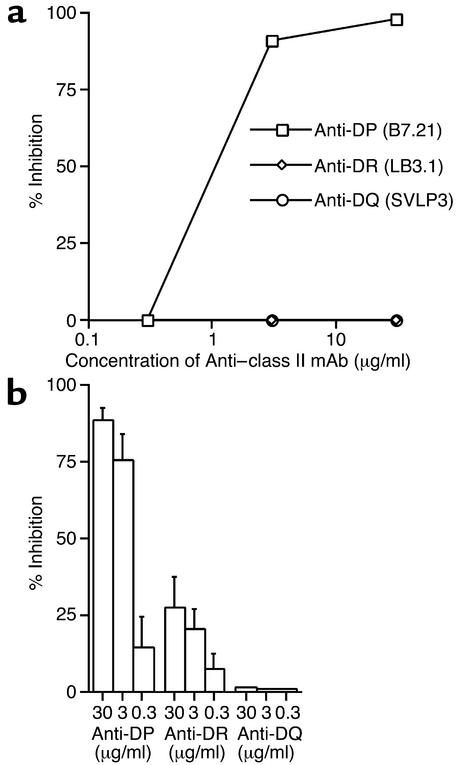
Previous studies have shown the importance of particular HLA-DP molecules in beryllium presentation to BAL CD4+ T cell lines from CBD patients (9, 10). In the current study of ex vivo BAL samples, we used blocking mAb’s to HLA-DP, -DR, and -DQ to determine the MHC class II molecules involved in beryllium stimulation of cytokine secretion. Figure 5a shows the results of an experiment with BAL cells from patient 12, in which beryllium-stimulated IFN-γ secretion was completely inhibited by the addition of the anti-DP mAb. No inhibition was noted with the anti-DR and -DQ mAb’s. Since CBD patient 12 is homozygous for HLA-DPB1*0201, the data indicate that beryllium recognition in the lung of this subject is restricted by DPB1*0201 and not by DR or DQ.
Figure 5.
Inhibition of beryllium-induced IFN-γ expression with mAb’s to MHC class II molecules. (a) BAL cells from CBD patient 12 were cultured with BeSO4 and increasing concentrations of mAb’s to HLA-DP, -DR, and -DQ. The data are presented as percent inhibition of IFN-γ expression. (b) Mean (± SEM) percent inhibition of beryllium-induced IFN-γ expression for the five CBD patients studied as shown in a.
In studies of BAL cells from five different CBD patients, the addition of 30 μg/ml of anti-DP mAb resulted in 88% ± 4% (range, 75–98%) inhibition of beryllium-stimulated IFN-γ expression (Figure 5b). Much less inhibition of IFN-γ expression (27% ± 10%) was noted with saturating amounts of the anti-DR mAb, and a lack of inhibition was observed with the anti-DQ mAb.
Assessment of beryllium-specific cytokine-producing cells in blood.
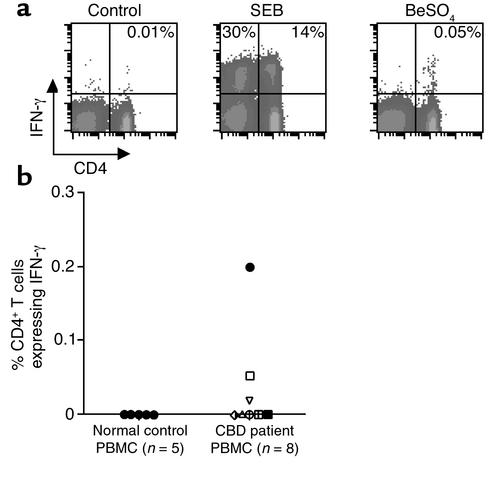
Most of these CBD patients demonstrated beryllium-specific T cells in the peripheral blood as detected in proliferation assays (see Figure 1b). However, previous studies of TCR expression suggested that expanded beryllium-responsive CD4+ T cell clones in the BAL of CBD patients are rarely present in the circulating pool (21). We measured intracellular cytokine expression after the stimulation of blood cells from CBD patients (Figure 6). Despite a frequency approaching 21% in the BAL of patient 2, the frequency of beryllium-specific CD4+ T cells for IFN-γ was 0.05% (1 in 2,000) (Figure 6a). Similar to the results in BAL, the beryllium-responsive T cells in blood were confined to the CD4-expressing T cells, although both CD4+ and CD8+ T cells expressed IFN-γ following SEB stimulation (Figure 6a). For the eight CBD patients evaluated, beryllium-responsive T cells were detected in blood in only three subjects (Figure 6b), and the highest frequency was only 1 in 500 cells.
Figure 6.
Intracellular expression of IFN-γ in peripheral blood CD4+ T cells after stimulation with SEB or BeSO4. (a) A representative experiment is shown for a flow cytometric analysis of PBMCs from CBD patient 2 stimulated with medium alone, SEB, or BeSO4 and stained for surface CD4 and intracellular IFN-γ expression. The number in the upper right quadrant of each density plot represents the percentage of CD4+ T cells expressing IFN-γ. (b) The percentage of peripheral blood CD4+ T cells that express IFN-γ after stimulation with BeSO4 is shown for eight CBD patients and five healthy control subjects. The symbols for each CBD patient correspond to those shown in Figure 4.
Discordance between the number of beryllium-responsive cytokine-secreting and proliferating cells.
We found no correlation between the frequency of beryllium-stimulated cytokine-producing CD4+ T cells and beryllium-induced lymphocyte proliferation for the eight CBD patients who had both assays performed (r2 = 0.028, P = 0.69). Three CBD patients (patients 4, 6, and 9) showed very high frequencies of beryllium-specific CD4+ T cells (16%, 24%, and 26%) but demonstrated minimal lymphocyte proliferation. These data suggest that certain antigen-specific CD4+ T cells in humans may be able to secrete Th1-type cytokines after stimulation but are temporarily or permanently unable to proliferate. A similar lack of correlation between cytokine secretion and proliferation was observed in the peripheral blood studies (compare Figures 1b and 6b).
Memory cell marker expression.
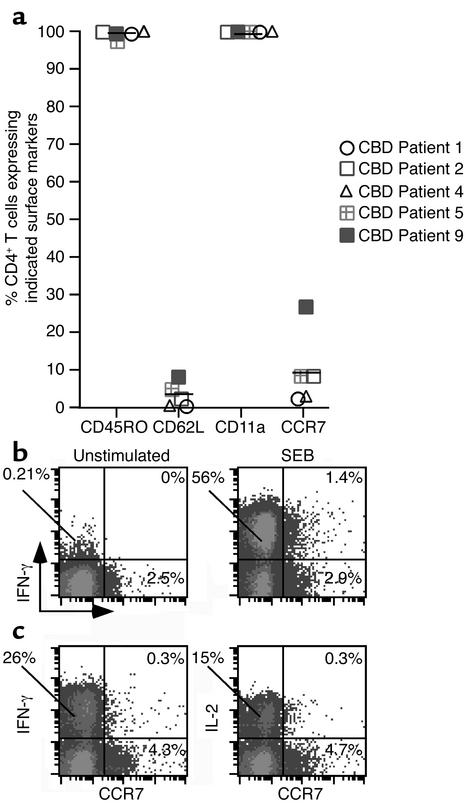
Due to the compartmentalization of beryllium-responsive T cells in the lung of CBD patients, we analyzed the expression of CD45RO, CD62L, CD11a, and CCR7 on BAL CD4+ T cells (Figure 7a). Greater than 99% of BAL CD4+ T cells express CD45RO and CD11a. In addition, these cells also express the adhesion molecule CD44 (data not shown). Surface expression of CD62L and CCR7 by BAL CD4+ T cells ranged from 0.7% to 8.3% (mean 3.5% ± 1.4%) and 2.3% to 27% (mean 9.8% ± 4.5%), respectively. Thus, the majority of BAL CD4+ T cells appear to be effector memory T cells, lacking expression of CD62L and CCR7. As shown in Figure 7, b and c, BAL CCR7+ CD4+ T cells lack the ability to express IFN-γ after short-term exposure to either SEB or BeSO4, and essentially all IFN-γ and IL-2 was produced by BAL CD4+ T cells that lacked CCR7 expression.
Figure 7.
Surface markers expressed by BAL CD4+ T cells from CBD patients. (a) The percentage of BAL CD4+ T cells that express CD45RO, CD62L, CD11a, and CCR7 is shown. The symbols for each CBD patient correspond to those shown in Figures 4 and 6, and the solid line represents the mean value for each marker. (b) Intracellular IFN-γ production by BAL CD4+ T cells in relation to expression of CCR7 following stimulation with medium alone or SEB. Data are shown for the percentage of BAL CD4+ T cells that express IFN-γ, CCR7, or both. (c) IFN-γ and IL-2 production by BAL CD4+ T cells in relation to expression of CCR7 following stimulation with BeSO4. Data are shown for the percentage of BAL CD4+ T cells that express IFN-γ, CCR7, or both.
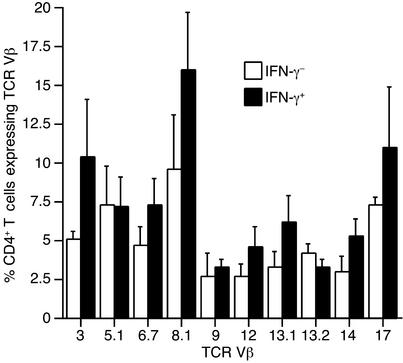
TCR Vβ repertoire of beryllium-specific memory effector BAL CD4+ T cells.
Although TCR Vβ subset expansions in the BAL of CBD patients have been previously identified, marked heterogeneity within the BAL CD4+ T cell repertoire characterizes this disease (20). In the current study, we examined whether TCR Vβ expression was selectively skewed in the beryllium-specific (IFN-γ–secreting) CD4+ T cells compared with other CD4+ T cells in BAL. A panel of ten anti-TCR mAb’s, covering approximately 35% of the normal CD4+ TCR Vβ repertoire, was used based on previous studies showing that these Vβ subsets encompassed most of the abnormalities found in the CBD population (20). In the current study, these antibodies covered over 70% of the repertoire expressed by beryllium-specific cells in the five CBD patients analyzed (Figure 8). The results showed that TCR Vβ expression is remarkably diverse in the beryllium-specific (IFN-γ+) population (Figure 8). No Vβ-expressing subset dominated this population in any individual. Consistent with previous studies (20, 21), we noted different individuals with percent-ages of Vβ3+, Vβ8+, Vβ13.1+, and Vβ17+ cells increased more than twofold in the IFN-γ+ versus the IFN-γ– populations, perhaps reflecting clonal or oligoclonal expansions expressing that particular Vβ. These individual differences were responsible for the increased mean values for the subsets shown in Figure 8, and for each TCR Vβ analyzed, no statistically significant differences were seen in the mean (± SEM) levels of TCR expression in the IFN-γ+ versus the IFN-γ– populations of all patients analyzed.
Figure 8.
TCR V regions expressed on BAL CD4+ T cells from five CBD patients. Data are expressed as the mean ± SEM percentage of IFN-γ– and IFN-γ+ CD4+ T cells that express the indicated TCR Vβ.
Discussion
Following exposure to beryllium in the workplace, a subset of individuals develop an accumulation of CD4+ T cells and granulomatous inflammation in the lung (2). The BAL CD4+ T cell population contains oligoclonal expansions of antigen-specific T cells that recognize beryllium in association with certain HLA-DP molecules on the surface of antigen-presenting cells (9). Following beryllium recognition, subsets of these cells proliferate and secrete Th1-type cytokines (2, 9, 21, 22). With the large number of lymphocytes accumulating in the lungs of CBD patients, this disease forms an ideal system in which to define the functional and phenotypic characteristics of antigen-specific CD4+ T cells in a target organ. In the present study, we used intracellular cytokine staining of BAL and blood cells to characterize beryllium-specific T cells in these compartments. Our results demonstrate a striking number of HLA-DP–restricted, beryllium-responsive, Th1-type cytokine–secreting CD4+ T cells compartmentalized to the lung in CBD patients. In addition, BAL CD4+ T cells expressed a surface marker pattern indicative of terminally differentiated effector memory T cells. Although it is possible that BAL CD8+ cells were unable to respond to beryllium under the experimental conditions used in this study, the data suggest that these cells play a minor, if any, role in the pathogenesis of this disease.
Due to the inaccessibility of antigen-specific T cells in target organs in humans as well as the absence of the known etiologic antigen or autoantigen in the majority of immune-mediated diseases, the prevalence of antigen-specific CD4+ T cells in target organs has not been well delineated. In the present work, we were able to study an immune-mediated disease with a known pathogenic antigen. Although our findings may not reflect the spectrum of CBD as a whole, our results demonstrate frequencies of IFN-γ–, TNF-α–, and IL-2–producing beryllium-specific CD4+ T cells in BAL ranging up to 29% of all of the CD4+ T cells in this organ. A lesser quantity of BAL T cells produced IL-2 after BeSO4 exposure as compared with either IFN-γ or TNF-α. These findings are consistent with the work of Reinhardt et al. (29), who found that memory CD4+ T cells in the lungs of C57BL/6 mice produced more IFN-γ, while memory cells from lymph nodes produced more IL-2, suggesting that memory T cells residing in lymph nodes and nonlymphoid organs possessed different functional capabilities. Thus, in an organ characterized by the infiltration of effector Th1-type memory T cells, similar expression of IFN-γ and TNF-α and less IL-2 after antigen exposure may be expected.
Compared with the precursor frequency of antigen-specific T cells found in target organs of other diseases, the quantity of beryllium-specific CD4+ T cells present in the lungs of CBD patients is striking. For example, the frequency of CD4+ T cells specific for Mycobacterium leprae antigens in tuberculoid skin lesions was estimated to be 1 in 50, which was 100-fold higher than in the blood of the same individuals (30, 31). In patients with treatment-resistant Lyme disease, CD4+ T cells specific for Borrelia burgdorferi peptide (OspA 164-183) were identified in the synovial fluid at frequencies ranging from 0.5% to 3.1% of CD4+ T cells (32).
Susceptibility to beryllium sensitization and CBD has been strongly associated with particular HLA-DP alleles (6–8, 33). Using T cell lines and clones derived from CBD patients, previous studies have shown that the MHC class II molecules involved in beryllium presentation match those HLA-DP alleles implicated in genetic susceptibility (9, 10). The present findings with freshly isolated BAL cells confirm that the great majority, and in some subjects all, of beryllium presentation is occurring through HLA-DP, with a minor role for HLA-DR and no involvement of HLA-DQ. Both proliferative and cytokine-secreting responses to beryllium appear to be mostly dependent on presentation through HLA-DP. The HLA-DP polymorphic charged residues important for beryllium presentation are predicted to be pointing into the peptide-binding cleft, thus affecting beryllium binding through the peptides bound in the groove (9). Together, this manner by which HLA-DP residues appear to affect beryllium presentation, the high frequency of beryllium-specific T cells in lung, and the diverse TCR Vβ repertoire among beryllium-specific CD4+ T cells suggest that a variety of peptides bound to HLA-DP are capable of interacting with beryllium and subsequently being recognized by CD4+ T cells.
Previous studies have suggested a separation of lung and blood CD4+ TCR repertoires in CBD patients (20, 21). The present study reinforces this compartmentalization of beryllium-specific CD4+ T cells within the lung, which appears to be explained by the large accumulation of Th1-type cytokine–secreting effector memory cells. In addition, our findings suggest that these CD4+ T cells may permanently reside in the lung and not continuously recirculate through the bloodstream. A potential mechanism underlying the differentiation and compartmentalization of antigen-specific CD4+ T cells in CBD may be the persistence of beryllium in the lungs of these patients, which can be detected years after removal from the workplace (1). Concordant findings have been reported in animal models following Sendai virus infection in C57BL/6 mice, where 13.2% of BAL CD4+ T cells were specific for an epitope of hemagglutinin-neuraminidase compared with 0.11% in mediastinal lymph nodes and 0.06% in the spleen (34). In addition, months to years after antigen challenge, Bice et al. (35, 36) observed the production of antibody by B cells localized to the lungs of beagles, confirming the maintenance of long-term antibody responses in the lung. Our findings have important implications for other CD4+ T cell–mediated diseases with inaccessible target organs, such as multiple sclerosis and type 1 diabetes. In addition, this study helps explain the findings of others that the frequency of antigen-specific CD4+ T cells in the peripheral blood of autoimmune disease patients may be too low to allow direct visualization (37).
Following exposure to antigen presented by dendritic cells in lymphoid tissues, naive T cells become activated, proliferate, and migrate to sites of inflammation. While the majority of antigen-primed T cells die, a subset develops into memory cells, which provide for a more rapid and effective secondary immune response (38). There appear to be at least two subsets of memory T cells, each possessing different migratory and functional capabilities (38–41). The effector memory T (TEM) cell represents a terminally differentiated cell that immediately produces cytokine after stimulation and lacks the lymph node homing receptors CD62L and CCR7. Conversely, the central memory T (TCM) cell expresses both CD62L and CCR7 and lacks immediate effector cell function. Once restimulated, TCM cells are capable of differentiating into TEM cells. Based on their cytokine-secreting responses and lack of CD62L and CCR7 expression, we conclude that the majority of beryllium-specific T cells in the lungs of CBD patients are highly differentiated effector memory T cells. These cells also express receptors such as CD11a that allow entry into inflamed tissues, and greater than 99% of BAL T cells express CD45RO and CD44. These observations are not specific for CBD patients, since BAL CD4+ T cells from normal subjects have also been found to express low levels of CCR7 (9.4% of those cells) (42). There also exists in the lung a much smaller population of central memory CCR7+ CD4+ T cells, which are trafficking through this organ and lack immediate effector function. Due to the low frequency of beryllium-specific T cells in blood, we were unable to perform studies of surface marker expression in fresh blood without some form of in vitro activation. Although CCR7+ cells in both BAL and blood contain few beryllium-specific cells capable of immediate cytokine production, we speculate that this cell population may indeed contain an antigen-specific subset with proliferative potential.
It is well established that naive and memory T cells possess different functional and phenotypic characteristics (40, 41). However, it has only recently become known that different subsets of memory CD4+ T cells also express different functional characteristics, which may correlate with some of the markers described above. Recently, Geginat et al. (43) showed that effector memory CD4+ T cells could be selectively expanded with IL-7 and IL-15 without the presence of antigen. However, it is unknown whether those cells would proliferate upon antigen exposure. Our data suggest that certain beryllium-specific BAL CD4+ T cells proliferate poorly despite the ability to secrete Th1 cytokines after exposure to BeSO4. In CD8+ T cells, there is evidence to suggest an inverse correlation between lymphocyte proliferation and cell differentiation, with increased perforin expression associated with less proliferative capacity (44). In a target organ, terminally differentiated effector memory T cells may indeed lose their proliferative capacity while maintaining the ability to secrete Th1-type cytokines in response to antigen exposure.
In summary, we have identified a large quantity of HLA-DP–restricted beryllium-specific effector memory CD4+ Th1-type cells compartmentalized to the lungs of CBD patients. We believe that these findings in the lung advance the current level of understanding of immunological memory in human disease. Although antigen-specific effector memory CD4+ T cells may be required for the rapid immune response to infection, their accumulation in target organs in autoimmune and hypersensitivity disorders likely contributes to the ongoing immune response and progressive organ damage.
Acknowledgments
This work was supported by NIH grants HL62410, ES06358, P01-ES011810, and HL03982, and General Clinical Research Center grant M01-RR-0051 from the Division of Research Resources.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: chronic beryllium disease (CBD); bronchoalveolar lavage (BAL); T cell receptor (TCR); white blood cell (WBC); staphylococcal enterotoxin B (SEB); stimulation index (SI).
References
- 1.Newman, L.S., Maier, L.A., and Nemery, B. 1998. Interstitial lung disorders due to beryllium and cobalt. In Interstitial lung disease. M.I. Schwarz and T.E. King, Jr., editors. B.C. Decker Inc. Hamilton, Ontario. 367–392.
- 2.Fontenot AP, Newman LS, Kotzin BL. Chronic beryllium disease: T cell recognition of a metal presented by HLA-DP. Clin Immunol. 2001;100:4–14. doi: 10.1006/clim.2001.5053. [DOI] [PubMed] [Google Scholar]
- 3.Kreiss K, Wasserman S, Mroz MM, Newman LS. Beryllium disease screening in the ceramics industry: blood test performance and exposure-disease relations. J Occup Med. 1993;35:267–274. [PubMed] [Google Scholar]
- 4.Kreiss K, Mroz MM, Zhen B, Martyny JW, Newman LS. Epidemiology of beryllium sensitization and disease in nuclear workers. Am Rev Respir Dis. 1993;148:985–991. doi: 10.1164/ajrccm/148.4_Pt_1.985. [DOI] [PubMed] [Google Scholar]
- 5.Kreiss K, Mroz MM, Newman LS, Martyny J, Zhen B. Machining risk of beryllium disease and sensitization with median exposures below 2 μg/m3. Am J Ind Med. 1996;30:16–25. doi: 10.1002/(SICI)1097-0274(199607)30:1<16::AID-AJIM3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 6.Richeldi L, Sorrentino R, Saltini C. HLA-DPB1 glutamate 69: a genetic marker of beryllium disease. Science. 1993;262:242–244. doi: 10.1126/science.8105536. [DOI] [PubMed] [Google Scholar]
- 7.Richeldi L, et al. Interaction of genetic and exposure factors in the prevalence of berylliosis. Am J Ind Med. 1997;32:337–340. doi: 10.1002/(sici)1097-0274(199710)32:4<337::aid-ajim3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, et al. Differential susceptibilities to chronic beryllium disease contributed by different Glu69 HLA-DPB1 and -DPA1 alleles. J Immunol. 1999;163:1647–1653. [PubMed] [Google Scholar]
- 9.Fontenot AP, Torres M, Marshall WH, Newman LS, Kotzin BL. Beryllium presentation to CD4+T cells underlies disease susceptibility HLA-DP alleles in chronic beryllium disease. Proc Natl Acad Sci USA. 2000;97:12717–12722. doi: 10.1073/pnas.220430797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lombardi G, et al. HLA-DP allele-specific T cell responses to beryllium account for DP-associated susceptibility to chronic beryllium disease. J Immunol. 2001;166:3549–3555. doi: 10.4049/jimmunol.166.5.3549. [DOI] [PubMed] [Google Scholar]
- 11.Newman LS, Kreiss K. Nonoccupational beryllium disease masquerading as sarcoidosis: identification by blood lymphocyte proliferation response to beryllium. Am Rev Respir Dis. 1992;145:1212–1214. doi: 10.1164/ajrccm/145.5.1212. [DOI] [PubMed] [Google Scholar]
- 12.Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997;336:1224–1234. doi: 10.1056/NEJM199704243361706. [DOI] [PubMed] [Google Scholar]
- 13.Saltini C, Winestock K, Kirby M, Pinkston P, Crystal RG. Maintenance of alveolitis in patients with chronic beryllium disease by beryllium-specific helper T cells. N Engl J Med. 1989;320:1103–1109. doi: 10.1056/NEJM198904273201702. [DOI] [PubMed] [Google Scholar]
- 14.Epstein PE, Dauber JH, Rossman MD, Daniele RP. Bronchoalveolar lavage in a patient with chronic berylliosis: evidence for hypersensitivity pneumonitis. Ann Intern Med. 1982;97:213–216. doi: 10.7326/0003-4819-97-2-213. [DOI] [PubMed] [Google Scholar]
- 15.Newman LS, et al. Compartmentalized immune response reflects clinical severity of beryllium disease. Am J Respir Crit Care Med. 1994;150:135–142. doi: 10.1164/ajrccm.150.1.8025739. [DOI] [PubMed] [Google Scholar]
- 16.Rossman MD, et al. Proliferative response of bronchoalveolar lymphocytes to beryllium. Ann Intern Med. 1988;108:687–693. doi: 10.7326/0003-4819-108-5-687. [DOI] [PubMed] [Google Scholar]
- 17.Kreiss K, Newman LS, Mroz M, Campbell PA. Screening blood test identifies subclinical beryllium disease. J Occup Med. 1989;31:603–608. doi: 10.1097/00043764-198907000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Saltini C, Kirby M, Trapnell BC, Tamura N, Crystal RG. Biased accumulation of T lymphocytes with “memory”-type CD45 leukocyte common antigen gene expression on the epithelial surface of the human lung. J Exp Med. 1990;171:1123–1140. doi: 10.1084/jem.171.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mroz MM, Kreiss K, Lezotte DC, Campbell PA, Newman LS. Reexamination of the blood lymphocyte transformation test in the diagnosis of chronic beryllium disease. J Allergy Clin Immunol. 1991;88:54–60. doi: 10.1016/0091-6749(91)90300-d. [DOI] [PubMed] [Google Scholar]
- 20.Fontenot AP, Kotzin BL, Comment C, Newman LS. Expansions of T-cell subsets expressing particular T cell receptor variable regions in chronic beryllium disease. Am J Respir Cell Mol Biol. 1998;18:581–589. doi: 10.1165/ajrcmb.18.4.2981. [DOI] [PubMed] [Google Scholar]
- 21.Fontenot AP, Falta MT, Freed BM, Newman LS, Kotzin BL. Identification of pathogenic T cells in patients with beryllium-induced lung disease. J Immunol. 1999;163:1019–1026. [PubMed] [Google Scholar]
- 22.Tinkle SS, Kittle LA, Schumacher BA, Newman LS. Beryllium induces IL-2 and IFN-γ in berylliosis. J Immunol. 1997;158:518–526. [PubMed] [Google Scholar]
- 23.Tinkle SS, Newman LS. Beryllium-stimulated release of tumor necrosis factor-alpha, interleukin-6, and their soluble receptors in chronic beryllium disease. Am J Respir Crit Care Med. 1997;156:1884–1891. doi: 10.1164/ajrccm.156.6.9610040. [DOI] [PubMed] [Google Scholar]
- 24.Newman LS, Kreiss K, King TE, Jr, Seay S, Campbell PA. Pathologic and immunologic alterations in early stages of beryllium disease: re-examination of disease definition and natural history. Am Rev Respir Dis. 1989;139:1479–1486. doi: 10.1164/ajrccm/139.6.1479. [DOI] [PubMed] [Google Scholar]
- 25.Bronchoalveolar lavage constituents in healthy individuals, idiopathic pulmonary fibrosis and selected comparison groups. The BAL Cooperative Group Steering Committee. Am Rev Respir Dis. 1990;141(Suppl. 5):S169–S202. doi: 10.1164/ajrccm/141.5_Pt_2.S169. [DOI] [PubMed] [Google Scholar]
- 26.Waldrop SL, Pitcher CJ, Peterson DM, Maino VC, Picker LJ. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest. 1997;99:1739–1750. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitcher CJ, et al. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 28.Wahlstrom J, et al. Analysis of intracellular cytokines in CD4+ and CD8+ lung and blood T cells in sarcoidosis. Am J Respir Crit Care Med. 2001;163:115–121. doi: 10.1164/ajrccm.163.1.9906071. [DOI] [PubMed] [Google Scholar]
- 29.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 30.Modlin RL, et al. Learning from lesions: patterns of tissue inflammation in leprosy. Proc Natl Acad Sci USA. 1988;85:1213–1217. doi: 10.1073/pnas.85.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melancon-Kaplan J, et al. Immunological significance of Mycobacterium leprae cell walls. Proc Natl Acad Sci USA. 1988;85:1917–1921. doi: 10.1073/pnas.85.6.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer AL, et al. Direct enumeration of Borrelia-reactive CD4 T cells ex vivo by using MHC class II tetramers. Proc Natl Acad Sci USA. 2000;97:11433–11438. doi: 10.1073/pnas.190335897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossman MD, et al. Human leukocyte antigen class II amino acid epitopes: susceptibility and progression markers for beryllium hypersensitivity. Am J Respir Crit Care Med. 2002;165:788–794. doi: 10.1164/ajrccm.165.6.2104002. [DOI] [PubMed] [Google Scholar]
- 34.Hogan RJ, et al. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 2001;193:981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bice DE, Weissman DN, Muggenburg BA. Long-term maintenance of localized antibody responses in the lung. Immunology. 1991;74:215–222. [PMC free article] [PubMed] [Google Scholar]
- 36.Bice DE, Muggenburg BA. Pulmonary immune memory: localized production of antibody in the lung after antigen challenge. Immunology. 1996;88:191–197. doi: 10.1111/j.1365-2567.1996.tb00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bill J, Kotzin BL. Use of soluble MHC class II/peptide multimers to detect antigen-specific T cells in human disease. Arthritis Res. 2002;4:261–265. doi: 10.1186/ar417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sallusto F, Lanzavecchia A. Exploring pathways for memory T cell generation. J Clin Invest. 2001;108:805–806. doi:10.1172/JCI200114005. doi: 10.1172/JCI14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 40.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290:92–97. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 41.Mackay CR, von Andrian UH. Memory T cells: local heroes in the struggle for immunity. Science. 2001;291:2323–2324. doi: 10.1126/science.1059984. [DOI] [PubMed] [Google Scholar]
- 42.Campbell JJ, et al. CCR7 expression and memory T cell diversity in humans. J Immunol. 2001;166:877–884. doi: 10.4049/jimmunol.166.2.877. [DOI] [PubMed] [Google Scholar]
- 43.Geginat J, Sallusto F, Lanzavechia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chamberlain WD, Falta MT, Kotzin BL. Functional subsets within clonally expanded CD8(+) memory T cells in elderly humans. Clin Immunol. 2000;94:160–172. doi: 10.1006/clim.1999.4832. [DOI] [PubMed] [Google Scholar]