Abstract
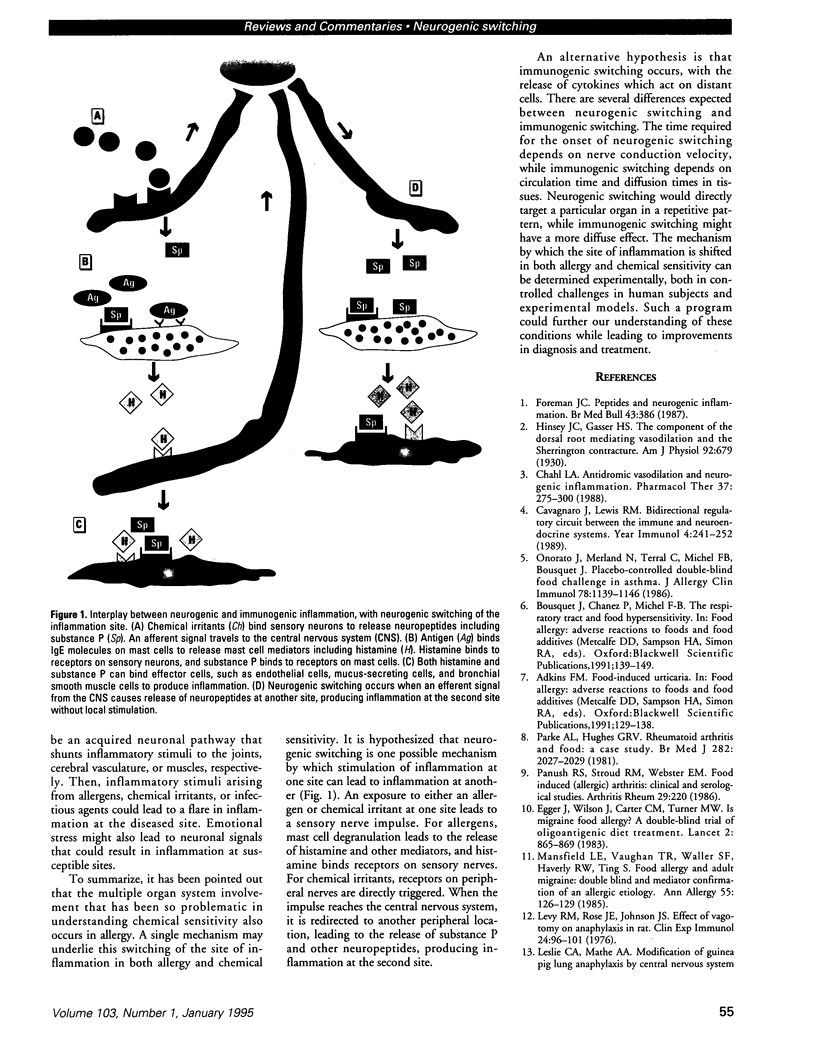
Neurogenic switching is proposed as a hypothesis for a mechanism by which a stimulus at one site can lead to inflammation at a distant site. Neurogenic inflammation occurs when substance P and other neuropeptides released from sensory neurons produce an inflammatory response, whereas immunogenic inflammation results from the binding of antigen to antibody or leukocyte receptors. There is a crossover mechanism between these two forms of inflammation. Neurogenic switching is proposed to result when a sensory impulse from a site of activation is rerouted via the central nervous system to a distant location to produce neurogenic inflammation at the second location. Neurogenic switching is a possible explanation for systemic anaphylaxis, in which inoculation of the skin or gut with antigen produces systemic symptoms involving the respiratory and circulatory systems, and an experimental model of anaphylaxis is consistent with this hypothesis. Food-allergy-iducing asthma, urticaria, arthritis, and fibromyalgia are other possible examples of neurogenic switching. Neurogenic switching provides a mechanism to explain how allergens, infectious agents, irritants, and possibly emotional stress can exacerbate conditions such as migraine, asthma, and arthritis. Because neurogenic inflammation is known to be triggered by chemical exposures, it may play a role in the sick building syndrome and the multiple chemical sensitivity syndrome. Thus neurogenic switching would explain how the respiratory irritants lead to symptoms at other sites in these disorders.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buzzi M. G., Moskowitz M. A. The trigemino-vascular system and migraine. Pathol Biol (Paris) 1992 Apr;40(4):313–317. [PubMed] [Google Scholar]
- Cavagnaro J., Lewis R. M. Bidirectional regulatory circuit between the immune and neuroendocrine systems. Year Immunol. 1989;4:241–252. [PubMed] [Google Scholar]
- Chahl L. A. Antidromic vasodilatation and neurogenic inflammation. Pharmacol Ther. 1988;37(2):275–300. doi: 10.1016/0163-7258(88)90029-0. [DOI] [PubMed] [Google Scholar]
- Cullen M. R. The worker with multiple chemical sensitivities: an overview. Occup Med. 1987 Oct-Dec;2(4):655–661. [PubMed] [Google Scholar]
- De Sarro G. B., Masuda Y., Ascioti C., Audino M. G., Nistico G. Behavioural and ECoG spectrum changes induced by intracerebral infusion of interferons and interleukin 2 in rats are antagonized by naloxone. Neuropharmacology. 1990 Feb;29(2):167–179. doi: 10.1016/0028-3908(90)90057-x. [DOI] [PubMed] [Google Scholar]
- Egger J., Carter C. M., Wilson J., Turner M. W., Soothill J. F. Is migraine food allergy? A double-blind controlled trial of oligoantigenic diet treatment. Lancet. 1983 Oct 15;2(8355):865–869. doi: 10.1016/s0140-6736(83)90866-8. [DOI] [PubMed] [Google Scholar]
- Foreman J. C. Peptides and neurogenic inflammation. Br Med Bull. 1987 Apr;43(2):386–400. doi: 10.1093/oxfordjournals.bmb.a072189. [DOI] [PubMed] [Google Scholar]
- Grönblad M., Konttinen Y. T., Korkala O., Liesi P., Hukkanen M., Polak J. M. Neuropeptides in synovium of patients with rheumatoid arthritis and osteoarthritis. J Rheumatol. 1988 Dec;15(12):1807–1810. [PubMed] [Google Scholar]
- Levy R. M., Rose J. E., Johnson J. S. Effect of vagotomy on anaphylaxis in the rat. Clin Exp Immunol. 1976 Apr;24(1):96–101. [PMC free article] [PubMed] [Google Scholar]
- Mansfield L. E., Vaughan T. R., Waller S. F., Haverly R. W., Ting S. Food allergy and adult migraine: double-blind and mediator confirmation of an allergic etiology. Ann Allergy. 1985 Aug;55(2):126–129. [PubMed] [Google Scholar]
- Meggs W. J. Multiple chemical sensitivities and the immune system. Toxicol Ind Health. 1992 Jul-Aug;8(4):203–214. [PubMed] [Google Scholar]
- Meggs W. J. Neurogenic inflammation and sensitivity to environmental chemicals. Environ Health Perspect. 1993 Aug;101(3):234–238. doi: 10.1289/ehp.93101234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obal F., Jr, Opp M., Cady A. B., Johannsen L., Postlethwaite A. E., Poppleton H. M., Seyer J. M., Krueger J. M. Interleukin 1 alpha and an interleukin 1 beta fragment are somnogenic. Am J Physiol. 1990 Sep;259(3 Pt 2):R439–R446. doi: 10.1152/ajpregu.1990.259.3.R439. [DOI] [PubMed] [Google Scholar]
- Onorato J., Merland N., Terral C., Michel F. B., Bousquet J. Placebo-controlled double-blind food challenge in asthma. J Allergy Clin Immunol. 1986 Dec;78(6):1139–1146. doi: 10.1016/0091-6749(86)90263-0. [DOI] [PubMed] [Google Scholar]
- Panush R. S., Stroud R. M., Webster E. M. Food-induced (allergic) arthritis. Inflammatory arthritis exacerbated by milk. Arthritis Rheum. 1986 Feb;29(2):220–226. doi: 10.1002/art.1780290210. [DOI] [PubMed] [Google Scholar]
- Parke A. L., Hughes G. R. Rheumatoid arthritis and food: a case study. Br Med J (Clin Res Ed) 1981 Jun 20;282(6281):2027–2029. doi: 10.1136/bmj.282.6281.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael G. D., Baraniuk J. N., Kaliner M. A. How and why the nose runs. J Allergy Clin Immunol. 1991 Feb;87(2):457–467. doi: 10.1016/0091-6749(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Silberstein S. D. Advances in understanding the pathophysiology of headache. Neurology. 1992 Mar;42(3 Suppl 2):6–10. [PubMed] [Google Scholar]
- Tabershaw I. R., Cooper W. C. Sequelae of acute organic phosphate poisoning. J Occup Med. 1966 Jan;8(1):5–20. [PubMed] [Google Scholar]