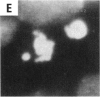
Abstract
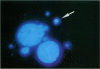
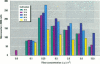
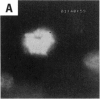
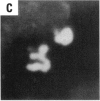
Asbestos and other mineral fibers have long been known to induce lung cancer and mesothelioma. However, the primary mechanisms of fiber-induced carcinogenesis still remain unclear. We investigated the occurrence of mitotic disturbances induced by asbestos (amosite, crocidolite, chrysotile) in an in vitro approach using Syrian hamster embryo (SHE) fibroblast cells. The following endpoints were investigated: micronucleus formation as a result of mitotic disturbances and characterization of the induced micronucleus population by kinetochore staining and visualization of the spindle apparatus. Supravital UV-microscopy was used to analyze changes in interphase chromatin structure, impaired chromatid separation, and blocked cytokinesis. All three asbestos fiber types induced a high frequency of micronucleus formation in SHE cells (> 200/2000 cells) in a dose-dependent manner (0.1-5.0 micrograms/cm2), with a maximum between 48 hr and 66 hr exposure time. At higher concentrations (more than 5.0 micrograms/cm2) the micronucleus formation decreased again as a result of increased toxicity. Kinetochore staining of micronuclei revealed that 48 +/- 2% of asbestos-induced micronuclei reacted positively with CREST (antikinetochore) serum. Furthermore, spindle apparatus deformations occurred in cells with disturbed metaphases and anaphases, while the spindle fiber morphology appeared unchanged. Our results show that asbestos fibers may cause both loss and breakage of chromosomes in the absence of direct interaction with spindle fibers.
Full text
PDF
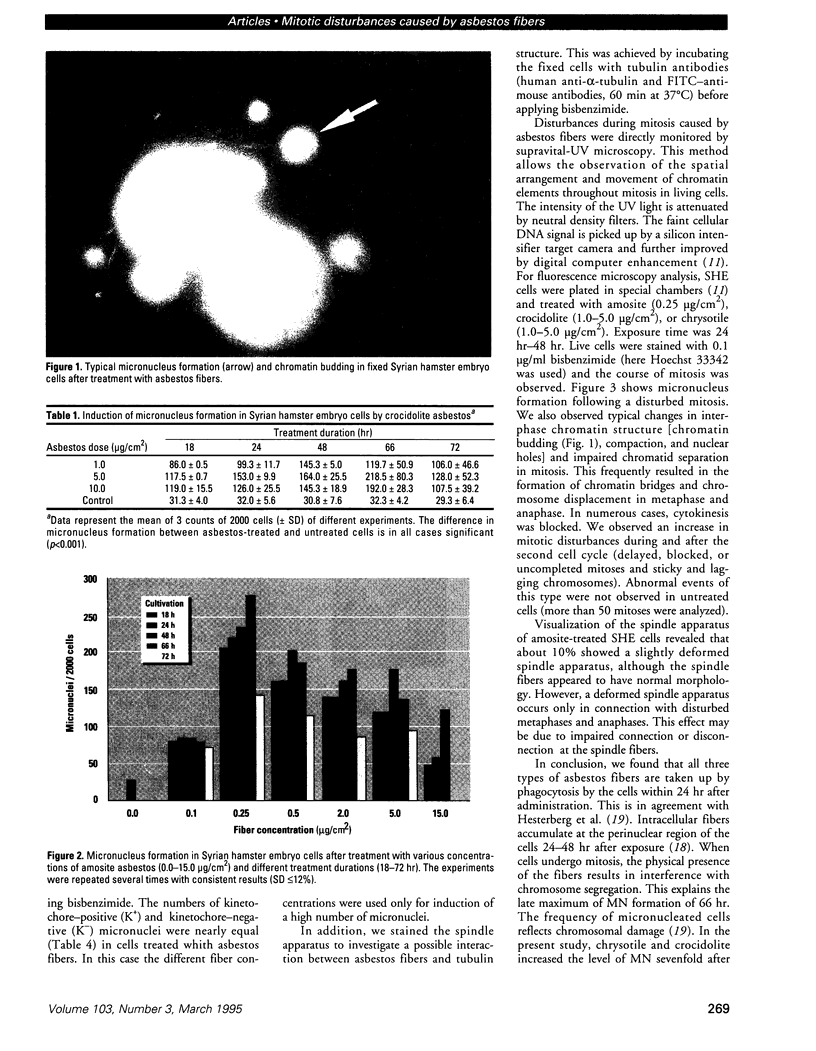
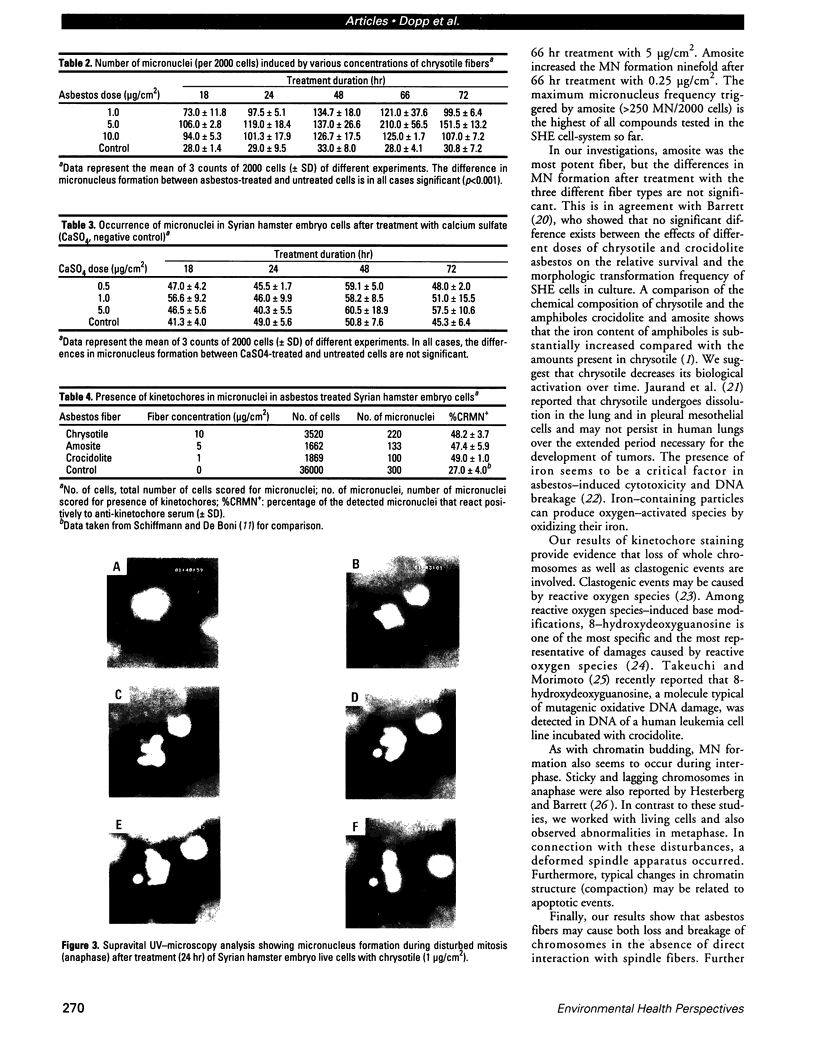

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. C., Lamb P. W., Wiseman R. W. Multiple mechanisms for the carcinogenic effects of asbestos and other mineral fibers. Environ Health Perspect. 1989 May;81:81–89. doi: 10.1289/ehp.898181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. C., Thomassen D. G., Hesterberg T. W. Role of gene and chromosomal mutations in cell transformation. Ann N Y Acad Sci. 1983;407:291–300. doi: 10.1111/j.1749-6632.1983.tb47834.x. [DOI] [PubMed] [Google Scholar]
- Degrassi F., Tanzarella C. Immunofluorescent staining of kinetochores in micronuclei: a new assay for the detection of aneuploidy. Mutat Res. 1988 Oct;203(5):339–345. doi: 10.1016/0165-1161(88)90030-1. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M. Chemical determination of free radical-induced damage to DNA. Free Radic Biol Med. 1991;10(3-4):225–242. doi: 10.1016/0891-5849(91)90080-m. [DOI] [PubMed] [Google Scholar]
- Fritzenschaf H., Kohlpoth M., Rusche B., Schiffmann D. Testing of known carcinogens and noncarcinogens in the Syrian hamster embryo (SHE) micronucleus test in vitro; correlations with in vivo micronucleus formation and cell transformation. Mutat Res. 1993 Sep;319(1):47–53. doi: 10.1016/0165-1218(93)90029-d. [DOI] [PubMed] [Google Scholar]
- Heddle J. A., Hite M., Kirkhart B., Mavournin K., MacGregor J. T., Newell G. W., Salamone M. F. The induction of micronuclei as a measure of genotoxicity. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat Res. 1983 Sep;123(1):61–118. doi: 10.1016/0165-1110(83)90047-7. [DOI] [PubMed] [Google Scholar]
- Hesterberg T. W., Barrett J. C. Dependence of asbestos- and mineral dust-induced transformation of mammalian cells in culture on fiber dimension. Cancer Res. 1984 May;44(5):2170–2180. [PubMed] [Google Scholar]
- Hesterberg T. W., Barrett J. C. Induction by asbestos fibers of anaphase abnormalities: mechanism for aneuploidy induction and possibly carcinogenesis. Carcinogenesis. 1985 Mar;6(3):473–475. doi: 10.1093/carcin/6.3.473. [DOI] [PubMed] [Google Scholar]
- Hesterberg T. W., Butterick C. J., Oshimura M., Brody A. R., Barrett J. C. Role of phagocytosis in Syrian hamster cell transformation and cytogenetic effects induced by asbestos and short and long glass fibers. Cancer Res. 1986 Nov;46(11):5795–5802. [PubMed] [Google Scholar]
- Jaurand M. C., Gaudichet A., Halpern S., Bignon J. In vitro biodegradation of chrysotile fibres by alveolar macrophages and mesothelial cells in culture: comparison with a pH effect. Br J Ind Med. 1984 Aug;41(3):389–395. doi: 10.1136/oem.41.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaurand M. C., Renier A., Gaudichet A., Kheuang L., Magne L., Bignon J. Short-term tests for the evaluation of potential cancer risk of modified asbestos fibers. Ann N Y Acad Sci. 1988;534:741–753. doi: 10.1111/j.1749-6632.1988.tb30163.x. [DOI] [PubMed] [Google Scholar]
- Kamp D. W., Graceffa P., Pryor W. A., Weitzman S. A. The role of free radicals in asbestos-induced diseases. Free Radic Biol Med. 1992;12(4):293–315. doi: 10.1016/0891-5849(92)90117-y. [DOI] [PubMed] [Google Scholar]
- Lechner J. F., Tokiwa T., LaVeck M., Benedict W. F., Banks-Schlegel S., Yeager H., Jr, Banerjee A., Harris C. C. Asbestos-associated chromosomal changes in human mesothelial cells. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3884–3888. doi: 10.1073/pnas.82.11.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund L. G., Aust A. E. Iron mobilization from crocidolite asbestos greatly enhances crocidolite-dependent formation of DNA single-strand breaks in phi X174 RFI DNA. Carcinogenesis. 1992 Apr;13(4):637–642. doi: 10.1093/carcin/13.4.637. [DOI] [PubMed] [Google Scholar]
- Oshimura M., Hesterberg T. W., Tsutsui T., Barrett J. C. Correlation of asbestos-induced cytogenetic effects with cell transformation of Syrian hamster embryo cells in culture. Cancer Res. 1984 Nov;44(11):5017–5022. [PubMed] [Google Scholar]
- Pienta R. J., Poiley J. A., Lebherz W. B., 3rd Morphological transformation of early passage golden Syrian hamster embryo cells derived from cryopreserved primary cultures as a reliable in vitro bioassay for identifying diverse carcinogens. Int J Cancer. 1977 May 15;19(5):642–655. doi: 10.1002/ijc.2910190508. [DOI] [PubMed] [Google Scholar]
- Schiffmann D., De Boni U. Dislocation of chromatin elements in prophase induced by diethylstilbestrol: a novel mechanism by which micronuclei can arise. Mutat Res. 1991 Jan;246(1):113–122. doi: 10.1016/0027-5107(91)90113-3. [DOI] [PubMed] [Google Scholar]
- Schmuck G., Lieb G., Wild D., Schiffmann D., Henschler D. Characterization of an in vitro micronucleus assay with Syrian hamster embryo fibroblasts. Mutat Res. 1988 Dec;203(6):397–404. doi: 10.1016/0165-1161(88)90012-x. [DOI] [PubMed] [Google Scholar]
- Sincock A., Seabright M. Induction of chromosome changes in Chinese hamster cells by exposure to asbestos fibres. Nature. 1975 Sep 4;257(5521):56–58. doi: 10.1038/257056a0. [DOI] [PubMed] [Google Scholar]
- Stanton M. F., Layard M., Tegeris A., Miller E., May M., Morgan E., Smith A. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J Natl Cancer Inst. 1981 Nov;67(5):965–975. [PubMed] [Google Scholar]
- Takeuchi T., Morimoto K. Crocidolite asbestos increased 8-hydroxydeoxyguanosine levels in cellular DNA of a human promyelocytic leukemia cell line, HL60. Carcinogenesis. 1994 Apr;15(4):635–639. doi: 10.1093/carcin/15.4.635. [DOI] [PubMed] [Google Scholar]