Abstract
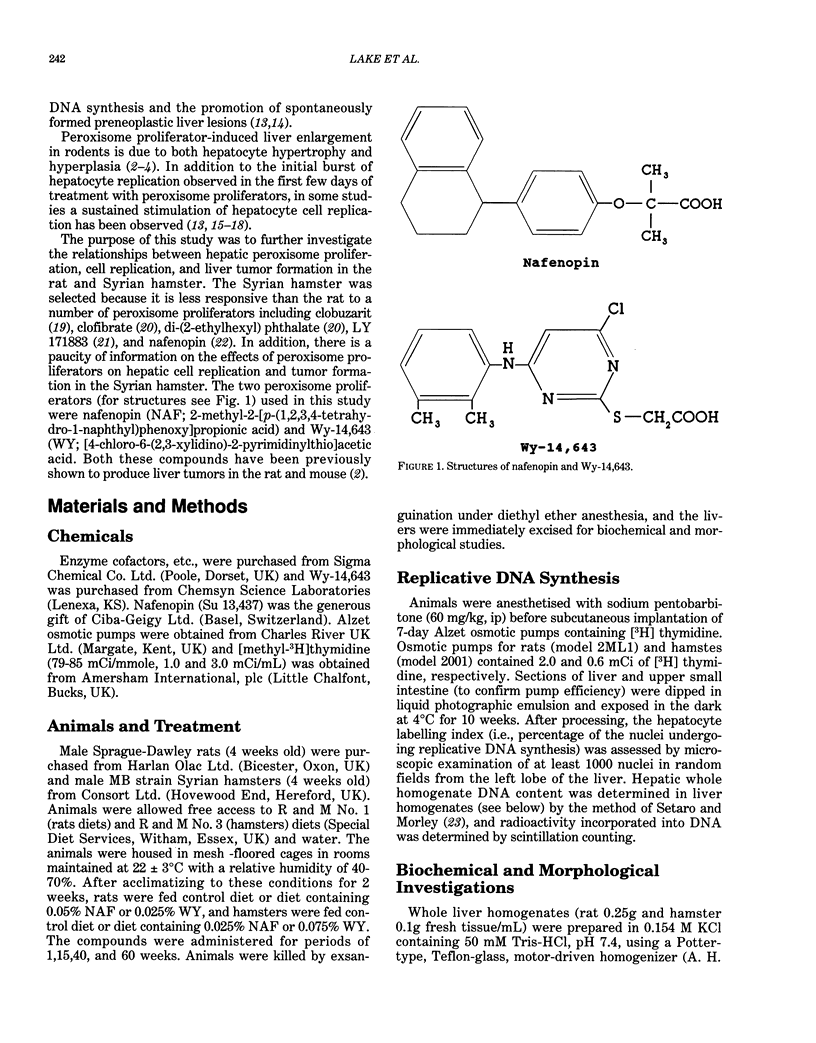
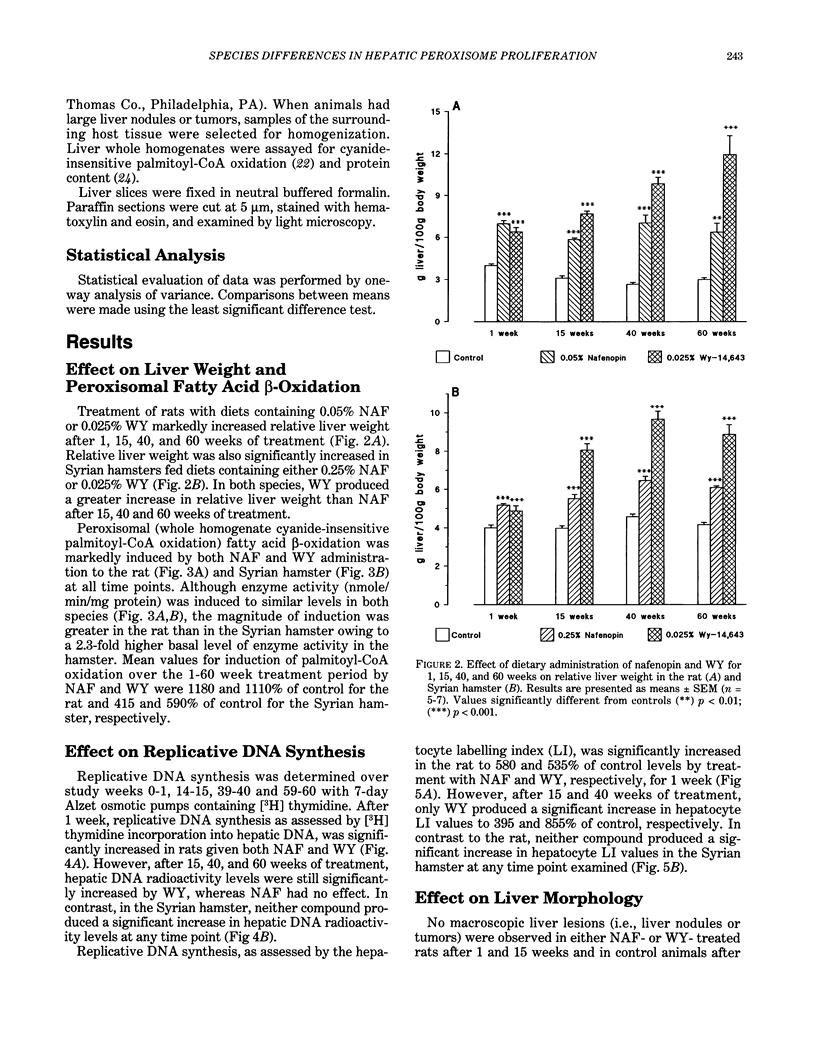
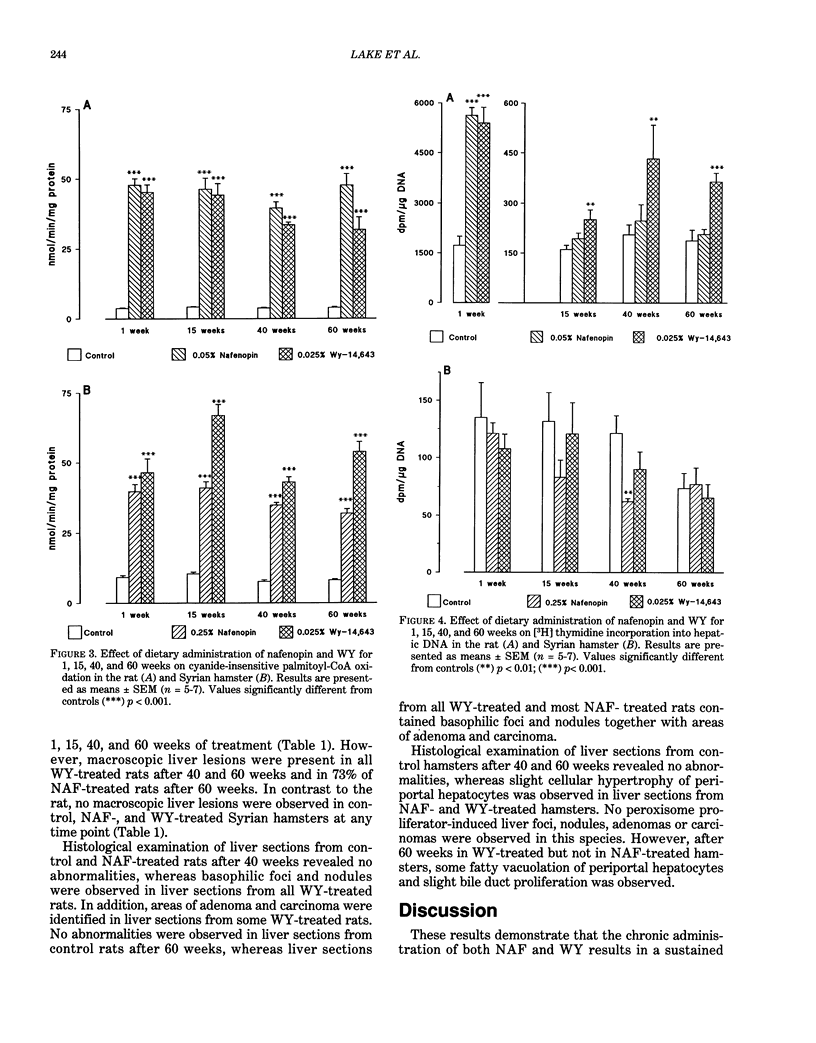
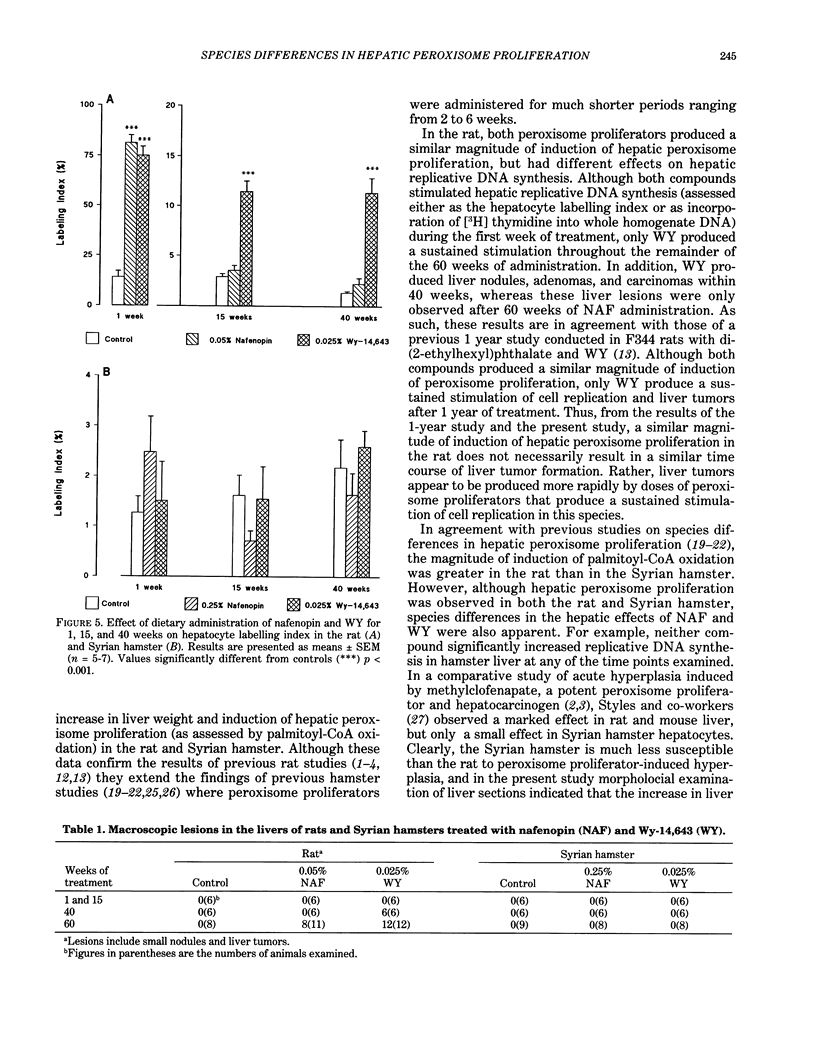
Male Sprague-Dawley rats were fed control diet or diet containing 0.05% nafenopin (NAF) or 0.025% WY-14,643 (WY) and male Syrian hamsters were fed control diet or diet containing 0.25% NAF or 0.025% WY for periods of 1, 15, 40, and 60 weeks. Both NAF and WY produced a sustained increase in liver weight and induction of peroxisomal fatty acid beta-oxidation in the rat and Syrian hamster. Replicative DNA synthesis was studied by implanting osmotic pumps containing [3H] thymidine during weeks 0-1, 14-15, 39-40, and 59-60. Cell replication, determined either as the hepatocyte labelling index or by incorporation of radioactivity into liver whole homogenate DNA, was increased in rats given NAF and WY for 1 week. However, only WY produced a sustained increased in cell replication after 15-60 weeks. After 40 weeks, liver nodules and tumors were present in WY-treated rats, and these lesions were observed in all WY-treated and some NAF-treated rats after 60 weeks. In contrast to the rat, no marked effect on replicative DNA synthesis and no liver nodules and tumors were observed in Syrian hamsters given NAF and WY for up to 60 weeks. The rat study demonstrates that liver tumors are produced more rapidly by doses of peroxisome proliferators that produce a sustained stimulation of cell replication, whereas the hamster study suggests that species differences may exist in both peroxisome proliferator-induced cell replication and liver tumor formation.
Full text
PDF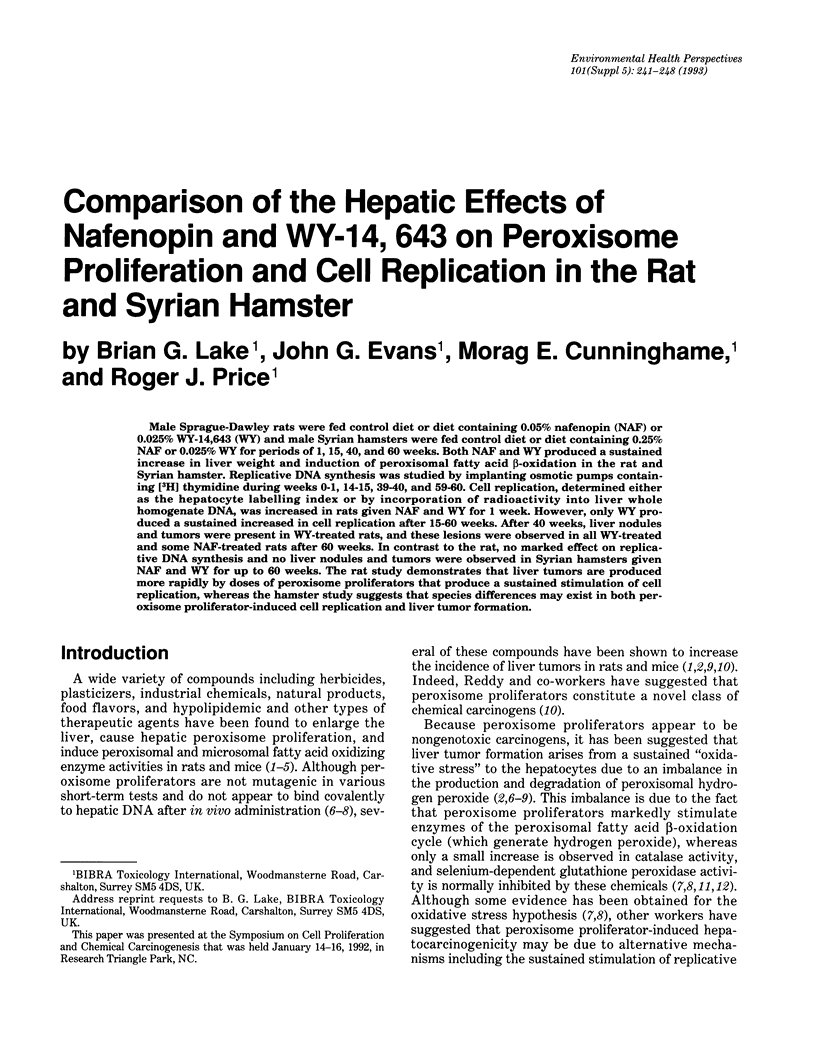


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen A. J., Grasso P. Review of the hepatic response to hypolipidaemic drugs in rodents and assessment of its toxicological significance to man. Food Cosmet Toxicol. 1981 Oct;19(5):585–605. doi: 10.1016/0015-6264(81)90509-5. [DOI] [PubMed] [Google Scholar]
- Eacho P. I., Foxworthy P. S., Johnson W. D., Hoover D. M., White S. L. Hepatic peroxisomal changes induced by a tetrazole-substituted alkoxyacetophenone in rats and comparison with other species. Toxicol Appl Pharmacol. 1986 May;83(3):430–437. doi: 10.1016/0041-008x(86)90225-5. [DOI] [PubMed] [Google Scholar]
- Eacho P. I., Lanier T. L., Brodhecker C. A. Hepatocellular DNA synthesis in rats given peroxisome proliferating agents: comparison of WY-14,643 to clofibric acid, nafenopin and LY171883. Carcinogenesis. 1991 Sep;12(9):1557–1561. doi: 10.1093/carcin/12.9.1557. [DOI] [PubMed] [Google Scholar]
- Goel S. K., Lalwani N. D., Reddy J. K. Peroxisome proliferation and lipid peroxidation in rat liver. Cancer Res. 1986 Mar;46(3):1324–1330. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lake B. G., Evans J. G., Gray T. J., Körösi S. A., North C. J. Comparative studies on nafenopin-induced hepatic peroxisome proliferation in the rat, Syrian hamster, guinea pig, and marmoset. Toxicol Appl Pharmacol. 1989 Jun 1;99(1):148–160. doi: 10.1016/0041-008x(89)90120-8. [DOI] [PubMed] [Google Scholar]
- Lake B. G., Gray T. J., Foster J. R., Stubberfield C. R., Gangolli S. D. Comparative studies on di-(2-ethylhexyl) phthalate-induced hepatic peroxisome proliferation in the rat and hamster. Toxicol Appl Pharmacol. 1984 Jan;72(1):46–60. doi: 10.1016/0041-008x(84)90248-5. [DOI] [PubMed] [Google Scholar]
- Lake B. G., Gray T. J., Smith A. G., Evans J. G. Hepatic peroxisome proliferation and oxidative stress. Biochem Soc Trans. 1990 Feb;18(1):94–97. doi: 10.1042/bst0180094. [DOI] [PubMed] [Google Scholar]
- Lake B. G., Kozlen S. L., Evans J. G., Gray T. J., Young P. J., Gangolli S. D. Effect of prolonged administration of clofibric acid and di-(2-ethylhexyl)phthalate on hepatic enzyme activities and lipid peroxidation in the rat. Toxicology. 1987 May;44(2):213–228. doi: 10.1016/0300-483x(87)90151-x. [DOI] [PubMed] [Google Scholar]
- Lock E. A., Mitchell A. M., Elcombe C. R. Biochemical mechanisms of induction of hepatic peroxisome proliferation. Annu Rev Pharmacol Toxicol. 1989;29:145–163. doi: 10.1146/annurev.pa.29.040189.001045. [DOI] [PubMed] [Google Scholar]
- Marsman D. S., Cattley R. C., Conway J. G., Popp J. A. Relationship of hepatic peroxisome proliferation and replicative DNA synthesis to the hepatocarcinogenicity of the peroxisome proliferators di(2-ethylhexyl)phthalate and [4-chloro-6-(2,3-xylidino)-2-pyrimidinylthio]acetic acid (Wy-14,643) in rats. Cancer Res. 1988 Dec 1;48(23):6739–6744. [PubMed] [Google Scholar]
- Moody D. E., Rao M. S., Reddy J. K. Mitogenic effect in mouse liver induced by a hypolipidemic drug, nafenopin. Virchows Arch B Cell Pathol. 1977 Apr 15;23(4):291–296. doi: 10.1007/BF02889139. [DOI] [PubMed] [Google Scholar]
- Moody D. E., Reddy J. K., Lake B. G., Popp J. A., Reese D. H. Peroxisome proliferation and nongenotoxic carcinogenesis: commentary on a symposium. Fundam Appl Toxicol. 1991 Feb;16(2):233–248. doi: 10.1016/0272-0590(91)90108-g. [DOI] [PubMed] [Google Scholar]
- Orton T. C., Adam H. K., Bentley M., Holloway B., Tucker M. J. Clobuzarit: species differences in the morphological and biochemical response of the liver following chronic administration. Toxicol Appl Pharmacol. 1984 Mar 30;73(1):138–151. doi: 10.1016/0041-008x(84)90062-0. [DOI] [PubMed] [Google Scholar]
- Pourbaix S., Heller F., Harvengt C. Effect of fenofibrate and LF 2151 on hepatic peroxisomes in hamsters. Biochem Pharmacol. 1984 Nov 15;33(22):3661–3666. doi: 10.1016/0006-2952(84)90154-0. [DOI] [PubMed] [Google Scholar]
- Rao M. S., Reddy J. K. An overview of peroxisome proliferator-induced hepatocarcinogenesis. Environ Health Perspect. 1991 Jun;93:205–209. doi: 10.1289/ehp.9193205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J. K., Azarnoff D. L., Hignite C. E. Hypolipidaemic hepatic peroxisome proliferators form a novel class of chemical carcinogens. Nature. 1980 Jan 24;283(5745):397–398. doi: 10.1038/283397a0. [DOI] [PubMed] [Google Scholar]
- Reddy J. K., Lalwai N. D. Carcinogenesis by hepatic peroxisome proliferators: evaluation of the risk of hypolipidemic drugs and industrial plasticizers to humans. Crit Rev Toxicol. 1983;12(1):1–58. doi: 10.3109/10408448309029317. [DOI] [PubMed] [Google Scholar]
- Reddy J. K., Lalwani N. D., Reddy M. K., Qureshi S. A. Excessive accumulation of autofluorescent lipofuscin in the liver during hepatocarcinogenesis by methyl clofenapate and other hypolipidemic peroxisome proliferators. Cancer Res. 1982 Jan;42(1):259–266. [PubMed] [Google Scholar]
- Reddy J. K., Rao M. S. Oxidative DNA damage caused by persistent peroxisome proliferation: its role in hepatocarcinogenesis. Mutat Res. 1989 Sep;214(1):63–68. doi: 10.1016/0027-5107(89)90198-x. [DOI] [PubMed] [Google Scholar]
- Reddy M. K., Lalwani N. D., Qureshi S. A., Reddy J. K. Induction of hamster hepatic peroxisomal beta-oxidation and peroxisome proliferation-associated 80000 mol. wt. polypeptide by hypolipidemic drugs. Hum Toxicol. 1982 Mar;1(2):135–147. doi: 10.1177/096032718200100205. [DOI] [PubMed] [Google Scholar]
- Schulte-Hermann R., Kraupp-Grasl B., Bursch W., Gerbracht U., Timmermann-Trosiener I. Effects of non-genotoxic hepatocarcinogens phenobarbital and nafenopin on phenotype and growth of different populations of altered foci in rat liver. Toxicol Pathol. 1989;17(4 Pt 1):642–650. doi: 10.1177/0192623389017004109. [DOI] [PubMed] [Google Scholar]
- Setaro F., Morley C. G. A modified fluorometric method for the determination of microgram quantities of DNA from cell or tissue cultures. Anal Biochem. 1976 Mar;71(1):313–317. doi: 10.1016/0003-2697(76)90043-9. [DOI] [PubMed] [Google Scholar]
- Sharma R., Lake B. G., Foster J., Gibson G. G. Microsomal cytochrome P-452 induction and peroxisome proliferation by hypolipidaemic agents in rat liver. A mechanistic inter-relationship. Biochem Pharmacol. 1988 Apr 1;37(7):1193–1201. doi: 10.1016/0006-2952(88)90770-8. [DOI] [PubMed] [Google Scholar]
- Styles J. A., Kelly M., Pritchard N. R., Elcombe C. R. A species comparison of acute hyperplasia induced by the peroxisome proliferator methylclofenapate: involvement of the binucleated hepatocyte. Carcinogenesis. 1988 Sep;9(9):1647–1655. doi: 10.1093/carcin/9.9.1647. [DOI] [PubMed] [Google Scholar]
- Yeldandi A. V., Milano M., Subbarao V., Reddy J. K., Rao M. S. Evaluation of liver cell proliferation during ciprofibrate-induced hepatocarcinogenesis. Cancer Lett. 1989 Sep 15;47(1-2):21–27. doi: 10.1016/0304-3835(89)90172-9. [DOI] [PubMed] [Google Scholar]


