Abstract
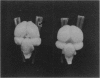
Great blue heron hatchlings from colonies in the Strait of Georgia, British Columbia, Canada are being monitored for environmental contaminant exposure and effects by the Canadian Wildlife Service. The contaminants of concern are polychlorinated dibenzodioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs), primarily derived from kraft pulp mill effluent. The levels of PCDDs and PCDFs in eggs from the most contaminated colonies peaked in 1988 and 1989 and dropped dramatically through 1990 to 1992. Brains of heron hatchlings (taken as eggs from the wild and hatched in the laboratory) were analyzed for gross morphological abnormalities. Brains from highly contaminated colonies (Crofton, British Columbia and University of British Columbia Endowment Lands) in 1988 exhibited a high frequency of intercerebral asymmetry. The frequency of this abnormality decreased in subsequent years as the levels of TCDD and TCDD-TEQs (toxic equivalence factors) decreased. The asymmetry was significantly correlated with the level of TCDD and TCDD-TEQs in eggs taken from the same nest. Yolk-free body weight negatively correlated and the brain somatic index positively correlated with the TCDD level in such pair-matched eggs. These results indicate that gross brain morphology, and specifically intercerebral asymmetry, may be useful as a biomarker for the developmental neurotoxic effects of PCDDs and related chemicals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellward G. D., Norstrom R. J., Whitehead P. E., Elliott J. E., Bandiera S. M., Dworschak C., Chang T., Forbes S., Cadario B., Hart L. E. Comparison of polychlorinated dibenzodioxin levels with hepatic mixed-function oxidase induction in great blue herons. J Toxicol Environ Health. 1990 May;30(1):33–52. doi: 10.1080/15287399009531408. [DOI] [PubMed] [Google Scholar]
- Couture L. A., Abbott B. D., Birnbaum L. S. A critical review of the developmental toxicity and teratogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin: recent advances toward understanding the mechanism. Teratology. 1990 Dec;42(6):619–627. doi: 10.1002/tera.1420420606. [DOI] [PubMed] [Google Scholar]
- Elliott J. E., Butler R. W., Norstrom R. J., Whitehead P. E. Environmental contaminants and reproductive success of great blue herons Ardea herodias in British Columbia, 1986-1987. Environ Pollut. 1989;59(2):91–114. doi: 10.1016/0269-7491(89)90099-7. [DOI] [PubMed] [Google Scholar]
- Flick D. F., Firestone D., Marliac J. P. Studies of the chick edema disease. 2. Preparation and biological effects of a crystalline chick edema factor concentrate. Poult Sci. 1965 Sep;44(5):1214–1222. doi: 10.3382/ps.0441214. [DOI] [PubMed] [Google Scholar]
- Hart L. E., Cheng K. M., Whitehead P. E., Shah R. M., Lewis R. J., Ruschkowski S. R., Blair R. W., Bennett D. C., Bandiera S. M., Norstrom R. J. Dioxin contamination and growth and development in great blue heron embryos. J Toxicol Environ Health. 1991 Mar;32(3):331–344. doi: 10.1080/15287399109531486. [DOI] [PubMed] [Google Scholar]
- Henry E. C., Gasiewicz T. A. Changes in thyroid hormones and thyroxine glucuronidation in hamsters compared with rats following treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1987 Jun 30;89(2):165–174. doi: 10.1016/0041-008x(87)90037-8. [DOI] [PubMed] [Google Scholar]
- Hoffman D. J., Rattner B. A., Bunck C. M., Krynitsky A., Ohlendorf H. M., Lowe R. W. Association between PCBs and lower embryonic weight in black-crowned night herons in San Francisco Bay. J Toxicol Environ Health. 1986;19(3):383–391. doi: 10.1080/15287398609530936. [DOI] [PubMed] [Google Scholar]
- McArthur M. L., Fox G. A., Peakall D. B., Philogène B. J. Ecological significance of behavioral and hormonal abnormalities in breeding ring doves fed an organochlorine chemical mixture. Arch Environ Contam Toxicol. 1983 May;12(3):343–353. doi: 10.1007/BF01059412. [DOI] [PubMed] [Google Scholar]
- Safe S. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs). Crit Rev Toxicol. 1990;21(1):51–88. doi: 10.3109/10408449009089873. [DOI] [PubMed] [Google Scholar]
- Sanderson J. T., Elliott J. E., Norstrom R. J., Whitehead P. E., Hart L. E., Cheng K. M., Bellward G. D. Monitoring biological effects of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls in great blue heron chicks (Ardea herodias) in British Columbia. J Toxicol Environ Health. 1994 Apr;41(4):435–450. doi: 10.1080/15287399409531855. [DOI] [PubMed] [Google Scholar]
- Sanderson J. T., Norstrom R. J., Elliott J. E., Hart L. E., Cheng K. M., Bellward G. D. Biological effects of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls in double-crested cormorant chicks (Phalacrocorax auritus). J Toxicol Environ Health. 1994 Feb;41(2):247–265. doi: 10.1080/15287399409531840. [DOI] [PubMed] [Google Scholar]
- Seegal R. F., Bush B., Shain W. Lightly chlorinated ortho-substituted PCB congeners decrease dopamine in nonhuman primate brain and in tissue culture. Toxicol Appl Pharmacol. 1990 Oct;106(1):136–144. doi: 10.1016/0041-008x(90)90113-9. [DOI] [PubMed] [Google Scholar]