Abstract
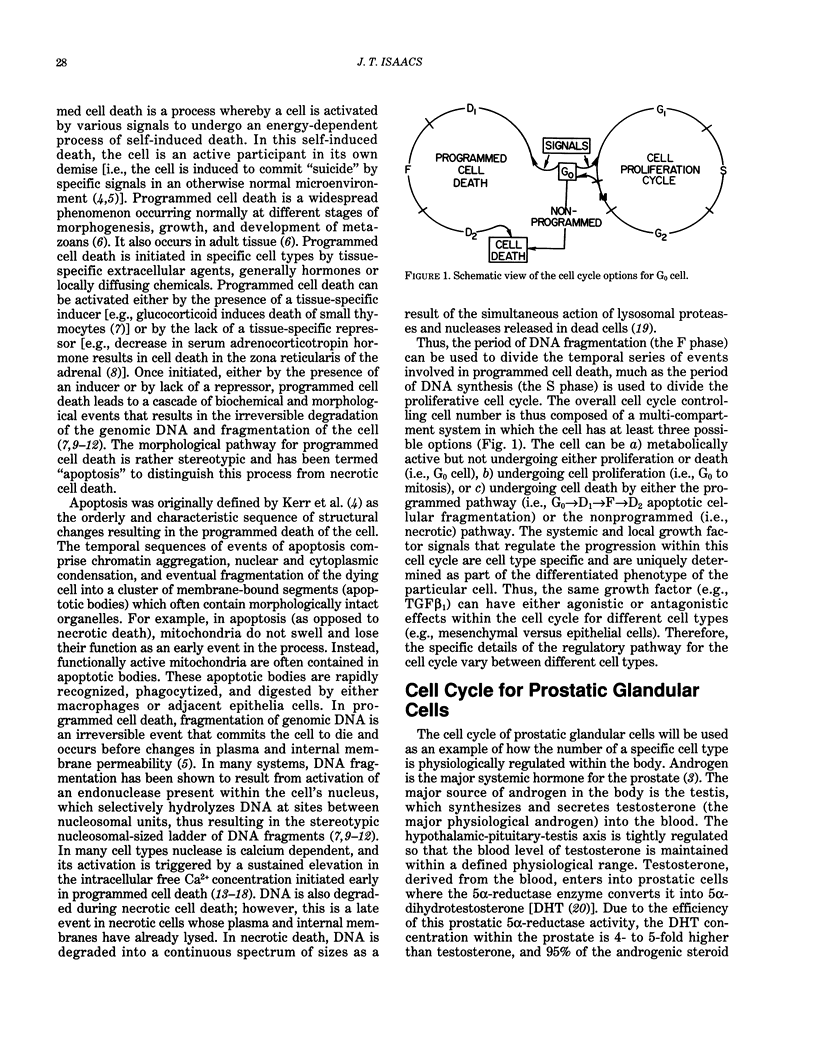
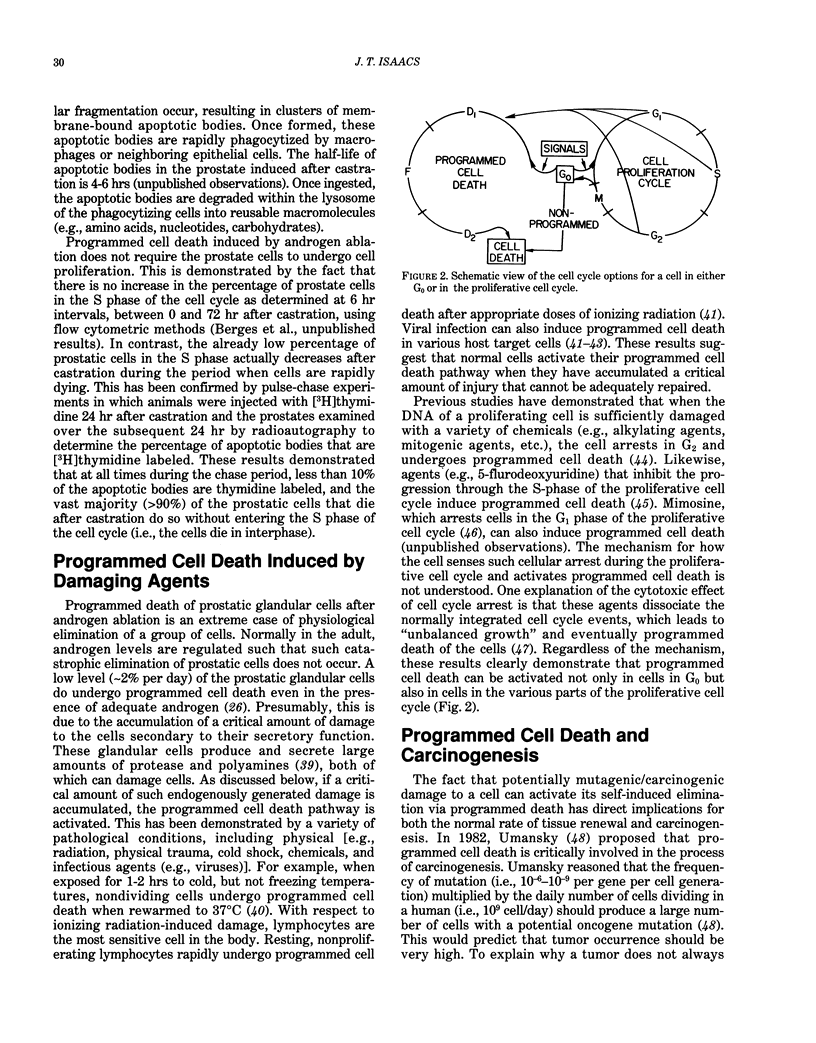
Cells possess within their repertoire of genetic programs the ability not only to proliferate and be functionally active, but also to activate and undergo a process of self-induced destruction. This process, called programmed cell death, involves a genetic reprogramming of the cell that results in an energy-dependent cascade of biochemical and morphological changes within the cell that result in its death and elimination. Activation of this programmed death process is controlled by a series of endogenous cell-type-specific signals. In addition, a variety of exogenous cell-damaging treatments (e.g., radiation, chemicals, and viruses) can activate this pathway if sufficient injury to the cell occurs. Because a cell must undergo a series of molecular changes to acquire the malignant phenotype and because these changes are often induced by agents or treatment that damage the cell over an extended period of time, anything that enhances the survival of initiated/damaged cells will promote the carcinogenic process. This paper presents an overview of the regulation and mechanism of programmed cell death and how derangement of this regulation may be involved in carcinogenesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allbritton N. L., Verret C. R., Wolley R. C., Eisen H. N. Calcium ion concentrations and DNA fragmentation in target cell destruction by murine cloned cytotoxic T lymphocytes. J Exp Med. 1988 Feb 1;167(2):514–527. doi: 10.1084/jem.167.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki S., Simada Y., Kaji K., Hayashi H. Role of protein kinase C in the inhibition by fibroblast growth factor of apoptosis in serum-depleted endothelial cells. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1081–1085. doi: 10.1016/0006-291x(90)91557-9. [DOI] [PubMed] [Google Scholar]
- Barry M. A., Behnke C. A., Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990 Nov 15;40(10):2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- Bruchovsky N. Comparison of the metabolites formed in rat prostate following the in vivo administration of seven natural androgens. Endocrinology. 1971 Nov;89(5):1212–1222. doi: 10.1210/endo-89-5-1212. [DOI] [PubMed] [Google Scholar]
- Caillet-Fauquet P., Perros M., Brandenburger A., Spegelaere P., Rommelaere J. Programmed killing of human cells by means of an inducible clone of parvoviral genes encoding non-structural proteins. EMBO J. 1990 Sep;9(9):2989–2995. doi: 10.1002/j.1460-2075.1990.tb07491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey D. S., Shimazaki J., Williams-Ashman H. G. Polymerization of deoxyribonucleotides in relation to androgen-induced prostatic growth. Arch Biochem Biophys. 1968 Mar 20;124(1):184–198. doi: 10.1016/0003-9861(68)90319-6. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- English H. F., Kyprianou N., Isaacs J. T. Relationship between DNA fragmentation and apoptosis in the programmed cell death in the rat prostate following castration. Prostate. 1989;15(3):233–250. doi: 10.1002/pros.2990150304. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Dive C., Henderson S., Smith C. A., Williams G. T., Gordon J., Rickinson A. B. Activation of Epstein-Barr virus latent genes protects human B cells from death by apoptosis. Nature. 1991 Feb 14;349(6310):612–614. doi: 10.1038/349612a0. [DOI] [PubMed] [Google Scholar]
- Henderson S., Rowe M., Gregory C., Croom-Carter D., Wang F., Longnecker R., Kieff E., Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991 Jun 28;65(7):1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. Cell. 1991 Sep 20;66(6):1071–1074. doi: 10.1016/0092-8674(91)90028-w. [DOI] [PubMed] [Google Scholar]
- Isaacs J. T. Antagonistic effect of androgen on prostatic cell death. Prostate. 1984;5(5):545–557. doi: 10.1002/pros.2990050510. [DOI] [PubMed] [Google Scholar]
- Isaacs J. T. Prostatic structure and function in relation to the etiology of prostatic cancer. Prostate. 1983;4(4):351–366. doi: 10.1002/pros.2990040405. [DOI] [PubMed] [Google Scholar]
- Kaiser N., Edelman I. S. Calcium dependence of glucocorticoid-induced lymphocytolysis. Proc Natl Acad Sci U S A. 1977 Feb;74(2):638–642. doi: 10.1073/pnas.74.2.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A. E., Benson M. C., Wise G. J., Olsson C. A., Bandyk M. G., Sawczuk I. S., Tomashefsky P., Buttyan R. Gene activity during the early phase of androgen-stimulated rat prostate regrowth. Cancer Res. 1989 Nov 1;49(21):5889–5894. [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung A. L., Zetterberg A., Sherwood S. W., Schimke R. T. Cytotoxic effects of cell cycle phase specific agents: result of cell cycle perturbation. Cancer Res. 1990 Nov 15;50(22):7307–7317. [PubMed] [Google Scholar]
- Kyprianou N., English H. F., Isaacs J. T. Activation of a Ca2+-Mg2+-dependent endonuclease as an early event in castration-induced prostatic cell death. Prostate. 1988;13(2):103–117. doi: 10.1002/pros.2990130203. [DOI] [PubMed] [Google Scholar]
- Kyprianou N., Isaacs J. T. "Thymineless" death in androgen-independent prostatic cancer cells. Biochem Biophys Res Commun. 1989 Nov 30;165(1):73–81. doi: 10.1016/0006-291x(89)91035-8. [DOI] [PubMed] [Google Scholar]
- Kyprianou N., Isaacs J. T. Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology. 1988 Feb;122(2):552–562. doi: 10.1210/endo-122-2-552. [DOI] [PubMed] [Google Scholar]
- Kyprianou N., Isaacs J. T. Expression of transforming growth factor-beta in the rat ventral prostate during castration-induced programmed cell death. Mol Endocrinol. 1989 Oct;3(10):1515–1522. doi: 10.1210/mend-3-10-1515. [DOI] [PubMed] [Google Scholar]
- Kyprianou N., Isaacs J. T. Identification of a cellular receptor for transforming growth factor-beta in rat ventral prostate and its negative regulation by androgens. Endocrinology. 1988 Oct;123(4):2124–2131. doi: 10.1210/endo-123-4-2124. [DOI] [PubMed] [Google Scholar]
- Lai Fatt R. B., Mak S. Mapping of an adenovirus function involved in the inhibition of DNA degradation. J Virol. 1982 Jun;42(3):969–977. doi: 10.1128/jvi.42.3.969-977.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalande M. A reversible arrest point in the late G1 phase of the mammalian cell cycle. Exp Cell Res. 1990 Feb;186(2):332–339. doi: 10.1016/0014-4827(90)90313-y. [DOI] [PubMed] [Google Scholar]
- Liao S., Fang S. Receptor-proteims for androgens and the mode of action of androgens on gene transcription in ventral prostate. Vitam Horm. 1969;27:17–90. doi: 10.1016/s0083-6729(08)61124-3. [DOI] [PubMed] [Google Scholar]
- Martikainen P., Isaacs J. Role of calcium in the programmed death of rat prostatic glandular cells. Prostate. 1990;17(3):175–187. doi: 10.1002/pros.2990170302. [DOI] [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Nicotera P., Orrenius S. Calcium-activated DNA fragmentation kills immature thymocytes. FASEB J. 1989 May;3(7):1843–1849. doi: 10.1096/fasebj.3.7.2497041. [DOI] [PubMed] [Google Scholar]
- McConkey D. J., Nicotera P., Hartzell P., Bellomo G., Wyllie A. H., Orrenius S. Glucocorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca2+ concentration. Arch Biochem Biophys. 1989 Feb 15;269(1):365–370. doi: 10.1016/0003-9861(89)90119-7. [DOI] [PubMed] [Google Scholar]
- Montpetit M. L., Lawless K. R., Tenniswood M. Androgen-repressed messages in the rat ventral prostate. Prostate. 1986;8(1):25–36. doi: 10.1002/pros.2990080105. [DOI] [PubMed] [Google Scholar]
- Nicotera P., Hartzell P., Davis G., Orrenius S. The formation of plasma membrane blebs in hepatocytes exposed to agents that increase cytosolic Ca2+ is mediated by the activation of a non-lysosomal proteolytic system. FEBS Lett. 1986 Dec 1;209(1):139–144. doi: 10.1016/0014-5793(86)81099-7. [DOI] [PubMed] [Google Scholar]
- Ojeda F., Guarda M. I., Maldonado C., Folch H. Protein kinase-C involvement in thymocyte apoptosis induced by hydrocortisone. Cell Immunol. 1990 Feb;125(2):535–539. doi: 10.1016/0008-8749(90)90106-2. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Rawson C., Cosola-Smith C., Barnes D. Death of serum-free mouse embryo cells caused by epidermal growth factor deprivation is prevented by cycloheximide, 12-O-tetradecanoylphorbol-13-acetate, or vanadate. Exp Cell Res. 1990 Jan;186(1):177–181. doi: 10.1016/0014-4827(90)90224-x. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Tarduchy G., López-Rivas A. Phorbol esters inhibit apoptosis in IL-2-dependent T lymphocytes. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1069–1075. doi: 10.1016/0006-291x(89)91778-6. [DOI] [PubMed] [Google Scholar]
- Saltzman A. G., Hiipakka R. A., Chang C., Liao S. Androgen repression of the production of a 29-kilodalton protein and its mRNA in the rat ventral prostate. J Biol Chem. 1987 Jan 5;262(1):432–437. [PubMed] [Google Scholar]
- Schulte-Hermann R., Timmermann-Trosiener I., Barthel G., Bursch W. DNA synthesis, apoptosis, and phenotypic expression as determinants of growth of altered foci in rat liver during phenobarbital promotion. Cancer Res. 1990 Aug 15;50(16):5127–5135. [PubMed] [Google Scholar]
- Sellins K. S., Cohen J. J. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol. 1987 Nov 15;139(10):3199–3206. [PubMed] [Google Scholar]
- Siiteri P. K., Wilson J. D. Dihydrotestosterone in prostatic hypertrophy. I. The formation and content of dihydrotestosterone in the hypertrophic prostate of man. J Clin Invest. 1970 Sep;49(9):1737–1745. doi: 10.1172/JCI106391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff B. L., Nagle W. A., Moss A. J., Jr, Henle K. J., Crawford J. T. Apoptosis induced by cold shock in vitro is dependent on cell growth phase. Biochem Biophys Res Commun. 1987 Jun 15;145(2):876–883. doi: 10.1016/0006-291x(87)91046-1. [DOI] [PubMed] [Google Scholar]
- Umansky S. R., Korol' B. A., Nelipovich P. A. In vivo DNA degradation in thymocytes of gamma-irradiated or hydrocortisone-treated rats. Biochim Biophys Acta. 1981 Aug 27;655(1):9–17. doi: 10.1016/0005-2787(81)90060-5. [DOI] [PubMed] [Google Scholar]
- Umansky S. R. The genetic program of cell death. Hypothesis and some applications: transformation, carcinogenesis, ageing. J Theor Biol. 1982 Aug 21;97(4):591–602. doi: 10.1016/0022-5193(82)90360-5. [DOI] [PubMed] [Google Scholar]
- White E., Cipriani R., Sabbatini P., Denton A. Adenovirus E1B 19-kilodalton protein overcomes the cytotoxicity of E1A proteins. J Virol. 1991 Jun;65(6):2968–2978. doi: 10.1128/jvi.65.6.2968-2978.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Grodzicker T., Stillman B. W. Mutations in the gene encoding the adenovirus early region 1B 19,000-molecular-weight tumor antigen cause the degradation of chromosomal DNA. J Virol. 1984 Nov;52(2):410–419. doi: 10.1128/jvi.52.2.410-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Sabbatini P., Debbas M., Wold W. S., Kusher D. I., Gooding L. R. The 19-kilodalton adenovirus E1B transforming protein inhibits programmed cell death and prevents cytolysis by tumor necrosis factor alpha. Mol Cell Biol. 1992 Jun;12(6):2570–2580. doi: 10.1128/mcb.12.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D., Gloyna R. E. The intranuclear metabolism of testosterone in the accessory organs of reproduction. Recent Prog Horm Res. 1970;26:309–336. doi: 10.1016/b978-0-12-571126-5.50012-1. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Macaskill I. A., Currie A. R. Adrenocortical cell deletion: the role of ACTH. J Pathol. 1973 Oct;111(2):85–94. doi: 10.1002/path.1711110203. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]


