Abstract
Decay-accelerating factor (DAF) is a glycosylphosphatidylinositol (GPI)-anchored membrane protein that inhibits both the classical and the alternative pathways of complement activation. DAF has been studied extensively in humans under two clinical settings: when absent from the erythrocytes of paroxysmal nocturnal hemoglobinuria (PNH) patients, who suffer from complement-mediated hemolytic anemia, and in transgenic pigs expressing human DAF, which have been developed to help overcome complement-mediated hyperacute rejection in xenotransplantation. Nevertheless, the exact role of DAF in regulating complement activation in vivo on the cell surface and the species specificity of this molecule remain to be fully characterized. To address these issues, we have used gene targeting to produce mice lacking GPI-anchored DAF. We found that erythrocytes from mice deficient in GPI-anchored DAF showed no increase in spontaneous complement activation in vivo but exhibited impaired regulation of zymosan-initiated bystander and antibody-triggered classical pathway complement activation in vitro, resulting in enhanced complement deposition. Despite a high level of C3 fixation, no homologous hemolysis occurred. It is noteworthy that GPI-linked DAF knockout erythrocytes, when tested with human and guinea pig sera, were more susceptible to heterologous complement lysis than were normal erythrocytes. These results suggest that DAF is capable of regulating homologous as well as heterologous complement activation via the alternative or the classical pathway. They also indicate that DAF deficiency alone is not sufficient to cause homologous hemolysis. In contrast, when the assembly of the membrane-attack complex is not properly regulated, as in the case of heterologous complement activation or in PNH patients, impaired erythrocyte DAF activity and enhanced C3 deposition could lead to increased hemolytic reaction.
Complement plays an essential role in host defense (1). To prevent complement-mediated autologous attack, host tissues express a number of fluid-phase and membrane-bound inhibitors (1, 2). The activities of the membrane-bound complement inhibitors are generally thought to be species-specific in that under normal circumstances, serum from one species is capable of lysing erythrocytes of another species but not of its own (1, 2). Decay-accelerating factor (DAF) is a glycosylphosphatidylinositol (GPI)-anchored membrane regulator of complement that inhibits the C3 convertases of both the classical and alternative pathways (3). DAF acts by facilitating subunit dissociation of preformed C3 convertases and by preventing the assembly of new C3 convertases (4–6). The protein originally was purified from human erythrocytes (4) and later was shown to be absent from the blood cells of patients with paroxysmal nocturnal hemoglobinuria (PNH) syndrome, a disease characterized by an increased sensitivity of red blood cells to autologous complement-mediated lysis (7, 8). It is now understood that the fundamental defect in PNH occurs at the stage of GPI anchor biosynthesis as a result of somatic mutations in the PIG-A gene in hematopoietic stem cells rather than a defect in the DAF gene per se (9). Thus, DAF and all other GPI-anchored proteins are absent from the affected blood cells of PNH patients (9).
The in vivo function of DAF in regulating complement activation on the cell surface, highlighted by its absence from the affected erythrocytes of PNH patients, remains an unsettled question. Medof et al. (10) and Wilcox et al. (11) have shown that purified human DAF, when incorporated into PNH erythrocytes in vitro (by virtue of its GPI anchor) could reduce the sensitivity of these cells to complement-mediated lysis. On the other hand, rare cases of specific DAF deficiency in human erythrocytes caused by germ line mutations in the DAF gene have also been identified (Inab phenotype), but individuals with these deficiencies do not develop PNH disease (12–14). Also, unlike cells from PNH patients, Inab erythrocytes are not sensitive to acidified serum lysis in vitro (15, 16). These observations have raised questions about the relevance of DAF deficiency in the pathogenesis of PNH syndrome and have led to the suggestion (17) that the DAF used in the reincorporation study of Medof et al. (10) might have been contaminated with CD59, a second GPI-anchored membrane complement regulator that also is deficient in PNH erythrocytes (9).
Another clinical setting in which human DAF has been studied intensively is that of xenotransplantation (18). A major obstacle in xenotransplantation is the hyperacute rejection mediated by human complement (18). It is generally assumed that activation of human complement on organs from a discordant species is partially a result of a lack of cross-species activity of membrane complement-regulating proteins such as DAF. Consequently, transgenic pigs overexpressing human DAF have been developed with the hope that organs from such animals, when transplanted, will be able to survive acute-phase rejection (18, 19). Despite the intense interest and research effort devoted to this subject, however, the issue of species specificity of DAF in regulating C3 convertase has not been properly addressed. Earlier studies dealing with this question that used either DAF proteins purified from erythrocytes (4, 20) or a soluble form of expressed human DAF (21) have produced mixed results.
To better define the in vivo function of DAF in regulating complement activation and to further characterize the species specificity of DAF, we have generated mice deficient in the GPI-anchored DAF by using homologous recombination in embryonic stem cells. In the mouse, two DAF genes encoding a GPI-anchored form of DAF (GPI-DAF) and a transmembrane form of DAF (TM-DAF), respectively, have been identified (22, 23). We chose to target the GPI-DAF gene because both its broad tissue-distribution pattern and the predicted GPI-anchored nature of the protein it encodes suggest that this gene, rather than the TM-DAF gene, is the true murine homolog of human DAF (22, 23). In this paper, we report the results of our study of GPI-DAF-deficient mouse erythrocytes with regard to their sensitivity to homologous and heterologous complement activation.
MATERIALS AND METHODS
Genomic Clone Isolation.
To clone the mouse GPI-DAF gene, the full-length mouse GPI-DAF cDNA was used as a probe to screen a 129/Sv λFixII murine genomic library (Stratagene). This strategy resulted in the isolation and cloning of a 13-kb genomic fragment (Fig. 1A). The two mouse DAF genes are overall highly homologous but can be distinguished by significant sequence divergence at the 5′ and 3′ ends of their cDNAs (22, 23). Selective sequence analysis of the isolated genomic clone showed it to represent the 5′ portion of the mouse GPI-DAF gene containing the first four exons (Fig. 1A). Exons 2 and 3 were found to encode the first and second short consensus repeats (SCRs) of mouse GPI-DAF (23), respectively.
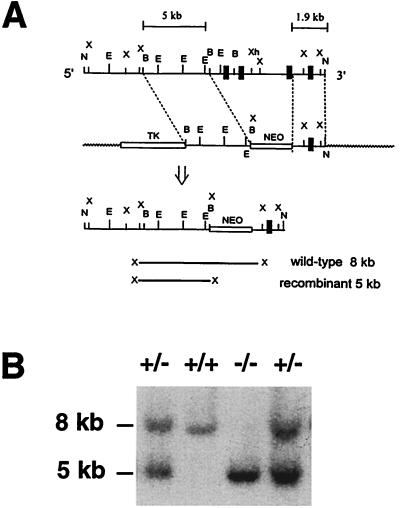
Figure 1.
Targeting of the GPI-DAF locus. (A) Partial restriction maps of the GPI-DAF gene fragment and the targeting vector. N, NotI; X, XbaI; E, EcoRI; B, BamHI; Xh, XhoI. After correct targeting, the first three exons (represented by thick vertical bars) would be deleted and replaced with the NEO gene. Targeted ES-cell clones were identified by the presence of an extra 5-kb band, in addition to the 8-kb wild-type band, on Southern blot analysis of XbaI-digested genomic DNAs. The probe used for Southern blot screening was a 300-bp XbaI-BamHI fragment immediately 5′ to the 5-kb BamHI fragment. (B) Representative Southern blot result of tail DNA showing the three genotypes of progeny between heterozygous mouse matings. +/+, wild-type; −/− homozygous; +/−, heterozygous.
Generation of GPI-DAF-Deficient Mice.
For construction of the targeting vector, a 5-kb BamHI fragment from the 5′-flanking region was subcloned into the unique BamHI site of the vector pPNT (24). The pPNT vector contains a positive (Neo) and a negative (thymidine kinase) selection marker. After identifying clones containing the 5-kb BamHI fragment in the correct orientation, we ligated a PCR-amplified 1.9-kb XhoI-NotI fragment encompassing exon 4 into the vector at the unique XhoI-NotI sites (Fig. 1A). The targeting vector was linearized by using NotI digestion and transfected by using electroporation (30 μg of DNA per 8 × 106 cells in 0.8 ml of PBS) into TL-1 embryonic stem (ES) cells (a gift from P. Labosky, Department of Cellular and Developmental Biology, University of Pennsylvania). Culturing and electroporation of ES cells were performed according to standard protocols (25, 26). Transfected ES cells were selected with neomycin (G418, 210 μg/ml) and gancyclovir (2 μM). Surviving cell clones were picked into 96 wells, expanded, and screened by Southern blot analysis of genomic DNAs after XbaI digestion with a 300-bp probe (Fig. 1A). Additional screening to confirm proper targeting was performed with a Neo probe. Selected ES cell clones were expanded and used for blastocyst microinjection to produce chimeric mice (26). Confirmation of germ-line transmission of the mutated GPI-DAF gene in chimeric mice and subsequent breeding experiments to produce homozygous mice (F2 generation) were carried out according to established procedures (26). The genotypes of the mice were determined by Southern blotting of tail DNA (26). All ensuing phenotype characterizations were carried out by using mice of the F2 or F3 generation that had mixed background (129/B6).
Northern Blot Detection of GPI-DAF and TM-DAF mRNAs.
Total tissue RNAs were extracted with Trizol reagent (GIBCO/BRL), fractionated in a 1% agarose gel, and transferred to Hybond-N+ nylon membranes. To detect GPI-DAF mRNAs, a 276-bp 3′ cDNA-specific fragment (23) was used as a probe for hybridization in QuickHyb solution (Stratagene). To detect TM-DAF mRNA, the membrane was stripped and rehybridized with a 180-bp specific probe corresponding to the 3′ cDNA of TM-DAF (23). Finally, the membrane was stripped again and hybridized with a control probe (glyceraldehyde-3-phosphate dehydrogenase) to confirm equal loading of RNAs.
Isolation of Blood Cells.
Blood (10–50 μl) was collected from the tail vein of wild-type or knockout mice into either normal saline (for red blood cell analysis) or 1% HCl to lyse and remove erythrocytes (for white blood cell analysis). Cells were harvested, washed with PBS, and counted with a hemocytometer or used for complement deposition or lysis assays.
Fluorescence-Activated Cell Sorter (FACS) Analysis of C3 Deposition.
To measure C3 deposition on the erythrocytes, cells (1 × 106 to 1 × 108/ml in FACS buffer) were incubated for 30 min with an FITC-conjugated polyclonal goat anti-mouse C3 antibody (Cappel/ICN), washed, and analyzed by FACScan (Becton Dickinson) by gating of red blood cells in forward and side scatterings. In the first experiment, erythrocytes were analyzed directly to assess the degree of spontaneous complement activation in vivo. In the second experiment, erythrocytes (1 × 108/ml) were first coincubated with zymosan particles in normal mouse serum at 37°C for 60 min [in a total volume of 100 μl, made by mixing an equal volume of serum and a 50% suspension of zymosan in gelatin-Veronal buffered saline containing 2 mM MgCl2 and 10 mM EGTA (GVBS-EGTA/Mg2+)]. The cells and zymosan particles were then washed in FACS buffer, incubated with an FITC-conjugated goat anti-mouse C3 antibody, and analyzed for C3 deposition. Total complement activation by zymosan particles in the fluid phase was determined by analyzing C3 cleavage by using double immunodiffusion electrophoresis. In the third experiment, regulation of classical-pathway complement activation on the erythrocyte surface was evaluated. Erythrocytes were first incubated in gelatin/Veronal buffered saline (GVBS2+) with a polyclonal rabbit anti-mouse erythrocyte antibody (1:200, Cappel/ICN). The cells were then washed and incubated with either normal mouse serum (1:4 dilution) or C5-deficient human serum (Sigma, 1:10 dilution) in GVBS2+. After washing and staining with an FITC-conjugated goat anti-mouse C3 or anti-human C3 antibody (Cappel/ICN), the levels of mouse or human C3 deposition on the cells were determined by FACS analysis.
Hemolytic Assays.
Hemolytic assays were carried out at 37°C for 30 min by using 2–3 × 107 erythrocytes in a 100-μl volume containing an appropriate amount of either mouse, human, or guinea pig serum (Sigma). The CH50 of a serum sample was first determined in a pilot experiment, and appropriate dilution of the serum was made so that 10–60% lysis was expected in the hemolytic assay. At the end of the incubation period, the cells were centrifuged and supernatants were measured spectrophometrically (at 414 nm) to determine hemoglobin release from hemolysis. Percent hemolysis was calculated by dividing the OD414 value by that of a sample in which total hemolysis was induced by hypotonic shock. Each sample was assayed in duplicate or triplicate. Hemolytic assays were carried out either in GVBS2+ (for classical or classical plus alternative pathways) or GVBS-EGTA/Mg2+ (for alternative pathway). For total or classical pathway hemolytic assays, erythrocytes were first sensitized with a polyclonal rabbit anti-mouse erythrocyte antibody (1:200, Cappel/ICN) as described above. For human classical pathway-mediated hemolysis (i.e., no alternative-pathway participation), serum was treated at 50°C for 20 min to inactivate factor B (27) and hence the alternative pathway activity. The effective and selective inactivation of factor B was verified by reconstitution experiments with purified factor B to 400 μg/ml.
RESULTS
Selective Inactivation of the GPI-DAF Gene.
Our strategy for targeting the GPI-DAF gene is illustrated in Fig. 1A. After correct targeting, the first three exons, as well as the intervening intron sequence and a portion of the 5′-flanking region, of the GPI-DAF gene were to be deleted and replaced by the Neo gene. No GPI-DAF mRNA was expected to be made, because the proximal promoter sequence necessary for RNA transcription would have been removed. Furthermore, even if alternatively spliced mRNAs and partial GPI-DAF proteins were made, no DAF activity would be displayed by such proteins, because the first two SCRs (encoded by exon 2 and 3, respectively) would be absent. Studies of human DAF have established that SCRs 2, 3, and 4 are essential for the activity of DAF (28). The successful generation of homozygous GPI-DAF-deficient mice is illustrated in Fig. 1B.
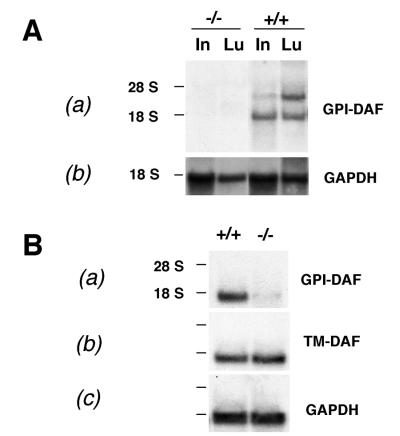
To confirm the inactivation of the GPI-DAF gene, Northern blot analysis was carried out with probes specific for the GPI-DAF and the TM-DAF genes. In prior studies, GPI-DAF, but not TM-DAF was shown to be expressed widely in normal mouse tissues (22, 23). By using specific cDNA probes, we detected GPI-DAF expression in the lung, liver, intestine, heart, kidney, spleen, uterus, and testis (data not shown). TM-DAF mRNA expression was detected only in mouse testis (ref. 23; data not shown). Comparative analysis of wild-type and homozygous knockout mouse tissues confirmed that no GPI-DAF mRNAs were made in the mutant mice (Fig. 2A). In contrast to the total abrogation of GPI-DAF gene expression, TM-DAF gene expression in the knockout mouse testis was unaffected (Fig. 2B). These results indicate that inactivation of the mouse GPI-DAF gene was complete and selective.
Figure 2.
Northern blot analysis of representative tissues confirming the complete and selective inactivation of the GPI-DAF gene. (A) (a) GPI-DAF mRNAs were expressed in the wild-type (+/+) but not the knockout (−/−) mouse intestine (In) or lung (Lu). Two alternatively spliced GPI-DAF mRNAs were detectible; (b) the membrane was stripped and rehybridized with a control cDNA (glyceraldehyde-3-phosphate dehydrogenase; GAPDH) probe to show equivalent RNA loading. (B) (a) GPI-DAF was expressed in wild-type but not in knockout mouse testis; (b) TM-DAF gene was expressed in both the wild-type and the GPI-DAF knockout mouse testis; (c) rehybridization of the membrane with a GAPDH probe. Positions of the 18S and 28S ribosomal RNAs are marked on the left.
The ratio of the three genotypes (wild-type, heterozygous, and homozygous) of the progeny from heterozygous matings showed no significant deviation from the expected 1:2:1 Mendelian distribution (23/117 were wild type, 67/117 were heterozygous, 27/117 were homozygous, 20:57:23). This observation indicates that there was no embryonic lethality associated with GPI-DAF gene inactivation. When housed in a nonspecific pathogen-free facility, GPI-DAF null mice were able to develop, grow, and reproduce, showing no overt abnormal phenotypes.
GPI-DAF-Deficient Erythrocytes Show No Increase in Autologous C3 Deposition in Vivo but Have Increased Bystander Complement Activation in Vitro.
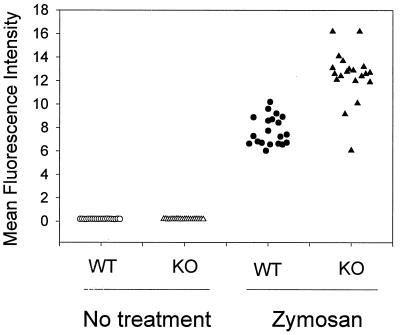
GPI-DAF-deficient mice showed no signs of hemolytic anemia. Total red blood cells and leukocytes were comparable for the wild-type and mutant mice (red blood cells, 9.32 ± 1.06 × 109/ml for wild-type and 9.63 ± 1.16 × 109/ml for mutant, n = 6; white blood cells, 6.15 ± 1.38 × 106/ml for wild-type and 7.71 ± 1.76 × 106/ml for mutant, n = 6). FACS analysis showed no spontaneous C3 deposition on the erythrocyte surface of knockout mice (Fig. 3). To determine whether knockout erythrocytes were more susceptible to C3 fixation from bystander complement activation, cells were coincubated ex vivo in normal mouse serum with zymosan particles, a potent alternative-pathway complement activator (29). Zymosan-initiated complement activation was confirmed by abundant C3 deposition on the particles and exhaustive C3 cleavage in the serum, as determined by FACScan and double immunodiffusion electrophoresis, respectively (data not shown). Fig. 3 shows that bystander C3 fixation was observed on both the wild-type and the knockout erythrocytes but, on average, C3 deposition was greater on the DAF knockout mouse erythrocytes (P < 0.001, Student’s t test).
Figure 3.
GPI-DAF knockout mouse erythrocytes had no spontaneous C3 deposition in vivo but were more susceptible to bystander complement fixation initiated by the alternative pathway complement activator, zymosan (P < 0.001, Student’s t test). Harvested wild-type (WT) or knockout (KO) erythrocytes were either stained directly (no treatment) for C3 or first coincubated in mouse serum with zymosan particles and then stained for C3 deposition. Erythrocytes from 10 animals from each group were analyzed, and values of duplicated experiment were presented.
GPI-DAF-Deficient Erythrocytes Also Have Impaired Regulation of Autologous Complement Activation via the Classical Pathway.
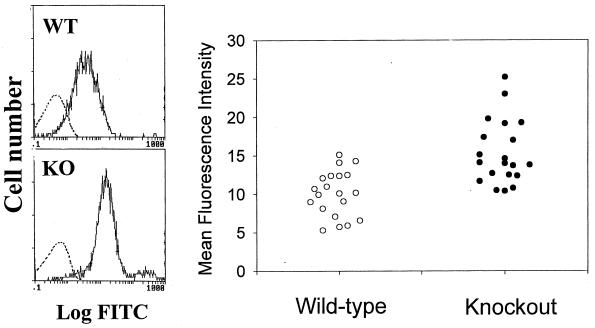
When wild-type and knockout erythrocytes were first sensitized with a rabbit anti-mouse erythrocyte antibody and then incubated with normal mouse serum, markedly increased C3 deposition was detected by FACS analysis onto both the wild-type and the knockout cells (Fig. 4). Again, the level of C3 deposited onto knockout erythrocytes was significantly greater than onto wild-type cells (Fig. 4; P < 0.001, Student’s t test). Despite high amounts of erythrocyte C3 deposition, no lysis of wild-type or GPI-DAF-deficient erythrocytes occurred in this experiment or in the zymosan-catalyzed bystander complement activation experiment (Fig. 3).
Figure 4.
GPI-DAF knockout mouse erythrocytes exhibited on average a higher level of classical pathway-initiated mouse C3 deposition ex vivo. (Left) Representative FACS analysis of C3 deposition on wild- type (WT) and knockout (KO) mouse erythrocytes, either with (solid line) or without (dashed line) antibody sensitization. (Right) Comparison of C3 deposition on erythrocytes from 10 wild-type and 10 knockout mice (duplicate analyses for each animal. P < 0.001, Student’s t test).
GPI-DAF-Deficient Erythrocytes Are More Susceptible than Are Wild-Type Erythrocytes to Heterologous Complement-Mediated Lysis.
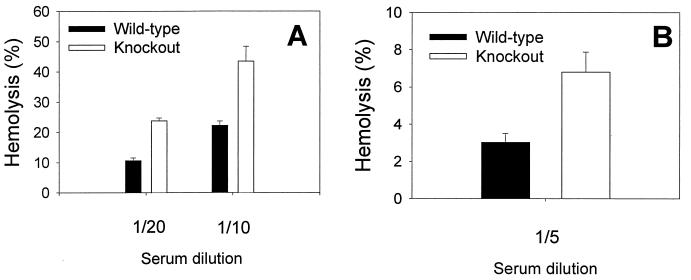
To determine whether the activity of mouse GPI-DAF in regulating complement activation on the erythrocyte surface is species-specific, antibody-sensitized wild-type and GPI-DAF-deficient mouse erythrocytes were incubated with normal human or guinea pig serum, and their degree of hemolysis was determined. A significantly greater percentage of GPI-DAF-deficient erythrocytes than wild-type erythrocytes was lysed by both human and guinea pig complement (Fig. 5). The enhanced susceptibility of GPI-DAF-deficient erythrocytes to heterologous complement lysis was correlated with, and was likely a result of, increased complement activation at the C3 level. When antibody-sensitized wild-type and knockout mouse erythrocytes were incubated with C5-deficient human serum (to prevent lysis) and then analyzed by FACS for C3 deposition, a significantly higher amount of human C3 was deposited onto the knockout erythrocytes than onto the wild-type erythrocytes (mean fluorescence intensity: wild-type, 406 ± 80; knockout, 1250 ± 108; n = 7 mice for each genotype).
Figure 5.
GPI-DAF knockout mouse erythrocytes were more sensitive to heterologous complement lysis. (A) Human complement (Diamedix, Miami; CH50= 205 units/ml, 1:10 or 1:20 dilution; n = 5 for wild-type, n = 4 for knockout mice, P < 0.005, Student’s t test). (B) Guinea pig complement (Sigma; CH50= 147 units/ml, 1:5 dilution; n = 7 for both wild-type and knockout mice, P < 0.05, Student’s t test). Antibody-sensitized mouse erythrocytes were incubated with human or guinea pig serum for 30 min at 37°C, and percent hemolysis was determined by measuring hemoglobin release. Erythrocytes from each mouse were assayed in duplicate.
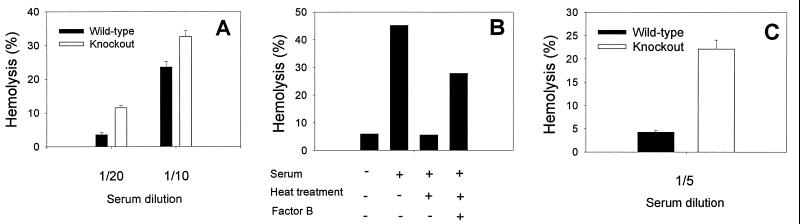
We next investigated the effects of mouse GPI-DAF on both the alternative and the classical pathways of human complement activation. For the alternative pathway assay, mouse erythrocytes were incubated with human serum in GVBS-EGTA/Mg2+ buffer. Knockout erythrocytes again displayed an increased sensitivity to lysis, indicating that mouse GPI-DAF is capable of inhibiting the alternative pathway of human complement (Fig. 6A). To assess the regulatory effect of GPI-DAF on the classical pathway, human serum was heat-treated at 50°C for 20 min to inactivate factor B (27) and therefore the alternative-pathway complement activity. The treated serum was confirmed to possess no alternative-pathway complement activity (Fig. 6B). When antibody-sensitized mouse erythrocytes were incubated with the factor B-depleted human serum, a pronounced difference was observed between the wild-type and GPI-DAF-deficient erythrocytes in their degrees of lysis (Fig. 6C). This result suggests that mouse GPI-DAF can regulate rather effectively the human classical pathway of complement.
Figure 6.
Hemolytic assays of wild-type and knockout mouse erythrocytes with human alternative and classical pathway complement. (A) Alternative-pathway assay (n = 6 for both wild-type and knockout mice, duplicate assays for each animal; P < 0.005, Student’s t test). (B) Human serum was heat-treated to inactivate factor B and thus the alternative pathway of complement. Inactivation of factor B was confirmed by alternative-pathway hemolytic assays (using erythrocytes from a wild-type mouse) with or without factor B reconstitution (to 400 μg/ml). Values shown are average of duplicate assays. (C) Classical-pathway assay using factor B-depleted serum prepared as shown in B (n = 8 for both wild-type and knockout mice, duplicate assays for each animal; P < 0.005, Student’s t test). A significant difference between wild-type and knockout erythrocytes also was observed in a separate experiment by using factor B-depleted serum obtained from a commercial source (Sigma). For the data presented, fresh human serum from a single donor was used, either directly at 1:20 or 1:10 dilution (A) or heat-treated and used at 1:5 dilution (B and C). Similar results were obtained from experiments using sera from three different donors.
DISCUSSION
DAF is a GPI-anchored membrane complement regulator that inhibits the C3 and C5 convertases of both the classical and alternative pathways. Reincorporation studies of PNH erythrocytes and purified human DAF have suggested a role for DAF deficiency in the PNH syndrome (10, 11), yet an absence of hematological disorders in Inab individuals has cast doubt on the relevance of DAF deficiency to complement-mediated hemolysis (12–14). Because the Inab genotype is so rare, relatively few studies on the sensitivity of Inab erythrocytes to complement activation have been published, and the reported results have not always been consistent (15–17, 30). In the present study, we have generated mice deficient in the GPI-anchored DAF by homologous recombination in ES cells and have used these mice to evaluate, in a systematic manner, the role of DAF in protecting erythrocytes from C3 deposition and lysis by homologous and heterologous complement.
The lack of increased mouse C3 fixation onto GPI-DAF knockout erythrocytes in vivo suggests that DAF does not play an indispensable role in regulating spontaneous complement activation on the erythrocyte surface. This result seems to be in agreement with the finding of a lack of spontaneous C3 deposition on human Inab erythrocytes (16). Nevertheless, there are important differences in the expression of complement-regulatory proteins on human and murine erythrocytes (31). It is known that complement receptor 1 (CR1), which has cofactor activity for factor I and decay-accelerating activity for C3 convertase, is expressed on human but not on murine erythrocytes (32). On the other hand, the rodent-specific complement-regulatory protein Crry/p65 is expressed abundantly on mouse erythrocytes (33). Although we detected no compensatory increase in Crry/p65 expression on the DAF knockout mouse erythrocytes (data not shown), the constitutive expression of Crry/p65 may be sufficient to prevent spontaneous complement activation on the erythrocyte surface. The concept that Crry/p65 is more important in controlling spontaneous complement activation is also supported by the finding that Crry/p65 knockout resulted in complement-mediated embryonic lethality (34). Conversely, the increased autologous C3 deposition ex vivo on knockout erythrocytes from bystander and antibody-initiated complement activation (Fig. 3 and 4) suggests that, under these circumstances, the role of GPI-DAF could not be totally compensated for by other complement-regulatory proteins. It is significant that despite high C3 deposition, no homologous hemolysis resulted.
The notion that the activities of membrane complement-regulatory proteins such as DAF are species-restrictive has led to the development of human DAF-transgenic pigs as a strategy for overcoming complement-mediated acute-phase rejection in xenotransplantation (18, 19). Our observation that mouse GPI-DAF can regulate the activation of human and guinea pig complements suggests that, contrary to what is generally believed, DAF may have significant cross-species activity. Indeed, the degree of inhibition of human classical pathway complement activation by mouse GPI-DAF, as revealed by the increased hemolytic response of the knockout erythrocytes (Fig. 6C), was quite remarkable. This finding raises the possibility that porcine DAF may likewise have a significant regulatory effect on human complement. Porcine DAF has not yet been cloned, and its species specificity remains unknown. However, a recent study on the cloning and expression of pig CD59 cDNA revealed no overt species specificity in the activity of porcine CD59 (35).
Our observation that GPI-DAF-deficient mice are fertile indicates that GPI-DAF is not indispensable for reproduction. This finding is of special interest because studies in humans have shown that DAF is expressed on trophoblasts and sperm cells (36–38). DAF has been thought to protect the sperm and the developing fetus from adverse complement-mediated immune reactions (36–38). In a previous study, we found that GPI-DAF was selectively up-regulated in the mouse uterus by the female sex hormone estrogen (23), suggesting a potential role for GPI-DAF in uterine biology. Our current study has shown that DAF is not essential for the female reproductive process. Because the TM-DAF gene is expressed abundantly in the mouse testis and is still intact in the GPI-DAF knockout mice, a role for DAF in testicular biology and/or sperm function cannot yet be ruled out.
In conclusion, our current study has shown that although DAF deficiency can lead to increased erythrocyte C3 deposition during complement activation, DAF deficiency alone is not sufficient to cause homologous hemolysis. On the other hand, increased lysis of GPI-DAF-deficient erythrocytes by heterologous complements suggests that when the assembly of the membrane-attack complex is not properly regulated (a situation that occurs in PNH syndrome as a result of CD59 deficiency and also that presumably occurs in heterologous complement regulation), DAF deficiency and increased C3 deposition could translate into enhanced hemolytic reaction. The greater sensitivity of GPI-DAF-deficient erythrocytes to classical pathway complement-mediated autologous C3 deposition also suggests that, although Inab individuals do not develop spontaneous intravascular hemolytic anemia, they may be more susceptible to complement receptor-mediated extravascular immune hemolytic disease.
Acknowledgments
We thank Dr. Doug Cines for advice on blood cell analysis and Dr. Michael Maldonado for help with FACS analysis. We also thank Dr. Michael Holers for kindly providing the anti-mouse Crry/p65 antibodies.
ABBREVIATIONS
- DAF
decay-accelerating factor
- GPI-DAF
glycosylphosphatidylinositol-linked DAF
- FACS
fluorescence-activated cell sorter
- FITC
fluoroscein isothiocyanate
- GVBS
gelatin veronal buffered saline
- PNH
paroxysmal nocturnal hemoglobinuria
- SCR
short consensus repeat
- TM-DAF
transmembrane decay-accelerating factor
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Volanakis J E, Frank M. The Human Complement System in Health and Disease. New York: Dekker; 1998. [Google Scholar]
- 2.Lambris J D. The Third Component of Complement: Chemistry and Biology. Berlin: Springer; 1990. [PubMed] [Google Scholar]
- 3.Nicholson-Weller A, Wang C E. J Lab Clin Med. 1994;123:485–491. [PubMed] [Google Scholar]
- 4.Nicholson-Weller A, Burge J, Fearon D T, Weller P F, Austen K F. J Immunol. 1982;129:184–189. [PubMed] [Google Scholar]
- 5.Medof M E, Kinoshita T, Nussenzweig V. J Exp Med. 1984;160:1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujita T, Inoue T, Ogawa K, Iida K, Tamura N. J Exp Med. 1987;166:1221–1228. doi: 10.1084/jem.166.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholson-Weller A, March J P, Rosenfeld S I, Austen K F. Proc Natl Acad Sci USA. 1983;80:5066–5071. doi: 10.1073/pnas.80.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pangburn M K, Muller-Eberhard H J. Proc Natl Acad Sci USA. 1983;80:5430–5434. doi: 10.1073/pnas.80.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyata T, Yamada N, Iida Y, Nishimura J, Takeda J, Kitani T, Kinoshita T. N Engl J Med. 1994;330(4):249–255. doi: 10.1056/NEJM199401273300404. [DOI] [PubMed] [Google Scholar]
- 10.Medof M A, Kinoshita T, Silber R, Nussenzweig V. Proc Natl Acad Sci USA. 1985;82:2980–2984. doi: 10.1073/pnas.82.9.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilcox L A, Ezzell J L, Bernshaw N J, Parker C J. Blood. 1991;78:820–829. [PubMed] [Google Scholar]
- 12.Telen M J, Hall S E, Green A M, Moulds J J, Rosse W F. J Exp Med. 1988;167:1993–1998. doi: 10.1084/jem.167.6.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lublin D M, Mallinson G, Poole J, Reid M E, Thompson E S, Ferdman B R, Telen M J, Anstee D J, Tanner M J A. Blood. 1994;84:1276–1282. [PubMed] [Google Scholar]
- 14.Wang L, Uchikawa M, Tsuneyama H, Tokunaga K, Tadokoro K, Juji T. Blood. 1998;91:680–684. [PubMed] [Google Scholar]
- 15.Reid M E, Mallinson G, Sim R B, Poole J, Pausch V, Merry A H, Liew Y W, Tanner M J A. Blood. 1991;78:3291–3297. [PubMed] [Google Scholar]
- 16.Holguin M H, Martin C B, Bernshaw N J, Paeker C J. J Immunol. 1992;148:498–502. [PubMed] [Google Scholar]
- 17.Merry A H, Rawlinson V I, Uchikawa M, Daha M R, Sim R B. Br J Hematol. 1989;73:248–253. doi: 10.1111/j.1365-2141.1989.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 18.Schmoeckel M, Bhatti F N K, Zaidi A, Cozzi A, Pino-Chavez G, Dunning J J, Wallwork J, White D J G. Transplant Proc. 1997;29:3157–3158. doi: 10.1016/s0041-1345(97)00823-3. [DOI] [PubMed] [Google Scholar]
- 19.McCurry K R, Kooyman D L, Alvarado C G, Cotterell A H, Martin M J, Logan J S, Platt J L. Nat Med. 1995;1:423–427. doi: 10.1038/nm0595-423. [DOI] [PubMed] [Google Scholar]
- 20.Kameyoshi Y, Matsushita M, Okada H. Immunology. 1989;68:439–444. [PMC free article] [PubMed] [Google Scholar]
- 21.Moran P, Beasley H, Gorrell A, Martin E, Gribling P, Fuchs H, Gillett N, Burton L E, Cara I W. J Immunol. 1992;149:1736–1743. [PubMed] [Google Scholar]
- 22.Spicer A P, Seldin M F, Gendler S J. J Immunol. 1995;155:3079–3091. [PubMed] [Google Scholar]
- 23.Song W-C, Deng C, Raszmann K, Moore R, Newbold E, McLachlan J A, Negishi M. J Immunol. 1996;157:4166–4172. [PubMed] [Google Scholar]
- 24.Tybulewicz V L J, Crawford C E, Jackson P K, Bronson R T, Mulligan R C. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 25.Wurst W, Joyner A L. In: Gene Targeting- A Practical Approach. Joyner A L, editor. Oxford: IRL; 1993. pp. 33–61. [Google Scholar]
- 26.Ramirez-Solis R, Davis A C, Bradley A. Methods Enzymol. 1993;225:855–878. doi: 10.1016/0076-6879(93)25054-6. [DOI] [PubMed] [Google Scholar]
- 27.Gotze O, Muller-Eberhard H J. J Exp Med. 1971;134:90S–108S. [PubMed] [Google Scholar]
- 28.Coyne K E, Hall S E, Thompson E S, Arce M A, Kinoshita T, Fujita T, Anstee D J, Rosse W, Lublin D M. J Immunol. 1992;149:2906–2913. [PubMed] [Google Scholar]
- 29.Matsumoto M, Fukuda W, Circolo A, Goellner J, Strauss-Schoenberger J, Wang X, Fujita S, Hidvegi T, Chaplin D D, Colten H R. Proc Natl Acad Sci USA. 1997;94:8720–8725. doi: 10.1073/pnas.94.16.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Telen M J, Green A. Blood. 1989;74:437–441. [PubMed] [Google Scholar]
- 31.Holers V M, Kinoshita T, Molina H. Immunol Today. 1992;13:231–236. doi: 10.1016/0167-5699(92)90160-9. [DOI] [PubMed] [Google Scholar]
- 32.Kinoshita T, Takeda J, Hong K, Kozono H, Sakai H, Inoue K. J Immunol. 1988;140:3066–3072. [PubMed] [Google Scholar]
- 33.Li B, Sallee C, Dehoff M, Foley S, Molina H, Holers V M. J Immunol. 1993;151:4295–4305. [PubMed] [Google Scholar]
- 34.Molina H, Mao D, Holers V M, Colten H, Xu C. Mol Immunol. 1998;35:382. (abstr.). [Google Scholar]
- 35.Hinchliffe S J, Rushmere N K, Hanna S M, Morgan B P. J Immunol. 1998;160:3924–3932. [PubMed] [Google Scholar]
- 36.Cervoni F, Oglesby T J, Fenichel P, Dohr G, Rossi B, Atkinson J P, Hsi B L. J Immunol. 1993;151:939–948. [PubMed] [Google Scholar]
- 37.Holmes C H, Simpson K L, Okada H, Okada N, Wainwright S D, Purcell D F J, Houlihan J M. Eur J Immunol. 1992;22:1579–1585. doi: 10.1002/eji.1830220635. [DOI] [PubMed] [Google Scholar]
- 38.Zarkadis I K, Omigbodun A, Forson A, Ziolkiewicz P, Kinoshita T, Lambris J D, Coutifaris C. J Soc Gynecol Invest. 1997;4:47–53. doi: 10.1016/S1071-5576(96)00061-5. [DOI] [PubMed] [Google Scholar]