The family of G protein–coupled receptors (GPCRs) responds selectively to ligands ranging from hormones to odorants and from neurotransmitters to photons. Following engagement of the ligand, these serpentine receptors selectively activate heterotrimeric G proteins that in turn transmit signals to distal effector pathways. The role of GPCRs in hypertension and cardiovascular diseases is well established (1). For example, pharmacological antagonists of GPCRs, such as the β-adrenergic and angiotensin receptors, are cornerstones of therapy in the treatment of hypertension and its complications.
GPCR signaling is triggered by ligand-induced conformational changes in intracellular portions of the receptor that promote exchange of guanosine 5′-diphosphate (GDP) for guanosine 5′-triphosphate (GTP) on the Gα subunit of the heterotrimeric G protein. This is followed by dissociation of the GTP-bound Gα from the Gβγ dimer (Figure 1). The dissociated subunits can then interact with effector molecules to propagate the intracellular signal. The duration and intensity of signaling are further regulated by GTPase-activating proteins (GAPs). GAPs accelerate the hydrolysis of Gα-bound GTP, returning the Gα subunit to its inactive form. The regulators of G protein signaling (RGSs) are a family of proteins with GAP activity. To date, more than 20 RGS proteins have been identified. These proteins are characterized by the presence of canonical RGS domains that exhibit G protein–GAP activity (2). Although physiological functions for most of the RGS family members have not been identified, recent studies have assigned roles to some RGS proteins. For example, RGS9-1 controls photosensitization in the eye (3). The RGS protein Sst2 mediates feedback inhibition of mating pheromone responses in yeast (4, 5). In this issue of the JCI, Heximer and associates describe a novel function of another RGS family member, RGS2, in regulation of blood pressure and vascular structure (6).
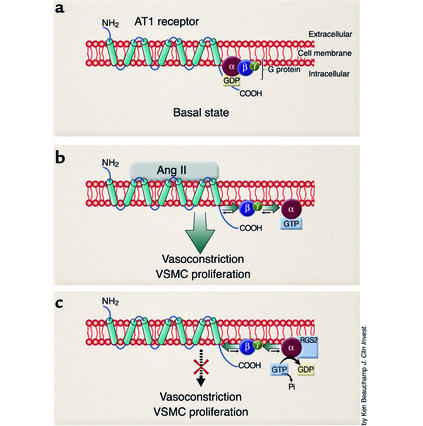
Figure 1.
Regulation of G protein signaling by RGS2. (a) During basal state the AT1 receptor spans the cell membrane and is associated with a heterotrimeric G protein. (b) Binding of hormone (angiotensin II) activates the G protein–coupled AT1 receptor, causing a conformational change and exchange of GDP for GTP on Gαq. The Gαq-GTP and Gβγ subunits dissociate and are free to activate downstream effector proteins, which eventually results in physiological actions such as vasoconstriction and vascular smooth muscle cell (VSMC) hypertrophy. Ang II, angiotensin II. (c) In the presence of RGS proteins, such as RGS2, the Gαq-catalyzed hydrolysis of GTP to GDP is accelerated, terminating the Gα-effector interactions. The Gα-GDP complex has high affinity for the Gβγ subunit, and reassociation of the Gαβγ-GDP unit with the receptor returns the system to the basal state (a).
RGS2 regulates blood pressure
RGS2 was originally identified as an early response gene that was upregulated in activated T cells (7). It was also found in CNS neurons, and its expression in the CNS was enhanced by stimuli associated with neuronal plasticity (8, 9). Functions of RGS2 in the immune and neurological systems were confirmed in RGS2-deficient mice, which were found to have impaired T cell responses, increased anxiety responses, and decreased male aggressiveness (10). Although abnormalities of the cardiovascular system were not reported in the original study of this mouse line, there was circumstantial evidence suggesting that RGS2 might play a role in cardiovascular regulation. First, RGS2 displays regulatory selectivity for the Gαq subclass of G proteins (11). Many important cardiovascular hormones such as angiotensin II, endothelin-1, thromboxane A2, and norepinephrine activate receptors that couple to Gαq. These hormones are potent vasoconstrictors and have been implicated in the pathogenesis of hypertension. Second, regulated expression of RGS2 has been described in tissues that are important for blood pressure regulation including the CNS (8, 9), vascular smooth muscle cells (12), and the kidney (13).
Using the RGS2-deficient mouse line (10), Heximer and associates showed that the absence of RGS2 causes hypertension. The level of blood pressure elevation in the RGS2-deficient animals is quite striking with increases in mean arterial pressure of 25 mm Hg in conscious in-strumented animals and up to 50 mm Hg in animals under anesthesia. The character of hypertension has several interesting features. At least in anesthetized mice, equivalent and substantial levels of blood pressure elevation are seen in both rgs2+/– and rgs2–/– animals, indicating that there may be a threshold level of RGS2 that is required for normal vascular homeostasis in vivo. Moreover, the presence of hypertension in the heterozygotes suggests that naturally occurring mutations that incrementally affect the level of RGS2 protein may have a significant impact on blood pressure regulation. Elevated blood pressure in RGS2-deficient mice, unlike in many other hypertensive mouse models, is not accompanied by a compensatory fall in heart rate, suggesting that the absence of normal RGS2 function may cause generalized disruption of cardiovascular reflexes.
The elevation in blood pressure in rgs2+/– and rgs2–/– animals is accompanied by modest medial expansion of renal arterioles. Yet the authors found no evidence of cardiac hypertrophy in these same animals despite the relatively substantial pressure overload on the heart. This apparent enhanced susceptibility for vascular versus cardiac hypertrophy may indicate a primary role for RGS2 in vascular homeostasis. However, these findings are seemingly at odds with previous studies suggesting that Gq-coupled receptor agonists cause cardiac hypertrophy primarily through their vascular actions (14). Further resolution of this issue will be one of many interesting areas for future exploration.
Does chronic vasoconstriction cause hypertension?
The authors conclude that the hypertensive phenotype in the RGS2 mutants is due to chronic constriction of the peripheral vasculature. In support of this notion are their observations that the rate of decline in blood pressure following vasopressor challenge was attenuated in RGS2-deficient mice. Furthermore, in vascular smooth muscle cells stimulated with agonist, peak intracellular calcium responses were higher, and the rate of decline of intracellular calcium was slower in RGS2 mutants than in wild-type controls. However, in apparent contrast to these in vitro data, pressor responses to vasoconstrictor agonists such as angiotensin II and phenylephrine were dramatically blunted in RGS2-deficient mice, indicating additional complexity of the system in vivo.
Against the notion that chronic vasoconstriction alone is sufficient to cause hypertension is extensive previous work by Guyton and others indicating that abnormal sodium handling by the kidney is required to maintain chronic elevation of arterial pressure irrespective of the nature of the initial stimulus for high blood pressure (15). This view is based on the idea that the sodium excretory capacity of the kidney provides a compensatory system with virtually infinite gain for countermanding elevations in blood pressure (16). This paradigm has been most convincingly established in models of hypertension that depend on enhanced activity of angiotensin II (17), and high blood pressure associated with RGS2-deficiency seems to fall into this category. Furthermore, virtually all of the genetic variants that have been linked to hypertension in humans are associated with alterations in renal sodium handling (18). As RGS2 is highly expressed in the kidney and is therefore positioned to affect Gq-dependent functions along the nephron, another area for future investigation will be defining precisely the role of RGS2 in the regulation of water and sodium homeostasis. We would suggest that the absence of these homeostatic actions of RGS2 is likely to contribute significantly to hypertension in RGS2-deficiency.
RGS2 and angiotensin II signaling
There is a particularly intriguing relationship between RGS2 and the angiotensin II type 1 (AT1) receptor, a GPCR that is part of a key pathway leading to progressive organ damage in patients with heart and kidney diseases. Hypertension in the RGS2-deficient animals, at least when they are under anesthesia, depends to a significant extent on angiotensin II signaling via the AT1 receptor. Moreover, previous studies in vascular smooth muscle cells have shown that angiotensin II stimulates expression of mRNA for RGS2 but not for other RGS proteins (12). These specific interactions between angiotensin II and RGS2 may have important implications for promoting or amplifying the contribution of angiotensin II to elevation of blood pressure and its associated tissue injury.
In summary, the studies of Heximer and associates clearly show that RGS2 is an important regulatory element acting to dampen signaling by Gαq-coupled receptors in vivo. Because these receptors are critical to the pathogenesis of hypertension and target organ damage, understanding the physiological actions of how RGS2 and other GAPs can “turn off” maladaptive signals should lead to new insights into genetic causes of hypertension and may suggest novel downstream targets for therapy.
Acknowledgments
The authors acknowledge support from the NIH and the Research Service of the Department of Veterans Affairs. They thank Howard Rockman and Robert Spurney for their comments and suggestions.
Footnotes
See the related article beginning on page 445.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: G protein–coupled receptor (GPCR); guanosine 5′-diphosphate (GDP); guanosine 5′-triphosphate (GTP); GTPase activating protein (GAP); regulator of G-protein signaling (RGS); angiotensin II type 1 (AT1).
References
- 1.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 2.Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- 3.Chen CK, et al. Slowed recovery of rod photoresponse in mice lacking the GTPase accelerating protein RGS9-1. Nature. 2000;403:557–560. doi: 10.1038/35000601. [DOI] [PubMed] [Google Scholar]
- 4.Chan RK, Otte CA. Physiological characterization of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol. Cell. Biol. 1982;2:21–29. doi: 10.1128/mcb.2.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dohlman HG, Song J, Ma D, Courchesne WE, Thorner J. Sst2, a negative regulator of pheromone signaling in the yeast Saccharomyces cerevisiae: expression, localization, and genetic interaction and physical association with Gpa1 (the G-protein αsubunit) Mol. Cell. Biol. 1996;16:5194–5209. doi: 10.1128/mcb.16.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heximer SP, et al. Hypertension and prolonged vasoconstrictor signaling in RGS2-deficient mice. J. Clin. Invest. 2003;111:445–452. doi:10.1172/JCI200315598. doi: 10.1172/JCI15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heximer SP, Cristillo AD, Forsdyke DR. Comparison of mRNA expression of two regulators of G-protein signaling, RGS1/BL34/1R20 and RGS2/G0S8, in cultured human blood mononuclear cells. DNA Cell Biol. 1997;16:589–598. doi: 10.1089/dna.1997.16.589. [DOI] [PubMed] [Google Scholar]
- 8.Ingi T, et al. Dynamic regulation of RGS2 suggests a novel mechanism in G-protein signaling and neuronal plasticity. J. Neurosci. 1998;18:7178–7188. doi: 10.1523/JNEUROSCI.18-18-07178.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burchett SA, Volk ML, Bannon MJ, Granneman JG. Regulators of G protein signaling: rapid changes in mRNA abundance in response to amphetamine. J. Neurochem. 1998;70:2216–2219. doi: 10.1046/j.1471-4159.1998.70052216.x. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira-Dos-Santos AJ, et al. Regulation of T cell activation, anxiety, and male aggression by RGS2. Proc. Natl. Acad. Sci. USA. 2000;97:12272–12277. doi: 10.1073/pnas.220414397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heximer SP, Watson N, Linder ME, Blumer KJ, Hepler JR. RGS2/G0S8 is a selective inhibitor of Gqalpha function. Proc. Natl. Acad. Sci. USA. 1997;94:14389–14393. doi: 10.1073/pnas.94.26.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant SL, et al. Specific regulation of RGS2 messenger RNA by angiotensin II in cultured vascular smooth muscle cells. Mol. Pharmacol. 2000;57:460–467. doi: 10.1124/mol.57.3.460. [DOI] [PubMed] [Google Scholar]
- 13.Chen C, Zheng B, Han J, Lin SC. Characterization of a novel mammalian RGS protein that binds to Galpha proteins and inhibits pheromone signaling in yeast. J. Biol. Chem. 1997;272:8679–8685. doi: 10.1074/jbc.272.13.8679. [DOI] [PubMed] [Google Scholar]
- 14.Keys JR, Greene EA, Koch WJ, Eckhart AD. Gq-coupled receptor agonists mediate cardiac hypertrophy via the vasculature. Hypertension. 2002;40:660–666. doi: 10.1161/01.hyp.0000035397.73223.ce. [DOI] [PubMed] [Google Scholar]
- 15.Guyton AC, et al. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am. J. Med. 1972;52:584–594. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 16.Cowley AW, Jr, Roman RJ. The role of the kidney in hypertension. JAMA. 1996;275:1581–1589. [PubMed] [Google Scholar]
- 17.Hall JE. Control of sodium excretion by angiotensin II: intrarenal mechanisms and blood pressure regulation. Am. J. Physiol. 1986;250:R960–R972. doi: 10.1152/ajpregu.1986.250.6.R960. [DOI] [PubMed] [Google Scholar]
- 18.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]