Abstract
The LexA protein of Escherichia coli represses the damage-inducible SOS regulon, which includes genes for repair of DNA. Surprisingly, lexA null mutations in Salmonella enterica are lethal even with a sulA mutation, which corrects lexA lethality in E. coli. Nine suppressors of lethality isolated in a sulA mutant of S. enterica had lost the Fels-2 prophage, and seven of these (which grew better) had also lost the Gifsy-1 and Gifsy-2 prophages. All three phage genomes included a homologue of the tum gene of coliphage 186, which encodes a LexA-repressed cI antirepressor. The tum homologue of Fels-2 was responsible for lexA lethality and had a LexA-repressed promoter. This basis of lexA lethality was unexpected because the four prophages of S. enterica LT2 are not strongly UV inducible and do not sensitize strains to UV killing. In S. enterica, lexA(Ind−) mutants have the same phenotypes as their E. coli counterparts. Although lexA null mutants express their error-prone DinB polymerase constitutively, they are not mutators in either S. enterica or E. coli.
The LexA-repressed SOS regulon includes genes that encode DNA repair enzymes (19). This regulon is induced when DNA damage produces single-stranded DNA, which interacts with RecA protein to form a complex that stimulates autocleavage of the LexA repressor protein (55). A mechanistically similar system regulates lysogeny of phage lambda (in Escherichia coli) and P22 (in Salmonella enterica) without direct involvement of LexA. For these phages, single-stranded DNA bound to RecA protein causes autocleavage of the major phage repressor protein instead of LexA (41, 42). In contrast, lysogens of coliphage 186 can be maintained only in the presence of LexA protein, which represses the phage tum (antirepressor) gene (27, 44). Phage 186 prophages are induced when cleavage of LexA allows tum expression and thereby upsets prophage repression.
The SOS system of S. enterica seems similar to that of E. coli (4). In both bacteria, lexA is the first gene in a two-gene operon (with dinF) that is repressed by LexA protein. In S. enterica, a series of genes (din) have been identified that show RecA-dependent induction by mitomycin C and are repressed by overproduction of the LexA protein (48). In E. coli, a lexA null mutation causes lethal cell filamentation, which can be prevented by a sulA mutation.
Evidence is presented that lexA null mutations in S. enterica are lethal even in the presence of a sulA mutation and that this lethal phenotype is due to induction of genes within resident prophages. Surprisingly, these prophages are not strongly UV inducible, and strains of S. enterica carrying all three LexA-regulated phages are not more sensitive to UV killing than is E. coli or isogenic Salmonella strains lacking the prophages. Induction of LexA-regulated genes of the Fels-2 and Gifsy prophages may inhibit cell growth without causing full prophage induction. Loss of prophages may be the most frequent event that provides resistance to this inhibition. Noninducible lexA(Ind−) mutants of S. enterica behave like their E. coli counterparts. Although lexA null mutations increase expression of the error-prone DinB polymerase in Salmonella spp., as in E. coli (53, 54), they do not cause a mutator phenotype. Thus, SOS induction of DinB appears to be necessary but not sufficient for mutagenesis (as is known for the error-prone UmuCD polymerase).
MATERIALS AND METHODS
Strains.
Except where noted, all strains used (Table 1) were derived from S. enterica serovar Typhimurium strain LT2. Key mutations are diagrammed in Fig. 1. The insertion mutations lexA40::Kan (Fig. 1B) and sulA46::Spc were constructed and provided by Montserrat Llagostera and Xavier Garriga (15). The reported structures were confirmed by sequencing. This lexA insertion proved to be lethal in our strains, so the received strains must carry a suppressor of lexA lethality; their genetic background is referred to as SLT2 (Spanish LT2).
TABLE 1.
Strain list
| Strain | Background if other than TR10000 | Genotypea | Source or reference |
|---|---|---|---|
| TR10000 | Wild type | S. enterica serovar Typhimurium strain LT2 | Laboratory collection |
| TR17000 | =DB7000 | Wild type (LT2 lacking Fels-2) | David Botstein |
| TR7178 | E. coli K-12 | ara thiA Rifr Δ13 lac proB/F′128 lacIqlacI33(fs) lacIZ(Ω fusion) pro′ | Cairns (14) |
| TR7235 | /pJWL21 lexA+ from E. coli | This study | |
| TR7236 | /pJWL26 lexA3(Ind−) from E. coli | This study | |
| TR7237 | E. coli | /pJWL21 lexA+ from E. coli | John Little |
| TR7238 | E. coli | /pJWL26 lexA3(Ind−) from E. coli | John Little |
| TT8371 | thr-469::MudA (blue) | Laboratory collection | |
| TT9048 | recA1 | Laboratory collection | |
| TT10268 | zai-808::Tn10 proC150 | C. Miller | |
| TT10725 | metE205 ara-9 metH2357::MudA (blue) | Laboratory collection | |
| TT10910 | DB9071 | hisO1242 Δ(Fels-2 Gifsy-1 Gifsy-2) | David Botstein |
| TT16803 | hisC1264::MudJ argR::Tn10dTet srl::Tn10dCam ΔhisG203 | Laboratory collection | |
| TT17200 | sulA zaj-9222::Tn10 din-9::MudJ | C. Mark Smith | |
| TT17202 | sulA zaj-9222::Tn10 din-80::MudJ | C. Mark Smith | |
| TT17207 | leuD21 din-84::MudJ | C. Mark Smith | |
| TT17212 | leuD21 din-10::MudJ | C. Mark Smith | |
| TT17213 | leuD21 din-11::MudJ | C. Mark Smith | |
| TT17214 | leuD21 din-14::MudJ | C. Mark Smith | |
| TT17215 | leuD21 din-220::MudJ | C. Mark Smith | |
| TT17216 | leuD21 din-240::MudJ | C. Mark Smith | |
| TT17217 | leuD21 din-243::MudJ | C. Mark Smith | |
| TT17218 | leuD21 din-292::MudJ | C. Mark Smith | |
| TT17499 | hisG618 cysA1585::MudA | Laboratory collection | |
| TT17652 | SLT2 | RifrsulA46::Spc (was UA1615) | Montserrat Llagostera and Xavier Garriga |
| TT17653 | SLT2 | RifrsulA46::Spc lexA40::Kan (was UA1685) | Montserrat Llagostera and Xavier Garriga |
| TT18829 | recN557::MudJ | David Thaler | |
| TT20492 | ATCC 14028 | S. enterica serovar Typhimurium ΔGifsy-1 | N. Bossi and N. Figueroa |
| TT20493 | ATCC 14028 | Δ(Gifsy-1 Gifsy-2) | N. Bossi and N. Figueroa |
| TT21685 | malB661::Tn10dTet | Laboratory collection | |
| TT22236 | /pTP223 | Tony Poteete | |
| TT22613 | SLT2 | sulA46::Spc lexA41::Cam(sw) | This study |
| TT22614 | SLT2 | sulA46::Spc lexA41::Cam(sw)/pTP223 | This study |
| TT22885 | /pCP20 lambdaC1857 FLP (Ampr Camr) | Laboratory collection | |
| TT22888 | DUP1995[(metH235) ∗MudA∗ (thr-469)] | This study | |
| TT22964 | lexA33::[Cam lexA3(Ind−)](sw) | This study | |
| TT23151 | zgc-9189::Cam recA281(Oc) recA+ | This study | |
| TT23199 | SLT2 | sulA46::Spc lexA42::(FRT-Cam-FRT) (sw) | This study |
| TT23200 | SLT2 | sulA46::Spc lexA42::FRT(sw) malB661::Tn10dTet | This study |
| TT23203 | sulA46::Spc DUP1995[(metH235) ∗MudA∗ (thr-469)] | This study | |
| TT23204 | SLT2 | sulA46::Spc malB661::Tn10dTet | This study |
| TT23205 | sulA46::Spc | This study | |
| TT23206 | zgc-9189::Cam recA281(Oc) recA+/pJWL21 plasmid (lexA+) | This study | |
| TT23207 | zgc-9189::Cam recA281(Oc) recA+/pJWL26 plasmid [lexA3(Ind−)] | This study | |
| TT23252 | lexA33::[Cam lexA3(Ind−)](sw) | This study | |
| TT23255 | sulA46::Spc lexA41::Cam(sw) Slx1 Δ(Gifsy-1 Gifsy-2 Fels-2) | This study | |
| TT23256 | sulA46::Spc lexA41::Cam(sw) Slx2 Δ(Gifsy-1 Gifsy-2 Fels-2) | This study | |
| TT23257 | sulA46::Spc lexA41::Cam(sw) Slx3 Δ(Gifsy-1 Gifsy-2 Fels-2) | This study | |
| TT23259 | sulA46::Spc lexA41::Cam(sw) Slx5 Δ(Gifsy-1 Gifsy-2 Fels-2) | This study | |
| TT23260 | sulA46::Spc lexA41::Cam(sw) Slx6 ΔFels-2 | This study | |
| TT23261 | sulA46::Spc lexA41::Cam(sw) Slx7 Δ(Gifsy-1 Gifsy-2 Fels-2) | This study | |
| TT23262 | sulA46::Spc lexA41::Cam(sw) Slx8 Δ(Gifsy-1 Gifsy-2 Fels-2) | This study | |
| TT23263 | sulA46::Spc lexA41::Cam(sw) Slx9 ΔFels-2 | This study | |
| TT23264 | sulA46::Spc lexA41::Cam(sw) Slx10 Δ(Gifsy-1 Gifsy-2 Fels-2) | This study | |
| TT23276 | sulA46::Spc lexA41::Cam(sw) recN557::MudJ Slx2 Δ(Gifsy-1 Gifsy-2 Fels-2) | This study | |
| TT23280 | sulA46::Spc lexA41::Cam(sw) recN557::MudJ Slx6 ΔFels-2 | This study | |
| TT23283 | sulA46::Spc lexA41::Cam(sw) recN557::MudJ Slx9 ΔFels-2 | This study | |
| TT23296 | DB9071 | sulA46::Spc hisO1242 ΔFels-2 | This study |
| TT23298 | ATCC 14028 | sulA46::Spc ΔGifsy-1 | This study |
| TT23299 | ATCC 14028 | sulA46::Spc Δ(Gifsy-1 Gifsy-2) | This study |
| TT23309 | sulA46::Spc malB661::Tn10dTet lexA+ Slx8 Δ(Gifsy-1 Gifsy-2 Fels-2) | This study | |
| TT23315 | recN557::MudJ | This study | |
| TT23317 | sulA46::Spc recN557::MudJ | This study | |
| TT23320 | sulA46::Spc malB661::Tn10dTet recN557::MudJ Slx2 Δ(Gifsy-1 Gifsy-2 Fels-2) | This study | |
| TT23324 | sulA46::Spc malB661::Tn10dTet recN557::MudJ Slx6 ΔFels-2 | This study | |
| TT23327 | sulA46::Spc malB661::Tn10dTet recN557::MudJ Slx9 ΔFels-2 | This study | |
| TT23351 | recN557::MudJ zgc-9189::Cam recA281(Oc) recA+ | This study | |
| TT23353 | sulA46::Spc recN557::MudJ zgc-9189::Cam recA281(Oc) recA+ | This study | |
| TT23355 | recN557::MudJ/pJWL21 lexA+ | This study | |
| TT23357 | sulA46::Spc recN557::MudJ/pJWL21 lexA+ | This study | |
| TT23361 | sulA46::Spc recN557::MudJ/pJWL26 lexA3(Ind−) | This study | |
| TT23379 | DB7000 | sulA46::Spc ΔFels-2 | This study |
| TT23381 | recN557::MudJ lexA33::[Cam lexA3(Ind−)](sw) | This study | |
| TT23383 | sulA46::Spc recN557::MudJ lexA33::[Cam lexA3(Ind−)](sw) | This study | |
| TT23388 | sulA46::Spc recN557::MudJ zgc-9189::Cam recA281(Oc) recA+/pJWL21 lexA+ | This study | |
| TT23392 | recN557::MudJ zgc-9189::Cam recA281(Oc) recA+/pJWL21 lexA+ | This study | |
| TT23393 | recN557::MudJ zgc-9189::Cam recA281(Oc) recA+/pJWL26 lexA3(Ind−) | This study | |
| TT23465 | recN557::MudJ recA462::T-POP | This study | |
| TT23489 | recN557::MudJ/pJWL26 lexA3(Ind−) | This study | |
| TT23491 | sulA46::Spc recN557::MudJ zgc-9198::Cam recA281(Oc) recA+/pJWL26lexA3(Ind−) | This study | |
| TT23515 | sulA46::Spc/pBADhisCdinF | This study | |
| TT23522 | Wild type | /pJWL26Cam derivative of pJWL26 lexA3(Ind−) (Camr cassette inserted 97 bp upstream of lexA start codon) | This study |
| TT23563 | gin-48(Fels-2)::Kan(sw) (was MA7273) | N. Bossi and N. Figueroa | |
| TT23569 | sulA46::Spc/pTP223 | This study | |
| TT23570 | SLT2 | sulA46::Spc/pTP223 | This study |
| TT23571 | SLT2 | sulA46::Spc lexA40::Kan/pTP223 | This study |
| TT23647 | trpB9hisC9955::MudJ::TPOP/pKD46 | This study | |
| TT23649 | tumFels-2::Cam(sw) | This study | |
| TT23650 | sulA46::Spc tumFels-2::Cam(sw) | This study | |
| TT23656 | Wild type | Made by making TT23205 sulA+ | This study |
| TT23657 | Δ(Fels-2 Gifsy-1 Gifsy-2) | This study | |
| TT23764 | proB1657::Tn10 sulA46::Spc Δ(Fels-2 Gifsy-1 Gifsy-2)/F′128 (Ptum::lacZ+) | This study | |
| TT23765 | proB1657::Tn10 sulA46::Spc Δ(Fels-2 Gifsy-1 Gifsy-2) lexA41::Cam(sw)/F′128 (Ptum::lacZ+) | This study | |
| TT23770 | metA22 metE551 trpD2 ilv-452 leu pro (leaky) hsdLT6 hsdSA29 hsdB strA120/pKD46/F′128 mhpC31::Tn10d-Tet | This study |
(sw) indicates a swap mutation for which the target gene has been deleted and replaced with a resistance gene.
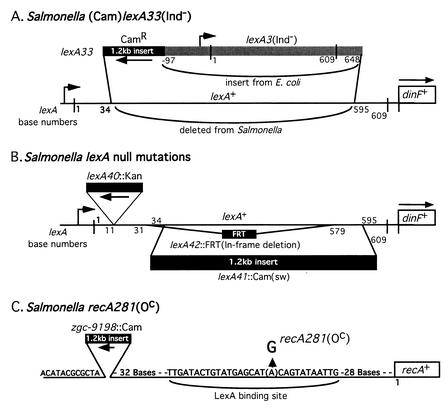
FIG. 1.
Structure of constructed lexA and recA mutations. (A) For the lexA33(Ind−) allele, bases 35 to 594 of the Salmonella lexA gene were replaced with a Camr gene and the complete lexA3(Ind−) allele of E. coli. (B) The lexA null mutation lexA40::Kan is an insertion of a kanamycin resistance gene after base 11 of lexA and was constructed by Montserrat Llagostera and Xavier Garriga (15). The lexA41::Cam(sw) allele is a replacement of lexA bases 35 to 594 with the above Camr gene. The lexA42::FRT mutation is an in-frame deletion that removes bases 32 to 578. (C) In the recA281(Oc) mutation, a G residue replaces A in the lexA binding site upstream of recA. The Camr gene was inserted upstream to allow selective transduction of this mutation into new strains. Construction methods are described in Materials and Methods.
Plasmids pJWL26 and pJWL21, carrying the lexA3(Ind−) and lexA+ alleles of E. coli, were provided by John Little (28, 33). A strain cured for Gifsy-1 (TT20492; was MA3408) and another lacking both Gifsy-1 and -2 (TT20493; was MA4587) were obtained from Lionello Bossi and Nara Figueroa, as were insertion mutations in the prophages of Fels-1 and Fels-2. Two strains lacking Fels-2 TR17000 (= DB7000) and TT10910 (= DB9071) were obtained from David Botstein; the second of these was found to also lack the Gifsy-1 and -2 prophages (see Results). The element Tn10dT-POP is a transposition-defective derivative of Tn10 that directs tetracycline-inducible transcription out of both ends (39). The recN::MudJ insertion was isolated and characterized by David Thaler (40). It is inserted at nucleotide 95 of the recN gene (Julianne Grose, personal communication).
Media and chemicals.
The minimal medium was NCE (6) containing glucose or lactose (0.2%) plus nutrient supplements at the concentrations recommended previously (17). Liquid rich medium was Luria-Bertani broth (LB), and the solid rich medium was nutrient broth (NB; Difco Laboratories) supplemented with 5 g of NaCl per liter and solidified with 1.5% BBL agar. Final concentrations of antibiotics in rich medium were: kanamycin sulfate (Kan), 50 μg/ml; tetracycline (Tet), 20 μg/ml; chloramphenicol (Cam), 25 μg/ml; rifampin (Rif; supplied by ICN), 80 μg/ml; spectinomycin (Spc), 150 μg/ml; and zeocin (Zeo; supplied by Invitrogen), 50 μg/ml. The chromogenic β-galactosidase substrate X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; Diagnostic Chemicals) was used at 25 μg/ml in NB for the colony sectoring tests. Restriction enzymes were from New England Biolabs. Unless otherwise specified, chemicals were obtained from Sigma Chemical Company.
Transduction and transformation methods.
Transduction crosses were mediated by the high-frequency generalized transducing mutant of phage P22 HT105-1 (43). Transductant clones were purified and freed of phage on green indicator plates (10). Phage sensitivity of cells was tested by cross-streaking with the P22 clear-plaque mutant H5 on green indicator plates. For transduction tests of a bacterial strain's ability to inherit a particular donor marker, the efficiency of the transducing lysate was demonstrated by its ability to correct the hisG mutation in strain TT17499 (hisG618 cysA1585::MudA). All lysates used produced greater than 1,000 His+ transductants per minimal cysteine plate under the conditions of our crosses.
For most of the linear transformation experiments (16, 61), the recipient strain carried plasmid pTP223, which includes genes for tetracycline resistance (Tetr) and expresses the recombination functions of phage lambda (red, gam, and exo) from a lac promoter. This plasmid was obtained from Tony Poteete (34, 35). To delete the tum gene of Fels-2 by linear transformation, the recipient strain (TT23647) carried plasmid pKD46, obtained from Barry Wanner (16). Plasmid pKD46 was also carried in the recipient strain used to create the Fels-2 tum regulatory fusion to lacZ (TT23770). Both methods were adapted for use in S. enterica by Eric Kofoid (personal communication).
Replacement of S. enterica lexA+ gene with E. coli lexA3(Ind−) allele.
The chloramphenicol resistance determinant (Camr) from plasmid pACYC184 was amplified by PCR and introduced by linear transformation just upstream of the E. coli lexA3(Ind−) gene in plasmid pJWL26. The resulting plasmid (pJWL26Cam; strain TT23522) was used as the template for PCR amplification of a fragment carrying both Camr and the E. coli lexA3 gene. The primers for this PCR had sequence at their 5′ ends that was homologous to the ends of the S. enterica lexA gene. The PCR product was introduced into the Salmonella chromosome by linear transformation. Transformants acquired Camr and the mutant lexA3 allele from E. coli in place of the S. enterica lexA+ gene. The structure of the final mutation, lexA33(Ind−) (Fig. 1A), was verified by sequencing.
Construction of an S. enterica lexA swap mutation.
The Camr gene from pACYC184 was PCR amplified with primers whose 5′ ends included sequence homologous to the ends of the S. enterica lexA gene. This product was introduced by linear transformation into an SLT2 strain (TT23570) which has sulA46::Spc in its chromosome and harbors plasmid pTP223 (encoding recombination functions of phage lambda). This genetic background (SLT2) carried an unknown suppressor of lexA lethality (later shown to be the lack of prophages). Transformants inherited Camr in place of the chromosomal lexA+ coding sequence; one of these, lexA41::Cam(sw), was used in subsequent experiments (Fig. 1B).
Construction of lexA in-frame deletion.
The Camr gene from pACYC184 was amplified by PCR. The 5′ ends of both primers contained FLP recognition target (FRT) sites and sequences that were homologous to the ends of the S. enterica lexA gene. The PCR product was introduced into the chromosome of an S. enterica (SLT2) strain by linear transformation, selecting Camr; transformants acquired a Camr determinant flanked by FRT sites in place of the lexA+ gene—an allele designated lexA42::(FRT-Cam-FRT; strain TT23199). A deletion between FRT sites generates an in-frame lexA deletion allele that encodes a 31-amino-acid product containing the first 10 and the last 9 amino acids of the LexA protein and 12 intervening amino acids encoded by the single remaining FRT sequence.
After moving the lexA42::(FRT-Cam-FRT) allele into a strain carrying insertion malB661::Tn10dTet (25 to 30% cotransducible with lexA), the Camr determinant was removed by Flp recombinase (16). Recombinase was provided by plasmid pCP20 (obtained from Barry Wanner), which is temperature sensitive for replication and encodes a heat-inducible Flp recombinase (12). At 30°C, this plasmid replicates without expressing Flp recombinase; at 42°C, the plasmid expresses the Flp recombinase and ceases replication. Strains carrying this plasmid were streaked on NB and incubated at 42°C overnight. The streaks were replica plated to NB-ampicillin (to score the presence of the plasmid), to NB-chloramphenicol (to score the presence of the Camr gene within lexA), and to NB. Strains were recovered with the FRT deletion and without the plasmid. The in-frame deletion (lexA42::FRT; strain TT23200) was verified by sequencing a PCR-amplified lexA fragment and is diagrammed in Fig. 1B.
The lethal phenotype of the in-frame deletion was demonstrated by a transduction cross with a donor carrying the insertion malB661::Tn10dTet closely linked to lexA42::FRT. The recipient carried a lexA33(Ind−) allele with its associated Camr marker and sulA but no suppressor of lexA lethality. None of the Tetr transductants lost Camr; that is, it was impossible to replace the recipient lexA(Ind−)-Camr allele with the donor lexA in-frame deletion. Without the lexA deletion in the donor, 30% of the transductants from this cross are Cams. This method was also used to show that the lexA in-frame deletion is lethal in strains carrying a Fels-2 prophage.
Construction of recA operator constitutive (Oc) mutation.
A previously characterized recA operator constitutive mutant of E. coli (recAo281) carries a point mutation in the LexA binding site between the −35 and −10 regions of the recA promoter region (51). This mutational change, an A/T to G/C transition at position −40, was introduced into the S. enterica genome by linear transformation (Fig. 1C). Primers for PCR were designed to amplify the pACYC184 Camr gene. At the 5′ end of one primer was the sequence −91 to −120 upstream of recA; at the 5′ end of the second primer were bases −1 to −39 upstream of recA, and then the 40th base of this primer introduces the point mutation within the LexA binding site. The PCR product was introduced into an SLT2 lexA40::Kan strain (TT23571) by linear transformation, selecting Camr. The presence of the recA Oc mutation (recAo281) was confirmed by sequencing.
Construction of chromosomal lexA+ duplication.
The chromosomal region between the metH and thr genes was transduced by two-fragment transduction (23). A P22 lysate was prepared on mutant metH2357::MudA(Amp Lac) (at 91.3 min) and another on mutant thr469::MudA(Amp Lac) (at 0 min). A 1:1 mixture of these lysates was used to transduce Ampr into strain LT2. While most transductants inherit one or the other of the donor insertions, some acquire a hybrid insertion at the joining point of a duplication that includes lexA as part of the region between the two insertions. This occurs if an appropriate pair of cotransduced fragments recombine with each other before recombining with the chromosome (23). Such duplication-bearing transductants were identified as Ampr clones with an unstable Lac+ phenotype (sectored blue colonies on NB-X-Gal). The presence of the duplication was confirmed by transducing the hybrid Mud-lac joining point into LT2 and observing that every transductant was unstably Lac+.
Cloning dinF under arabinose control.
The dinF gene of S. enterica was PCR amplified with one primer with an NcoI site near its 5′ end, the first 20 bp of dinF at its 3′ end and a second primer with 61 bp just downstream of dinF at its 3′ end. A 1.4-kb dinF+ sequence was amplified with these primers, digested with the endonucleases NcoI and EcoRI (which cuts 41 bp downstream of dinF), and ligated into the NcoI and EcoRI sites of the plasmid vector pBADhisC (Invitrogen) to produce plasmid pBADhisCdinF. The cloned dinF gene is controlled by the arabinose-inducible pBAD promoter. The integrity of the gene was confirmed by sequencing. This dinF-bearing plasmid did not correct the lethality of lexA null mutations with or without induction by l-arabinose (0.2%). Thus, the lethal phenotype of lexA null mutations is not due to lack of DinF.
Construction of a dinF::Zeo swap mutation.
The zeocin resistance gene (Zeor) from pCR-BluntII-Topo (Invitrogen) was PCR amplified with primers whose 5′ ends included sequence homologous to the ends of the S. enterica dinF gene. This linear product was introduced by electroporation, and transformants that carried the Zeor determinant in place of most of the dinF gene were selected; only 13 amino acids of the DinF protein sequence are left, and the lexA gene is intact. The dinF1012::Zeo swap is 100% linked to lexA+ in crosses with a recipient strain carrying the lexA41::Cam swap mutation.
Deleting the tum homolog of the Fels-2 prophage.
The Camr gene from pACYC184 was PCR amplified with primers whose 5′ ends contained sequence that is homologous to the ends of the Fels-2 tum-like gene. Linear transformation was used to introduce this fragment into the chromosome of the LT2-derived strain TT23647. The swap was confirmed by PCR and by linkage with another Fels-2 marker, gin-48::Kan(sw), obtained from Bossi and Figueroa.
Fusing the Fels-2 tum promoter to the lac operon.
A sequence including 282 bp immediately upstream of the tum gene and 126 bp of the gene itself was PCR amplified and placed immediately upstream of the lacZ gene on an F′ lac plasmid to create a tum-lacZ fusion. This was done by the two steps diagrammed in Fig. 2. First, the region was cloned upstream of lacZ in a high-copy-number cloning plasmid, pTrchis2-Topo-lacZ (Invitrogen) (Fig. 2A). The lacZ gene in this recombinant plasmid (Fig. 2B) was constitutively expressed, suggesting that the plasmid copy number was so high (about 200) that it exceeded the repression capacity of the LexA protein produced by a single chromosomal lexA gene.
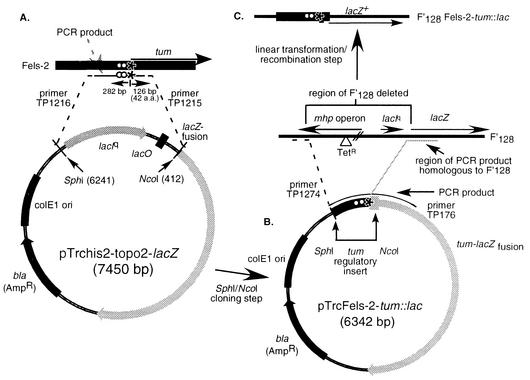
FIG. 2.
Construction of a tum promoter-lacZ reporter construct. (A) The control region of the Fels-2 tum gene was PCR amplified and cloned adjacent to the lacZ gene of the Topo cloning vector (Stratogene). Strains with this high-copy-number plasmid show constitutive lacZ expression. The tum control region was amplified from the above plasmid (B) and inserted (by recombination following linear transformation) adjacent to the lacZ gene of the low-copy-number plasmid F′128 (C). Strains with this plasmid show LexA-controlled expression of LacZ.
Therefore, the tum control region and part of the lacZ gene from the first plasmid were PCR amplified to produce a fragment with ends homologous to sites in a region near the upstream end of the lac operon in F′128. Selection was made for loss of tetracycline resistance (7) from the recipient strain, which carried a Tn10dTet insertion near lac in the mphC gene of F′128. This required that the tum control region be inserted in place of about 6 kb of plasmid sequence (including mphC::Tn10dTet) immediately upstream of the lacZ gene (Fig. 2C).
Assay of β-galactosidase.
Overnight cultures were diluted 100-fold into LB and grown with shaking to an optical density at 650 nm (OD650) of 0.6. Where appropriate, tetracycline (20 μg/ml) was added to maintain plasmids, and mitomycin C (1 μg/ml) was added to liquid growth medium. β-Galactosidase activity was determined in chloroform-permeabilized cells as described by Miller (32). Enzyme activity was expressed as nanomoles of nitrophenol produced per minute per OD650 unit of cell culture.
UV survival assay.
Overnight cultures grown in LB medium were diluted 100-fold and grown to an OD650 of 0.6 (in duplicate). Serial dilutions of these cultures were spread on NB plates and irradiated with UV light. UV fluence was measured with a short-wave UV meter (model J-225; Ultra-Violet Products, Upland, Calif.). Plates (NB) spread with the irradiated cells were incubated for 48 h at 37°C, and cells were counted.
Preparation of DNA for pulsed-field gel electrophoresis.
Cells were grown in 1.0 ml of LB broth, harvested by centrifugation, and treated as follows. After washing twice in TEN buffer (10 mM Tris [pH 7.5], 100 mM EDTA [pH 8.0], 250 mM NaCl) and resuspension in 0.5 ml of TEN, cells were mixed with 0.75 ml of 0.9% SeaKem agarose (suspended in TEN) and dispensed into 100-μl plug molds (Bio-Rad). Agarose plugs were treated for 2 h at 65°C in lysing solution (0.2% sodium dodecyl sulfate, 0.5% Sarkosyl, 10 mM Tris [pH 7.2], 100 mM EDTA [pH 8.0], 50 mM NaCl) and then at least 8 h in lysing solution with 0.1% lysozyme at room temperature. Plugs were subsequently treated with 1 mg of proteinase K per ml in wash solution (10 mM Tris [pH 8], 50 mM EDTA [pH 8.0]) for 48 h at 42°C. To inactivate the proteinase K, plugs were treated with 0.01 mM phenylmethylsulfonyl fluoride in wash solution for 1 h, washed five times, and stored in 0.1× wash solution; this method was adapted from that of Bergthorsson and Ochman (5). Before digestion, agarose plugs were washed seven times in 3 ml of sterile water, cut into fragments, and incubated for 30 min in 2 volumes of the appropriate buffer. Plugs were digested with 30 U of XbaI or BlnI.
Procedure for pulsed-field gel electrophoresis.
Electrophoresis was performed in a noncommercial apparatus with 0.5× Tris-borate-EDTA (TBE) buffer at 14°C. Approximately 15 μl of a plug was loaded into a 0.9% agarose gel. Electrophoresis was run for 24 h at 150 V in a noncommercial apparatus with hexagonally arrayed electrodes. To resolve high-molecular-weight bands, pulse times were initially 60 s and decreased linearly with time to 30 s over the 24-h period of electrophoresis. To resolve low-molecular-weight XbaI bands, a constant 7-s pulse time was used over the entire 24-h period. Fragments of phage lambda (New England Biolabs) were used as low-range molecular weight standards.
Determining the insertion sites of din::MudJ elements.
DNA was isolated from insertion mutants grown overnight in 1 ml of NB broth. Cells were pelleted and resuspended in 200 μl of Quick DNA buffer (10 mM Tris-HCl [pH 8.5], 1 mM EDTA, 0.2% sodium dodecyl sulfate) and incubated for 5 min at 100°C. Debris was removed by centrifugation (10 min at 13,000 rpm), and the supernatants were stored at −20°C. These DNA samples were diluted 100-fold and used in single-primer PCRs (see below).
The region adjacent to one end of the inserted element was PCR amplified with a single primer carrying sequence at the attL end of MudJ (21). Initial cycles are performed at high stringency to linearly amplify the junction region, and later cycles are performed at low stringency to allow adventitious initiation on the chromosomal sequences and subsequent logarithmic amplification. Amplification conditions (in an Idaho Technologies air cycler) were 20 cycles of <1 s at 94°C, <1 s at 55°C, and 1 min at 72°C, followed by 30 cycles of <1 s at 94°C, <1 s at 40°C, and 1 min at 72°C, followed by 30 cycles of <1 s at 94°C, <1 s at 55°C, and 1 min at 72°C. The primer TP251 (5′-GCAAGCCCCACCAAATCTAATCCCA-3′) directed replication outward from the attL end of MudJ into the adjacent chromosomal region. PCR products were treated with single-strand exonuclease (1 μl of a 1-U/μl solution of exonuclease I) for 1 h at 37°C to remove excess primers, purified with a Qiaquick PCR purification kit, and sequenced with a nested primer, TP240 (5′-CCGAATAATCCAATGTCC-3′).
RESULTS
Characterization of a lexA::Kan insertion mutation.
A strain carrying a lexA insertion mutation after base 11 of the coding sequence (lexA40::Kan; Fig. 1B) was constructed in vitro and kindly provided to us by Llagostera and Garriga (15). This strain (TT17653; = UA1685) also carries a constructed sulA46::Spc (spectinomycin resistance) insertion that was rendered essential by the lexA defect and could not be removed by transduction. The original sulA lexA mutant shows no growth defect. This behavior is similar to that of E. coli lexA null mutants, whose lethal phenotype is corrected by a sulA mutation (22, 26).
Unlike E. coli, our wild-type S. enterica LT2 strain could not inherit the lexA40::Kan insertion whether or not the recipient carried a sulA mutation. Our sulA+ wild-type LT2 strain (TR10000), when used as a transduction recipient for the lexA40::Kan insertion, gave about 1% of the expected transductant number (Table 2). These few transductants were found to carry a duplication of the lexA region, based on the fact that they gave rise to frequent kanamycin-sensitive segregants and showed both a lexA+ and a lexA40::Kan allele by PCR analysis. Apparently a cell with an existing lexA+ duplication inherited the donor lexA40::Kan allele by recombination with one copy and retained a lexA+ allele in the other copy. This suggests that duplications were present in about 1% of the cells in the recipient population, a fraction that is typical of genes in this region of the chromosome (2). The lexA null mutation appears to cause a recessive lethal phenotype in our wild type and could be maintained only by cells with a second wild-type lexA+ allele.
TABLE 2.
Lethality of lexA null mutations in strain LT2
| Transductional recipient
|
No. of selected transductants obtained with indicated donor (constant phage input, 3 × 109 PFU)
|
||||
|---|---|---|---|---|---|
| Strain | Relevant genotype | Genetic background if other than LT2 | lexA40::Kan (TT17653)a | lexA41::Cam (TT22613)b | (Cam)lexA33(Ind−) (TT22964)b |
| TR10000 | Wild type (LT2) recA+lexA+ | 1c | 3c | 444 | |
| TT23205 | sulA46::Spc | 12d | 22d | 524 | |
| TT23204 | sulA46::Spc (no Fels-2 or Gifsy-1 or -2) | SLT2 | 476 | 368 | 492 |
| TT22888 | Duplication (lexA+/lexA+) | 348 | 396 | 532 | |
| TT23203 | sulA46::Spc duplication (lexA+/lexA+) | 460 | 364 | 428 | |
| TT23296 | sulA46::Spc (no Fels-2 or Gifsy-1 or -2) | DB9071 | 480 | 512 | 500 |
| TT23298 | sulA46::Spc (no Gifsy-2) | ATCC 14028 | 43d | 6d | 552 |
| TT23299 | sulA46::Spc (no Gifsy-1 or -2) | ATCC 14028 | 20d | 10d | 548 |
| TT23379 | sulA46::Spc (no Fels-2) | DB7000 | 340e | 516e | 515 |
| TT23649 | tum(Fels-2)::Cam(sw) sulA+ | 3c | (656)f | ||
| TT23650 | tum(Fels-2)::Cam(sw) sulA46::Spc | 306e | (678)f | ||
Selection was for resistance to kanamycin on NB containing 50 μg of kanamycin per ml.
Selection was for resistance to chloramphenicol (Cam) on NB containing 25 μg of chloramphenicol per ml.
Healthy colonies that contained a duplication of the lexA gene.
Mixture of healthy colonies and flat, small colonies. Healthy colonies contained a duplication of the lexA gene.
Colonies were virtually all flat and small.
Since this recipient was already Camr, a hisC::Kan (TT16803) marker strain was used as the donor instead of (Cam)lexA33; transductants were selected on NB containing kanamycin (50 μg/ml).
In support of this conclusion, the lexA40::Kan insertion could be efficiently transduced into a strain that carried a constructed lexA+ duplication (Table 2) (see Materials and Methods for duplication construction). Duplication strains that inherited the lexA40::Kan insertion gave rise to segregants that lost the join-point markers (Lac+ and Ampr); all such segregants also lost the Kanr phenotype associated with the lexA mutation. That is, no haploid segregants that maintained the lexA::Kan insertion were recovered. This demonstrated that a lexA null mutation had a lethal phenotype in a SulA+ background of S. enterica (as known for E. coli) and that that this lethality is recessive to a wild-type lexA allele.
Unexpectedly, recipients carrying a sulA mutation also yielded few lexA40::Kan transductants (TT23205 [Table 2]). Some of the few transductants carried a spontaneous lexA duplication, as described above. However, a slightly greater number formed small flat colonies that were not seen for the SulA+ recipient. When these small colonies were streaked on rich medium containing kanamycin, they gave rise mostly to small, flat, single colonies like the original transductants, but a small percentage of the new colonies were large and appeared healthy. The fast-growth phenotype was stable and was not due to a lexA duplication; the donor lexA40::Kan allele had been inherited in place of the recipient lexA+ allele (determined by PCR). This suggested that lexA mutations in S. enterica (unlike those in E. coli) were lethal even in a sulA background and that suppressors of this lethality arose at high frequency.
Phenotype of a lexA deletion (swap) mutation.
To be sure that the behavior of lexA40::Kan was not a unique property of this insertion, a deletion of the S. enterica lexA gene was constructed by linear transformation (16, 38, 61). A chloramphenicol resistance gene (Camr) was used to replace 561 of the 609 bases of the lexA coding sequence in the SLT2 background, received from Llagostera and Garriga (see Materials and Methods). When transduced into our genetic background, the new mutation, lexA41::Cam, behaved exactly as did the lexA40::Kan mutation described above (Table 2). Thus, both the deletion and insertion alleles of lexA had a recessive lethal phenotype that was corrected, in a sulA background, by frequent suppressors. Neither these suppressors nor the sulA mutation was separately sufficient to correct lethality.
Lethality of lexA null mutations is not due to a polar effect on dinF.
In E. coli, where lexA lethality was not seen, most work employed point mutations that may be less strongly polar than the insertion or swap alleles used here (26, 56). The possibility that lethality in S. enterica was due to stronger polarity on the dinF gene was eliminated because an in-frame lexA deletion, expected to be nonpolar, was also lethal in S. enterica. In addition, overexpression of dinF from a plasmid did not allow inheritance of lexA null mutations (see Materials and Methods).
Isolation of suppressors of lexA null mutations.
Ten independent lexA suppressor strains (Slx) were isolated in a sulA LT2 strain by transducing in the lexA41::Cam swap mutation, selecting for chloramphenicol resistance on NB plates. The rare flat colonies that arose were restreaked on selective medium and (as seen before) gave rise primarily to flat colonies plus an occasional healthy colony. It is presumed that the initial transductants arose from recipient cells that happened to carry a weak suppressor of lexA lethality and that secondary growth-enhancing suppressors arose during restreaking. Both primary and secondary suppressor strains were included in our set of 10.
Preliminary characterization of suppressors.
All 10 suppressor strains were prototrophic. Two strains (Slx6 and Slx9) formed flat, slower-growing colonies and were expected to carry only the primary weak suppressor of lexA. Seven strains (Slx1, -2, -3, -5, -7, -8, and -10) formed healthy colonies and are inferred to carry both primary and secondary suppressors. (The Slx4 strain proved phage resistant and was not studied further.) None of the nine characterized suppressor strains contained a duplication of the lexA region; PCR amplification of lexA showed only the swap mutant allele.
All nine suppressor strains expressed the SOS-induced recN::MudJ operon fusion constitutively, as expected for strains lacking LexA repressor (Table 3). Thus, the suppressors correct the lethal phenotype without restoring repression of the SOS regulon. When the lexA+ allele was reintroduced into the Slx strains (by cotransduction with malB661::Tn10dTet), the resulting lexA+ suppressor strains again repressed the recN::lac fusion. These lexA+ suppressor strains (like the SLT2 background) supported efficient introduction of a lexA null allele.
TABLE 3.
Effects of lexA alleles on induction of a recN::lac fusiona
| Strain | Relevant genotype | β-Galactosidase in recN::lac strains grown without (−) or with (+) mitomycin C (1 μg/ml)
|
|||
|---|---|---|---|---|---|
|
sulA+
|
sulA46::Spc
|
||||
| − | + | − | + | ||
| TR10000 | recA+lexA+ (without recN::lac) | 0 | 0 | 0 | 0 |
| TT23315 | recA+lexA+ (with recN::lac) | 13.1 | 268.5 | 9.8 | 162.8 |
| TT23465 | recA643::Tn10T-POP | 2.7 | 2.2 | ND | ND |
| TT23381 | lexA33(Ind−) | 2.2 | 1.9 | 2.3 | 2.0 |
| TT23351 | recA281(Oc) | 9.5 | 368.2 | 10.8 | 197.0 |
| TT23355 | /pJWL21 lexA+ | 3.7 | 4.0 | 4.3 | 4.5 |
| TT23489 | /pJWL26 lexA3(Ind−) | 3.8 | 4.4 | 4.2 | 21.9 |
| TT23392 | recA281(Oc)/pJWL21 lexA+ | 5.2 | 56.5 | 5.3 | 54.7 |
| TT23393 | recA281(Oc)/pJWL26 lexA3(Ind−) | 4.2 | 4.0 | 4.2 | 4.6 |
| TT23276 | Slx2 sulA46::Spc lexA41::Cam | ND | ND | 215.5 | 289.1 |
| TT23320 | Slx2 sulA46::Spc lexA+ | ND | ND | 5.9 | 202.9 |
| TT23280 | Slx6 sulA46::Spc lexA41::Cam | ND | ND | 144.8 | 173.5 |
| TT23324 | Slx6 sulA46::Spc lexA+ | ND | ND | 2.8 | 133.1 |
| TT23283 | Slx9 sulA46::Spc lexA41::Cam | ND | ND | 149.1 | 175.8 |
| TT23327 | Slx9 sulA46::Spc lexA+ | ND | ND | 3.6 | 89.1 |
All strains except TR10000 (top line) contained the recN551::MudJ insertion. Assays were done with at least two cultures, each tested in duplicate. Units are nanomoles of nitrophenol produced per minute per unit of optical density at 650 nm of cell suspension. ND, not done.
Difficulty in mapping these suppressors and the high frequency of new suppressor mutations suggested that they might reflect gene duplication or deletion. Pulsed-field gel electrophoresis revealed the prophage loss described below.
Suppression of lexA null mutations by loss of prophages.
Strain LT2 of S. enterica carries four active phages—Fels-1, Fels-2, Gifsy-1, and Gifsy-2. All nine suppressor strains lacked the Fels-2 prophage; the seven healthier suppressor strains had also lost the Gifsy-1 and Gifsy-2 prophages. The Fels-1 prophage remained in all strains.
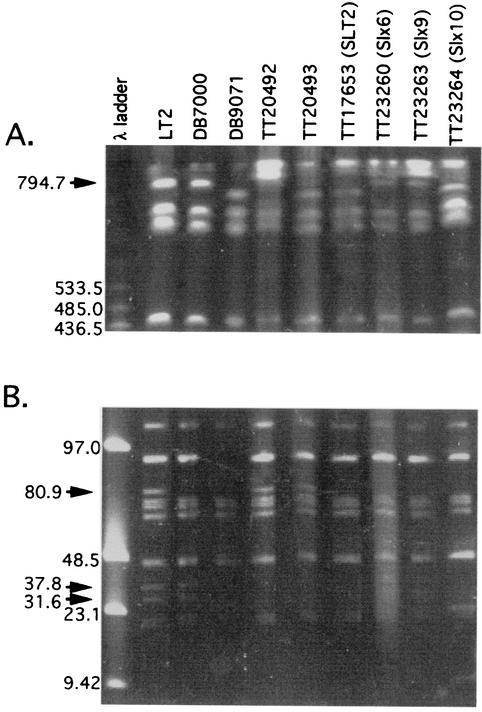
The Fels-2 prophage was contained within an 80.9-kb XbaI fragment that was absent from both the healthy and slow-growing suppressor strains. All of the healthy suppressor strains showed a 40-kb decrease in the size of an XbaI fragment that, in the wild type, is 794.7 kb and includes both the Fels-1 and Gifsy-2 prophages. (Representative strain Slx10 is shown in Fig. 3A.) Strains with this deletion produced a large BlnI fragment (data not shown; diagrammed in Fig. 4) whose size demonstrated removal of two BlnI sites known to be within the Gifsy-2 prophage (18). The deletion size (∼40 kb) and the loss of these BlnI sites suggested that Gifsy-2 (45.8 kb) is missing and Fels-1 was still present in the healthy suppressor strains. The Gifsy-1 prophage spans two XbaI fragments (37.9 kb and 31.6 kb), both of which were missing from the healthy suppressor strains (Fig. 3B). The restriction map in Fig. 4 indicates the fragments described above.
FIG. 3.
Pulsed-field gel electrophoresis of genomic XbaI fragments from various strains of S. enterica. Arrows indicate bands of interest. For DNA preparation and electrophoresis conditions, see Materials and Methods. (A) High-molecular-weight XbaI bands were separated with pulse times decreasing from 60 to 30 s over a 24-h period. (B) Low-molecular-weight XbaI bands were separated with 7-s pulses over a 24-h period. Strain DB7000 lacks the 90-kb band (due to plasmid pSLT) and also the chromosomal fragments indicated by arrows (see text).
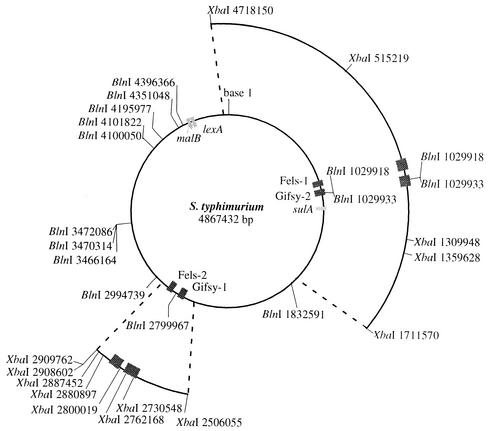
FIG. 4.
Genome map of S. enterica, indicating locations of prophages and XbaI and BlnI restriction sites. Boxes represent complete prophage inserted in the Salmonella genome and genes relevant to this study. The published sequence of S. enterica serovar Typhimurium strain LT2 was used to construct this map; sequence coordinates are for a genome that includes all four active prophages (29).
The slow-growing suppressor strains TT23260 (Slx6) and TT23263 (Slx9) lacked only the Fels-2 prophage (Fig. 3). The 37.9- and 31.6-kb bands indicative of Gifsy-1 were present but faint. This proved to reflect frequent loss of Gifsy-1 from the slow-growing suppressor strains. This instability is consistent with the high frequency with which these strains gave rise to healthy derivatives that have lost Gifsy-1 and -2. The Gifsy-2 prophage seemed to be present but was particularly unstable in Slx9. This conclusion is based on the fact that both the 794.7-kb XbaI band containing the Gifsy-2 insertion and the smaller derived deletion ∼755-kb band were present. This behavior was seen for multiple single-colony isolates of strain TT23263. Thus, strains lacking Gifsy-2 arose and overgrew when the suppressor strain was cultured to make the pulsed-field gel electrophoresis sample. The original slow-growing suppressor strains appeared to have lost only Fels-2. Data below suggest that both Gifsy-1 and -2 are unstable (or are counterselected) in lexA null strains lacking Fels-2. It is unclear why Gifsy-2 appears to be more unstable in Slx6 than in Slx9.
Wild-type S. enterica strain LT2 and several derived strains known to lack particular prophages were used as controls for the pulsed-field gel electrophoresis analysis. Strain DB7000 (TR17000) from David Botstein is known to lack Fels-2; the derived strain TT10910 (DB9071) was found, in the course of these studies, to lack three phages, Fels-2, Gifsy-1, and Gifsy-2, although it was never placed under selection to maintain lexA null (Fig. 3). Strain TT20492 (= MA3408 from Bossi and Figueroa) lacks Gifsy-1, and strain TT20493 (= MA4587) lacks both Gifsy-1 and -2.
The above conclusions were verified by PCR amplification of the bacterial attachment sites of the Fels-2 and Gifsy-1 and -2 phages and amplification of both of the phage-bacterium junctions of Gifsy-1 and -2. Strains inferred above to lack Fels-2, Gifsy-1, and Gifsy-2 all showed bacterial attachment site fragments of a size expected if the phage was perfectly excised. Slow-growing suppressor strains inferred to still possess Gifsy prophages gave amplification of both the empty attachment site and the phage-bacterial junction fragments, suggesting that some cells in the culture retained the Gifsy phages while others excised them. This is consistent with the frequent loss of Gifsy prophages in lexA null strains lacking Fels-2 (data not shown).
The fully viable original lexA40::Kan insertion mutant received from Llagostera and Garriga (SLT2; = TT17653) was assumed to carry some suppressor of lexA lethality. These strains showed a pulsed-field gel electrophoresis pattern identical to that of the healthy suppressor strains described above (Fig. 3) and therefore, like them, appears to have lost Fels-2 and both Gifsy phages. Either the SLT2 wild type had lost these phages prior to construction of the lexA40::Kan insertion, or these phages were lost in the process of that construction.
Genetic evidence that suppressors of lexA null mutations are prophage deletions.
Transposon insertions are available for all four of the prophages present in the genome S. enterica (LT2)—Fels-1, Fels-2, Gifsy-1, and Gifsy-2 (1, 18, 59). The Gifsy-1 and Gifsy-2 insertions (din-11::MudJ and din-243::MudJ, respectively) are described below. The Fels-1::MudJ and the Fels-2::Kan insertions were provided by Bossi and Figueroa.
One expects it to be difficult to transduce an insertion mutation within a donor prophage into a recipient strain lacking that prophage. If the recipient possesses the prophage, the transducing phage is required to package only the insertion and some flanking material to support recombination. However, if the recipient lacks the target prophage, then the donated fragment must include all of the donor prophage with the inserted resistance determinant and material to support recombination. In addition, there is the possibility that introduction of a prophage into a recipient that lacks it (or its immunity region) will cause zygotic prophage induction and consequent lethality.
Fels-1::MudJ(Kanr) could be transduced with high efficiency into all of the suppressor strains, indicating that the Fels-1 prophage was present in all suppressor strains. The Gifsy-1::MudJ and Gifsy-2::MudJ elements could not be transduced into any of the healthy suppressor strains Slx1, -2, -3, -5, -7, -8, or -10, indicating that these suppressor strains lacked both of these prophages and supporting the conclusions based on pulsed-field gel electrophoresis. Both Gifsy phage insertions could be transduced into the unhealthy suppressor strains Slx6 and Slx9, though the Gifsy-1 insertion transduced at a lower efficiency, probably due to loss of the phage during growth of the cultures. This result is consistent with the pulsed-field gel electrophoresis and PCR results, suggesting that these suppressors retained the Gifsy prophages, albeit unstably. Consistent with the pulsed-field gel electrophoresis results, the SLT2 background, in which the original lexA40::Kan insertion was received, proved to be a poor recipient for insertions in Fels-2, Gifsy-1, and Gifsy-2 (Table 4 and Fig. 3).
TABLE 4.
Transduction tests for presence of Gifsy and Fels prophagesa
| Transductional recipient
|
No. of selected transductants obtained with indicated donor (constant phage input)
|
||||
|---|---|---|---|---|---|
| Strain | Relevant genotype | Gifsy-1 (din-9)::MudJ (TT17200) | Gifsy-2 (din-243)::MudJ (TT17217) | Fels-1::MudJ (TT23252) | Fels-2 (gin-48)::Kan (TT23563) |
| TT23205 | sulA46::Spc lexA+ | 488 | 180 | 544 | 87 |
| TT23204 | sulA46::Spc lexA+ (SLT2) | 0 | 0 | 346 | 0 |
| TT23255 | Slx1 | 0 | 0 | 612 | 0 |
| TT23256 | Slx2 | 0 | 1 | 284 | 0 |
| TT23257 | Slx3 | 0 | 3 | 412 | 0 |
| TT23259 | Slx5 | 0 | 0 | 500 | 0 |
| TT23260 | Slx6b | 48 | 255 | 66 | 0 |
| TT23261 | Slx7 | 0 | 1 | 404 | 0 |
| TT23262 | Slx8 | 0 | 0 | 508 | 0 |
| TT23263 | Slx9b | 33 | 266 | 90 | 0 |
| TT23264 | Slx10 | 0 | 0 | 416 | 0 |
All strains carried a sulA46::Spc insertion, and all but the first two carried the lexA41::Cam deletion/insertion. Selection was for resistance to kanamycin on NB.
The growth rate of these strains was often considerably lower than for any of the other suppressor strains.
Loss of prophages is sufficient to correct lethality of lexA null mutations.
The lexA null mutations were transduced into the four control strains that had lost various combinations of these prophages but had not previously been under selection to grow with a lexA null mutation. The sulA46::Spc mutation was introduced into these strains prior to testing (Table 2). The lexA null mutations are lethal in strains that lack only the Gifsy prophages. The strain lacking only Fels-2 inherited the lexA null mutations efficiently, but all recombinants formed the small flat colonies characteristic of lexA mutants carrying Gifsy-1 and -2. The strain lacking Fels-2 and both Gifsy phages (TT23296) was comparable to the SLT2 strain in which the lexA mutation was received; both strains inherited lexA insertion mutations efficiently and gave healthy recombinant strains. Since these strains were never under selection to carry a lexA null mutation, we conclude that loss of the prophages (in addition to a sulA mutation) is sufficient to suppress the lethal phenotype of a lexA null mutation.
The nonpolar, in-frame deletion mutation lexA42::FRT showed the same behavior as the insertion and swap alleles. (See Materials and Methods for a description of the crosses.) The deletion was efficiently inherited by all of the suppressor (Slx) strains tested and also by tester strains lacking the Fels-2 prophage; it could not be inherited by strains lacking only Gifsy prophages. Coinheritance of lexA null with mal::Tn10dTet was lower in a recipient lacking only Fels-2 (15%) than was seen for the lexA+ allele (27%) or for the lexA null allele in any of the healthy suppressor backgrounds (24 to 29%), probably due to poor growth of lexA strains lacking only Fels-2.
P22 prophage does not cause lexA lethality.
A lexA+ allele and a P22 prophage were introduced into all seven strong suppressor strains (lacking sulA, Fels-2, Gifsy-1, and Gifsy-2). The introduced P22 phage carried an sie mutation, which allows the lysogen to serve as a transductional recipient. Each such lysogen (e.g., TT23340 to TT23343) efficiently inherited either the lexA40::Kan insertion or the lexA41::Cam swap deletion. Thus, a P22 prophage does not cause lexA to show a lethal phenotype. This confirms previous conclusions that P22 regulation is independent of LexA (49).
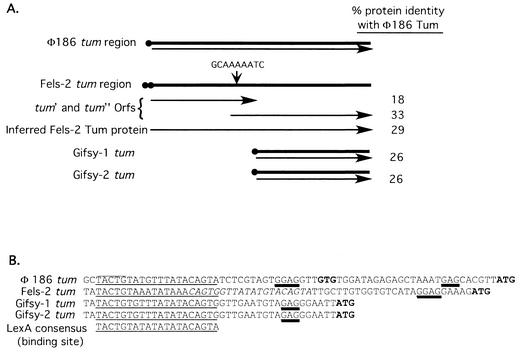
Fels-2, Gifsy-1, and Gifsy-2 but not Fels-1 or P22 encode homologues of the coliphage 186 cI antirepressor Tum.
Phage 186, unlike its close relative P2, forms lysogens that are UV inducible (58). Inducibility is due not to direct RecA-mediated cleavage of the phage repressor (as in phage lambda and P22), but to induction of a LexA-repressed phage gene for an antirepressor (Tum) that binds reversibly to the phage 186 phage repressor protein and prevents it from binding to its operator (8, 27, 44). Homologues of the phage 186 tum gene are found in all three of the phages Fels-2, Gifsy-1, and Gifsy-2 that contribute to lexA lethality in S. enterica. Homologs of tum were not found in the genomes of Fels-1, P22, or P2.
The tum gene of Fels-2 is similar in length to that of coliphage 186 but includes a frameshift mutation (Fig. 5A); alternatively, this region may include two genes, each encoding a protein homologous to part of Tum. The upstream region (or gene) encodes the actual antirepressor function, while the second region (or gene) encodes a homologue of the E. coli DinI protein (see Discussion) (Gail E. Christie, personal communication). The tum region of the Gifsy phages is smaller, encoding a protein that is homologous to DinI and the carboxy-terminal portion of coliphage 186 Tum.
FIG. 5.
Comparison of the tum region of coliphage 186 with those from Fels-2, Gifsy-1, and Gifsy-2. (A) Schematic representation of the tum genes of these prophages. Black circles indicate potential LexA binding sites. Arrows indicate the predicted open reading frame(s) for each gene. The potential site for frameshifting in the Fels-2 tum gene is indicated. (B) Potential LexA binding sites (underlined) upstream of the Fels-2, Gifsy-1, and Gifsy-2 tum genes. Sequences are compared to the demonstrated LexA binding region of coliphage 186 tum (8) and the LexA binding site consensus sequence from the review by Walker (56). Italicized bases in the Fels-2 sequence indicate a second potential LexA binding site. Potential start sites for translation are in bold. Predicted Shine-Dalgarno sequences are double underlined. Overlines denote the −10 promoter region identified by Brumby et al. (8).
Potential LexA binding sites were identified immediately upstream of all of these genes (Fig. 5B). This suggested that lexA null mutations might cause expression of the Fels-2 tum antirepressor function, leading to phage induction or expression of phage proteins that inhibit bacterial growth. In either case, removal of the Fels-2 tum region should eliminate the lexA lethal phenotype. The genome of P22 lacks a tum homologue but encodes another antirepressor (ant) that does not appear to be regulated by LexA (49).
Fels-2 tum deletion suppresses the lethal phenotype of lexA null mutations.
A deletion of the entire tum region from the Fels-2 prophage (see Materials and Methods), together with a sulA mutation, allowed efficient inheritance of the original lexA40::Kan insertion mutation by strains possessing all four of the LT2 prophages. The transduction frequency was comparable to that seen for recipients with a lexA+ duplication (TT22888 [Table 2]) and that seen for the SLT2 suppressor background. Transductants of the tum sulA mutant formed small flat colonies that threw off healthy suppressor strains upon restreaking (presumably due to the subsequent loss of the Gifsy prophages). This supports the model above and suggests that lexA null mutations cause expression of the Fels-2 tum antirepressor, which either causes production of phage proteins that inhibit cell growth or causes cell lysis by at least occasional full induction of the Fels-2 prophage.
We have not demonstrated that the residual growth defect in the presence of Gifsy prophages is due to induction of their tum genes. The tum gene homologue in these prophages does not include the portion of the phage 186 tum gene thought to encode the antirepressor, only the DinI homologue (see Discussion).
Control region of Fels-2 tum gene includes a LexA-repressed promoter.
To further test the idea that LexA regulates the Fels-2 tum promoter, we placed this promoter region adjacent to the lacZ gene on plasmid F′128 (see Materials and Methods). Strains with this fusion showed induction by mitomycin C if the strain was lexA+ and constitutive high expression if the strain carried a lexA null mutation (Table 5). By similar methods, we fused the promoter region of both Gifsy phages to a lac reporter and saw similar evidence for LexA-mediated induction by mitomycin (data not shown).
TABLE 5.
LexA-dependent control of a Fels-2 tum::lac fusiona
| Strain | Relevant genotype | β-Galactosidase activity in F′128-Fels-2 tum::lac strains
|
|
|---|---|---|---|
| LB | LB + mitomycin C (1 μg/ml) | ||
| TT23764 | recA+lexA+ | 3.6 | 95.2 |
| TT23765 | lexA41::Cam | 168.2 | 180.3 |
Both strains contained the proB1567::Tn10 and sulA46::Spc insertions and lacked prophages Fels-2, Gifsy-1, and Gifsy-2. They also contained the plasmid F′128 (Ptum::lacZ+), which encodes a lacZ gene regulated by the control region of the Fels-2 tum gene. Assays were done with at least two cultures, each tested in duplicate. Units are nanomoles of nitrophenol produced per minute per optical density unit at 650 nm of cell suspension.
Most of the known SOS-regulated (din) genes in S. enterica are within prophages.
If phage transcription is controlled in part by the LexA repressor, one might expect that the set of damage-inducible (din) genes in S. enterica (48) would include some within the affected prophages. These din genes were identified as MudJ(Lac) insertions that showed mitomycin-inducible levels of LacZ. They were inferred to be LexA (SOS) regulated because their induction is prevented by a recA mutation and induced by overexpression of LexA (48).
Eight of the 12 available S. enterica din::MudJ insertions (48) were sequenced as described in Materials and Methods. Seven of the sequenced insertions lay within the prophage of either Gifsy-1 or Gifsy-2 and only one lay in a standard chromosomal gene (Table 6). We failed to obtain sequence for four of the other insertions, but two of these (din-220::MudJ and din-292::MudJ) are also inferred to lie within prophages because they could not be transduced into a strain (TT23309) that lacked the Gifsy-1 and -2 and Fels-2 prophages (Shawn Gerum, personal communication). Thus, 9 of the 10 din insertions tested appeared to be within prophages.
TABLE 6.
Sequenced damage-inducible (din) gene insertions
| Damage-inducible gene insertiona | Gene containing the MudJ insertionb | Location of identified gene | Repression by LexA overexpressionc | Inducibility by mitomycin Cd
|
|
|---|---|---|---|---|---|
| lexA+ | lexA(Ind−) | ||||
| din-240::MudJ | recE | Prophage Gifsy-2e | Weak | Yes | None |
| din-11::MudJ (identical to din-80::MudJ) | recE | Prophage Gifsy-2e | Weak | Yes | ± |
| din-14::MudJ | Gene for putative T4-like terminase large subunit | Prophage Gifsy-2 | ND | Yes | None |
| din-243::MudJf | Gene resembling the H gene of phage lambda (tail component) | Prophage Gifsy-2 | Strong | Yes | None |
| din-10::MudJ | ORF between genes that resemble the J and tail fiber genes of phage lambda | Prophage Gifsy-2 | Strong | Yes | None |
| din-9::MudJ | Between genes that resemble the “gpshp” and gp-7 genes of phage 21 | Prophage Gifsy-1 | Strong | Yes | None |
| din-84::MudJ | ybhA | Chromosome, 18.3 min | Weak | Yes | None |
Isolated by Smith et al. (48).
Determined by single-primer PCR and sequencing (see Materials and Methods). ORF, open reading frame.
Data from Smith et al. (48). ND, not done.
Determined by replica plating duplicate patches of strains to NB-X-Gal plates containing 0, 50, 100, 150 and 200 ng of mitomycin C per ml.
The recE genes of Gifsy-1 and -2 are identical, and therefore linkage (or lack thereof) with an nadB insertion (nadB215::Tn10) approximately 11 kb from recE in Gifsy-1 was used to determined if the recE insertions were in Gifsy-1 or -2.
With no DNA damage and a wild-type LexA protein, this gene is fully repressed; in a lexA(Ind−) background, the gene is transcribed at a low level but does not appear to be upregulated in the presence of DNA damage.
lexA(Ind−) mutation in S. enterica prevents induction of SOS-regulated genes.
The above results suggested that the lethality of lexA null mutations in S. enterica (LT2) is due to prophages carried by that strain and does not reflect a difference between the SOS control systems of S. enterica and E. coli. To verify this, a constructed lexA(Ind−) mutation (see Materials and Methods) was tested for its effect on the SOS-controlled recN gene (Table 3). In lexA+ strains, the recN::lac fusion was induced by mitomycin C, and that induction was prevented by a recA (TT23465) mutation. Induction was also prevented by the lexA(Ind−) allele placed either in the chromosome or on a plasmid (strains TT23381 and TT23489, respectively). The plasmid lexA(Ind−) allele prevented recN induction even when RecA protein (the LexA coprotease) was expressed constitutively (TT23393 [Table 3]). Overexpression of lexA+ (from a plasmid) also prevented induction of recN by mitomycin C (TT23355) in an otherwise wild-type background and partially repressed recN in a strain carrying a recA operator mutation (TT23392). The SOS response of the recN fusion was not altered by the presence of a sulA46::Spc mutation. This behavior is like that shown previously for the SOS system of E. coli (55) A chromosomal lexA(Ind−) mutation also prevented mitomycin induction of din::lac fusions in Gifsy prophages (Table 6).
Plasmids carrying the lexA+ or lexA(Ind−) gene reduce recombination by superrepression of the recA gene.
The RecA protein of E. coli is superrepressed by overexpression of the LexA+ or LexA(Ind−) repressor protein (14, 52), and this repression is sufficient to reduce recombination rates (55). In S. enterica, plasmids carrying either lexA+ or lexA33(Ind−) reduced recombination at least 500-fold, as judged by transductional inheritance of a drug resistance marker (recipients TR7235 and TR7236 [Table 7]). The recombination defect in these plasmid-bearing strains was increased about 200-fold by a recA operator constitutive mutation (TT23206 and TT23207) and thus appeared to be due to superrepression of recA. This operator mutation (51) is known to reduce the apparent affinity of LexA for the recA control region by a factor of 10 (13). Residual ability of LexA to bind the mutant recA operator may explain the slight decrease in recombinant yield caused by the plasmids in a recA(Oc) strain. A single chromosomal copy of the lexA(Ind−) allele caused no defect in recombination that could be detected by the methods used (TT22964 [Table 7]). These results suggest that the SOS system of S. enterica is like that of E. coli. Construction of the recA(Oc) and lexA(Ind−) mutations is described in Materials and Methods.
TABLE 7.
Recombination reduction by plasmids carrying lexA+ or lexA3(Ind−)
| Transductional recipient
|
No. of Kanr transductants per plate (constant phage input)b | |
|---|---|---|
| Strain | Relevant genotype | |
| TR10000 | recA+lexA+, wild type | >1,500 |
| TT9048 | recA1 | 0 |
| TR7235 | recA+lexA+/pJWL21 lexA+ | 31 |
| TR7236 | recA+lexA+/pJWL26 lexA3(Ind−) | 12 |
| TT23151 | recA281(Oc) | >1,500 |
| TT23206 | recA281(Oc)/pJWL21 lexA+ | 624 |
| TT23207 | recA281(Oc)/pJWL26 lexA(Ind−) | 422 |
| TT22613 | sulA46::Spc lexA41::Cam(sw) (SLT2)a | >1,500 |
| TT22964 | lexA33(Ind−) | >1,500 |
SLT2 background from Montserrat Llagostera and Xavier Garriga.
Donor strain TT10286 carries a hisD::Kan marker; transduction was done on NB containing kanamycin.
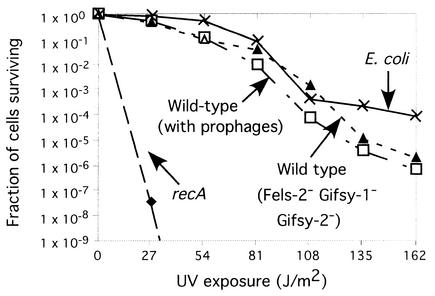
LexA-regulated prophages do not make S. enterica sensitive to UV killing.
Wild-type strains of S. enterica are not notably sensitive to UV despite the fact that they carry the phages discussed here. This was retested directly, comparing E. coli K-12 to S. enterica LT2 strains with and without these prophages (Fig. 6). Strains of S. enterica with the prophages were very slightly more sensitive to UV than were either E. coli or an S. enterica strain lacking the phages. Apparently UV irradiation does not effectively induce these prophages. Thus, lexA null mutations may reduce the growth rate of lysogens rather than inducing lysis.
FIG. 6.
UV killing of E. coli and S. enterica. Strains tested were as follows: wild-type S. enterica (recA+ lexA+ sulA+, with all prophages present [TT23656]); S. enterica Fels-2− Gifsy-1− Gifsy-2− (lexA suppressor strain Slx10 made lexA+ and sulA+ [TT23657]); S. enterica recA (recA1 [TT9048]); E. coli K-12 strain, lexA+ sulA+ recA+, without a lambda lysogen [TR7178]).
LexA null mutations do not cause a mutator phenotype.
The error-prone polymerase DinB is part of the LexA-controlled SOS regulon (24, 50). The DinB polymerase contributes to lac reversion under selective conditions in the Cairns experiment (9, 30, 31) and is responsible for the associated general mutagenesis suffered by lac revertants in that experiment (E. S. Slechta, K. L. Bunny, E. Kofoid, K. Sivaraman, D. I. Andersson, and J. R. Roth, unpublished data). This associated mutagenesis appears to requires SOS induction (presumably of DinB) because it is eliminated by a lexA(Ind−) mutation as well as by a dinB mutation (47). However, this induction was not sufficient for mutagenesis, as judged by mutation rates measured in a lexA null mutant.
Seven suppressor strains carrying the lexA41::Cam null mutation were compared to isogenic lexA+ strains for the effect of the mutations on both base substitution and frameshift mutation rate. The base substitution rate was scored by selecting rifampin-resistant (Rifr) mutants. Frameshift mutation (−1) rate was scored by assessing reversion of a lac +1 frameshift allele (lacIZ33) inserted in the Salmonella chromosome as described previously (46). Ten independent cultures of each strain were grown in LB and plated on selective medium (LB with rifampin or NCE with 0.2% lactose). The number of mutations per 108 plated cells varied from culture to culture, as expected, but lexA+ and lexA strains showed no detectable difference in the median number of either base substitution or frameshift mutants (data not shown). This experiment could have detected a twofold increase in the frequency of either mutation type. Thus, DinB-dependent mutagenesis appears to require some second factor in addition to SOS (DinB) induction.
DISCUSSION
The SOS regulon of S. enterica (LT2) appears to be controlled by the LexA protein as originally described for E. coli (K-12). The lethal phenotype of lexA null mutations in sulA mutants of S. enterica (LT2) is inferred to be a consequence of prophages carried in the genome of S. enterica. Lethality is caused primarily by the Fels-2 prophage, and growth is secondarily impaired by the Gifsy-1 and -2 prophages, which appear to be destabilized (or counterselected) in the absence of LexA.
All three of these S. enterica prophages carry homologues of the tum cI antirepressor gene of phage 186, and in all three phages, the promoter region of the tum homologue includes an apparent LexA binding site. Thus, the tum antirepressor gene of the Fels-2 prophage is necessary for the observed lethal phenotype of lexA null mutations, and the tum gene is regulated by LexA. The growth rate of lexA null mutants lacking Fels-2 was improved by loss of the Gifsy phages.
It seems reasonable that lack of LexA protein might cause full induction of Fels-2 and thereby lead to cell killing. However, since this prophage is not strongly induced by UV irradiation, it seems more likely that the lack of LexA causes expression of some subset of phage genes (perhaps mediated by the Tum antirepressor) whose products restrict cell growth without causing full phage induction. Cells that inherit the lexA null mutation might be able to grow only if they have spontaneously lost their Fels-2 prophage prior to acquiring the lexA null mutation. In either case, UV irradiation (and consequent LexA cutting) appears to be less effective at inducing the phage or its host-inhibitory gene products than is complete loss of the LexA protein. The same considerations apply to the Gifsy phages. Expression of genes from these phages (in a lexA mutant) could destabilize the prophages or cause expression of phage genes that inhibit cell growth and the consequent positive selection for spontaneous prophage loss.
The Fels-2 prophage is related to phages P2 and 186 of E. coli. P2 lacks a tum gene and is not induced by UV; phage 186 is induced by UV, and this induction is mediated by derepression of its tum gene following LexA inactivation (44). While removal of the Fels-2 tum gene (plus a sulA mutation) corrects the lethal phenotype of lexA null mutations in S. enterica, the phage tum gene sequence appears to carry a frameshift mutation that divides the reading frame (as seen in phage 186) into two approximately equal-sized parts. It is not clear whether these represent distinct genes or require spontaneous frameshifting in order to produce a functional product. Genes for transposases have also been found to include a frameshift and a “shifty” sequence that is thought to make transposition depend on occasional spontaneous frameshifting during translation (11). Fels-2 may hedge its bets in this way and cause a low-probability induction during a standard UV exposure. Deletion of the lexA gene may allow more expression and thereby lead to more frequent induction.
An alternative explanation is that the two open reading frames seen in Fels-2 actually encode two distinct proteins with independent functions. This interpretation is supported by the recent finding in the P2-related phage PSP3 that the sequence corresponding to the phage 186 tum gene encodes two distinct genes (G. E. Christie, personal communication). The upstream gene encodes the antirepressor activity, and the downstream gene encodes a homologue of the DinI protein of E. coli, which is SOS induced and may limit the consequences of an SOS induction (60). If the two portions of the tum region have different functions, Gifsy prophages have only the distal (DinI-like) gene and may lack an antirepressor activity (Fig. 5). The Gifsy phages are inefficiently induced by UV, and the weak observed induction appears to depend on RecA function (L. Bossi and N. Figueroa, personal communication).
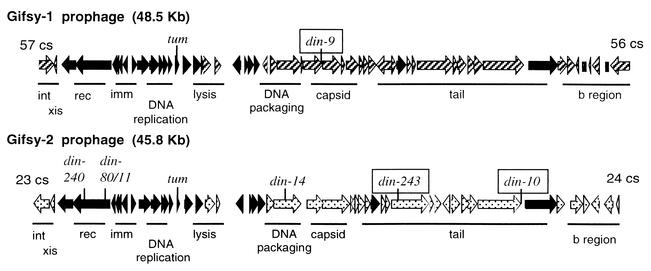
Fusions of lac on the right side of the Gifsy prophage (Fig. 7) are those reported previously to be strongly induced by mitomycin C and strongly repressed by overproduction of the LexA protein (48). It seems likely that these Gifsy transcripts are induced following DNA damage and by lexA null mutations. In contrast, lac fusions on the left side of these prophages are only weakly induced by mitomycin, and that induction is only partially prevented by overproduced LexA. It is possible that induction of Gifsy phages may require two signals for full induction. The system-repressing genes on the left side in Fig. 7 may not be induced in a LecA-dependent manner.
FIG. 7.
Genetic map of the Gifsy phages, with the damage-inducible (din) insertions indicated. Allele numbers point to the site of a din::MudJ insertion. Alleles that are strongly repressed by overexpression of LexA are boxed. Open reading frames in black are nearly identical in both phages, patterned open reading frames show little similarity between Gifsy-1 and Gifsy-2. Nomenclature is as for lambda-like phages: int, integration; xis, excision; red, recombination; imm, immunity; b region, nonessential genes. The positions of the phages in the Salmonella genome are shown in centisomes (cs). (Basic diagram kindly provided by Lionello Bossi and Nara Figueroa.)
Null lexA mutations are not strong mutators even though they do cause induction the SOS regulon and thereby the error-prone polymerases DinB and UmuC. This suggests that both polymerases require an additional second factor to activate their mutagenic ability. For UmuCD, the second factor is known to be activation of the UmuCD enzyme by RecA-mediated cleavage of the UmuD protein (3, 25, 37, 57). The second factor for DinB must be different, since umuCD mutations prevent virtually all UV mutagenesis in both E. coli (36, 45) and S. enterica (25). The second factor required by DinB-dependent mutagenesis is provided when a leaky lac mutant grows under selection to amplify its lac operon (20, 46, 47). Our preliminary results suggest that the second factor for DinB-dependent mutagenesis is coamplification of the dinB gene with lac (20; Slechta et al., unpublished); these genes are located close together on the F′128 plasmid (E. Kofoid, U. Bergthorsson, E. S. Slechta, and J. R. Roth, submitted for publication).
Acknowledgments
We thank Montserrat Llagostera, Xavier Garriga, Nello Bossi, Nara Figueroa, Tony Poteete, David Botstein, and John Little for strains. We also thank Nello Bossi, Nara Figueroa, and Sherwood Casjens for valuable discussions regarding the prophages found in the S. enterica genome. We thank Gail E. Christie, Virginia Commonwealth University, for communication of unpublished results. Tyrel Goodman and Shawn Gerum sequenced the din::mudJ fusions. Ulfar Bergthorsson provided guidance for the pulsed-field gel electrophoresis, and T. Wallow assisted with preparation of the Photoshop pulsed-field gel electrophoresis figure.
This work was supported in part by NIH grant GM34804.
REFERENCES
- 1.Akiyoshi, H., and N. Yamamoto. 1970. Genetic evolution of bacteriophage, II. Physical length of the homologous region in a hybrid between serologically and morphologically unrelated phages. Proc. Natl. Acad. Sci. USA 66:385-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, R. P., and J. R. Roth. 1981. Spontaneous tandem genetic duplications in Salmonella typhimurium arise by unequal recombination between ribosomal RNA (rrn) cistrons. Proc. Natl. Acad. Sci. USA 78:3113-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagg, A., C. J. Kenyon, and G. C. Walker. 1981. Inducibility of a gene product required for UV and chemical mutagenesis in Escherichia coli. Proc. Natl. Acad. Sci. USA 78:5749-5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson, N. R., R. M. Wong, and M. McClelland. 2000. Analysis of the SOS response in Salmonella enterica serovar Typhimurium with RNA fingerprinting by arbitrarily primed PCR. J. Bacteriol. 182:3490-3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergthorsson, U., and H. Ochman. 1995. Heterogeneity of genome sizes among natural isolates of Escherichia coli. J. Bacteriol. 177:5784-5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berkowitz, D., J. M. Hushon, H. J. Whitfield, J. Roth, and B. N. Ames. 1968. Procedure for identifying nonsense mutations. J. Bacteriol. 96:215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bochner, B. R., H. C. Huang, G. L. Schieven, and B. N. Ames. 1980. Positive selection for loss of tetracycline resistance. J. Bacteriol. 143:926-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brumby, A. M., I. Lamont, I. B. Dodd, and J. B. Egan. 1996. Defining the SOS operon of coliphage 186. Virology 219:105-114. [DOI] [PubMed] [Google Scholar]
- 9.Cairns, J., and P. L. Foster. 1991. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics 128:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan, R. K., D. Botstein, T. Watanabe, and Y. Ogata. 1972. Specialized transduction of tetracycline resistance by phage P22 in Salmonella typhimurium. II. Properties of a high-frequency-transducing lysate. Virology 50:883-898. [DOI] [PubMed] [Google Scholar]
- 11.Chandler, M., and O. Fayet. 1993. Translational frameshifting in the control of transposition in bacteria. Mol. Microbiol 7:497-503. [DOI] [PubMed] [Google Scholar]
- 12.Cherepanov, P. P., and W. Wackernagel. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9-14. [DOI] [PubMed] [Google Scholar]
- 13.Clark, A. J. 1982. recA operator mutations and their usefulness. Biochimie 64:669-675. [DOI] [PubMed] [Google Scholar]
- 14.Clark, A. J., M. R. Volkert, L. J. Margossian, and H. Nagaishi. 1982. Effects of a recA operator mutation on mutant phenotypes conferred by lexA and recF mutations. Mutat. Res. 106:11-26. [DOI] [PubMed] [Google Scholar]
- 15.Clerch, B., X. Garriga, E. Torrents, C. M. Rosales, and M. Llagostera. 1996. Construction and characterization of two lexA mutants of Salmonella typhimurium with different UV sensitivities and UV mutabilities. J. Bacteriol. 178:2890-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 with PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis, R. W., D. Botstein, and J. R. Roth. 1980. Advanced bacterial genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 18.Figueroa-Bossi, N., E. Coissac, P. Netter, and L. Bossi. 1997. Unsuspected prophage-like elements in Salmonella typhimurium. Mol. Microbiol 25:161-173. [DOI] [PubMed] [Google Scholar]
- 19.Friedberg, E. C., G. C. Walker, and W. Siede. 1995. DNA repair and mutagenesis. ASM Press, Washington, D.C.
- 20.Hendrickson, H., E. S. Slechta, U. Bergthorsson, D. I. Andersson, and J. R. Roth. 2002. Amplification mutagenesis: evidence that growth with a selected gene amplification causes adaptive mutation and hypermutability. Proc. Natl. Acad. Sci. USA 99:2164-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermann, S. R., J. A. Miller, S. O'Neill, T. T. Tsao, R. M. Harding, and J. L. Dale. 2000. Single primer amplification of flanking sequences. BioTechniques 29:1176-1178, 1180. [DOI] [PubMed]
- 22.Hill, S. A., and J. W. Little. 1988. Allele replacement in Escherichia coli by use of a selectable marker for resistance to spectinomycin: replacement of the lexA gene. J. Bacteriol. 170:5913-5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes, K. T., and J. R. Roth. 1985. Directed formation of deletions and duplications with Mud(Ap, Lac). Genetics 109:263-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenyon, C. J., and G. C. Walker. 1980. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc. Natl. Acad. Sci. USA 77:2819-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch, W. H., T. A. Cebula, P. L. Foster, and E. Eisenstadt. 1992. UV mutagenesis in Salmonella typhimurium is umuDC dependent despite the presence of samAB. J. Bacteriol. 174:2809-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krueger, J. H., S. J. Elledge, and G. C. Walker. 1983. Isolation and characterization of Tn5 insertion mutations in the lexA gene of Escherichia coli. J. Bacteriol. 153:1368-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamont, I., A. M. Brumby, and J. B. Egan. 1989. UV induction of coliphage 186: prophage induction as an SOS function. Proc. Natl. Acad. Sci. USA 86:5492-5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Little, J. W. 1980. Isolation of recombinant plasmids and phage carrying the lexA gene of Escherichia coli K-12. Gene 10:237-247. [DOI] [PubMed] [Google Scholar]
- 29.McClelland, M., K. E. Sanderson, J. Spieth, S. W. Clifton, P. Latreille, L. Courtney, S. Porwollik, J. Ali, M. Dante, F. Du, S. Hou, D. Layman, S. Leonard, C. Nguyen, K. Scott, A. Holmes, N. Grewal, E. Mulvaney, E. Ryan, H. Sun, L. Florea, W. Miller, T. Stoneking, M. Nhan, R. Waterston, and R. K. Wilson. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852-856. [DOI] [PubMed] [Google Scholar]
- 30.McKenzie, G., P. Lee, M.-J. Lombardo, P. Hastings, and S. Rosenberg. 2001. SOS mutator DNA polymerase IV functions in adaptive mutation and not adaptive amplification. Mol. Cell 7:571-579. [DOI] [PubMed] [Google Scholar]
- 31.McKenzie, G. J., R. S. Harris, P. L. Lee, and S. M. Rosenberg. 2000. The SOS response regulates adaptive mutation. Proc. Natl. Acad. Sci. USA 97:6646-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller, J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N. Y.
- 33.Mount, D. W., J. W. Little, and S. H. Edmiston. 1980. Influence of plasmids carrying the lexA gene on DNA repair and related processes in Escherichia coli K-12. Mol. Gen. Genet 177:477-483. [DOI] [PubMed] [Google Scholar]
- 34.Murphy, K. C. 1998. Use of bacteriophage lambda recombination functions to promote gene replacement in Escherichia coli. J. Bacteriol. 180:2063-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy, K. C., K. G. Campellone, and A. R. Poteete. 2000. PCR-mediated gene replacement in Escherichia coli. Gene 246:321-330. [DOI] [PubMed] [Google Scholar]
- 36.Nohmi, T., L. Battista, A. Dodson, and G. C. Walker. 1988. RecA-mediated cleavage activates UmuD for mutagenesis: mechanistic relationship between transcriptional derepression and posttranslational activation. Proc. Natl. Acad. Sci. USA 85:1816-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nohmi, T., M. Yamada, M. Watanabe, S. Y. Murayama, and T. Sofuni. 1992. Roles of Salmonella typhimurium umuDC and samAB in UV mutagenesis and UV sensitivity. J. Bacteriol. 174:6948-6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poteete, A., and A. Fenton. 1984. Lambda rec-dependent growth and recombination of phage P22. Virology 134:161-167. [DOI] [PubMed] [Google Scholar]
- 39.Rappleye, C. A., and J. R. Roth. 1997. A Tn10 derivative (T-POP) for isolation of insertions with conditional (tetracycline-dependent) phenotypes. J. Bacteriol. 179:5827-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rayssiguier, C., D. S. Thaler, and M. Radman. 1989. The barrier to recombination between Escherichia coli and Salmonella typhimurium is disrupted in mismatch-repair mutants. Nature 342:396-401. [DOI] [PubMed] [Google Scholar]
- 41.Roberts, J. W., and R. Devoret. 1983. Lysogenic induction. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 42.Sauer, R. T., M. J. Ross, and M. Ptashne. 1982. Cleavage of the lambda and P22 repressors by RecA protein. J. Biol. Chem. 257:4458-4462. [PubMed] [Google Scholar]
- 43.Schmieger, H. 1971. A method for detection of phage mutants with altered transducing ability. Mol. Gen. Genet. 110:378-381. [DOI] [PubMed] [Google Scholar]
- 44.Shearwin, K. E., A. M. Brumby, and J. B. Egan. 1998. The Tum protein of coliphage 186 is an antirepressor. J. Biol. Chem. 273:5708-5715. [DOI] [PubMed] [Google Scholar]
- 45.Shinagawa, H., H. Iwasaki, T. Kato, and A. Nakata. 1988. RecA protein-dependent cleavage of UmuD protein and SOS mutagenesis. Proc. Natl. Acad. Sci. USA 85:1806-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slechta, S., J. Harold, D. I. Andersson, and J. R. Roth. 2002. Effect of genome position on reversion during growth under selection. Mol. Microbiol. 44:1017-1032. [DOI] [PubMed] [Google Scholar]
- 47.Slechta, S., J. Liu, D. I. Andersson, and J. R. Roth. 2002. Evidence that selected amplification of a bacterial lac frameshift allele can stimulate Lac+ reversion (adaptive mutation) with or without general hypermutability. Genetics 191:945-956. [DOI] [PMC free article] [PubMed]
- 48.Smith, C. M., Z. Arany, C. Orrego, and E. Eisenstadt. 1991. DNA damage-inducible loci in Salmonella typhimurium. J. Bacteriol. 173:3587-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Susskind, M. M., and D. Botstein. 1978. Molecular genetics of bacteriophage P22. Microbiol. Rev. 42:385-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thoms, B., and W. Wackernagel. 1987. Regulatory role of recF in the SOS response of Escherichia coli: impaired induction of SOS genes by UV irradiation and nalidixic acid in a recF mutant. J. Bacteriol. 169:1731-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uhlin, B. E., M. R. Volkert, A. J. Clark, A. Sancar, and W. D. Rupp. 1982. Nucleotide sequence of a recA operator mutation. LexA/operator-repressor binding/inducible repair. Mol. Gen. Genet. 185:251-254. [DOI] [PubMed] [Google Scholar]
- 52.Volkert, M. R., L. J. Margossian, and A. J. Clark. 1981. Evidence that rnmB is the operator of the Escherichia coli recA gene. Proc. Natl. Acad. Sci. USA 78:1786-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner, J., P. Gruz, S. R. Kim, M. Yamada, T. Matsui, R. P. Fuchs, and T. Nohmi. 1999. The dinB gene encodes a novel E. coli DNA polymerase, DNA pol IV, involved in mutagenesis. Mol. Cell 4:281-286. [DOI] [PubMed] [Google Scholar]
- 54.Wagner, J., and T. Nohmi. 2000. Escherichia coli DNA polymerase IV mutator activity: genetic requirements and mutational specificity. J. Bacteriol. 182:4587-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker, G. 1996. The SOS response of Escherichia coli, p. 1400-1416. In F. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C.
- 56.Walker, G. C. 1984. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol. Rev. 48:60-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woodgate, R., and S. G. Sedgwick. 1992. Mutagenesis induced by bacterial UmuDC proteins and their plasmid homologues. Mol. Microbiol. 6:2213-2218. [DOI] [PubMed] [Google Scholar]
- 58.Woods, W. H., and J. B. Egan. 1974. Prophage induction of noninducible coliphage 186. J. Virol. 14:1349-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamoto, N. 1969. Genetic evolution of bacteriophage. I. Hybrids between unrelated bacteriophages P22 and Fels 2. Proc. Natl. Acad. Sci. USA 62:63-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yasuda, T., K. Morimatsu, T. Horii, T. Nagata, and H. Ohmori. 1998. Inhibition of Escherichia coli RecA coprotease activites by DinI. EMBO J. 17:3207-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]