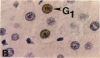
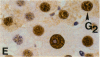
Abstract
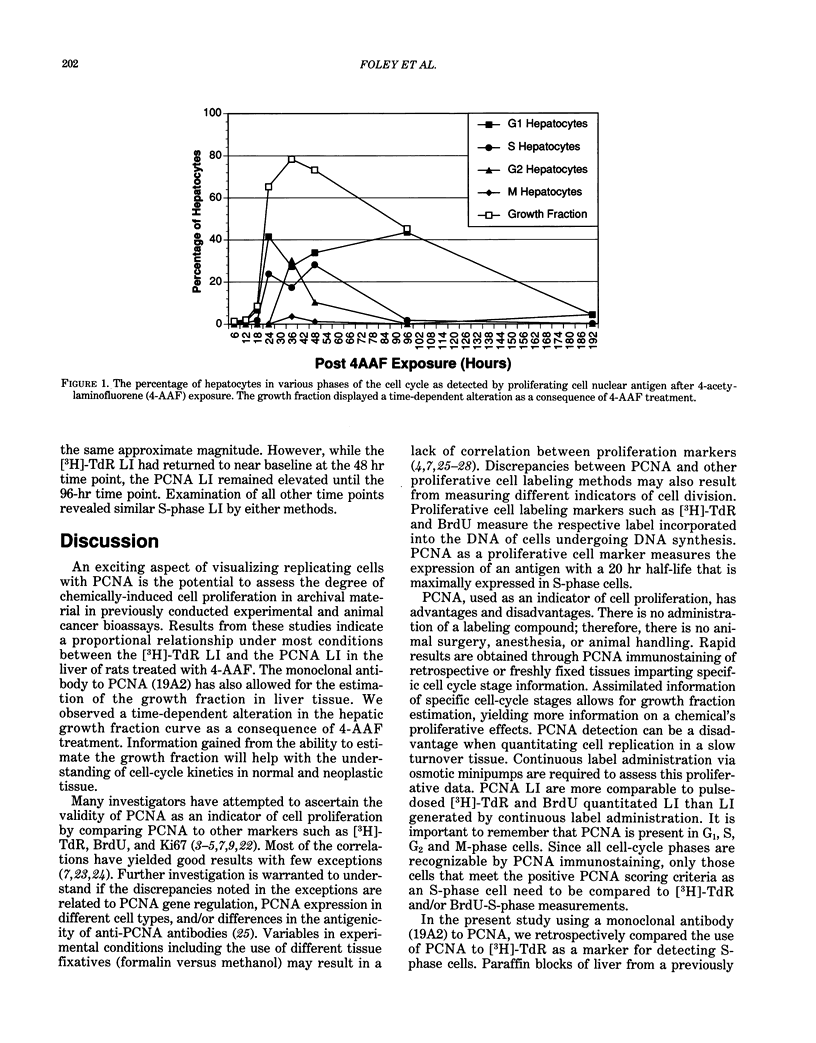
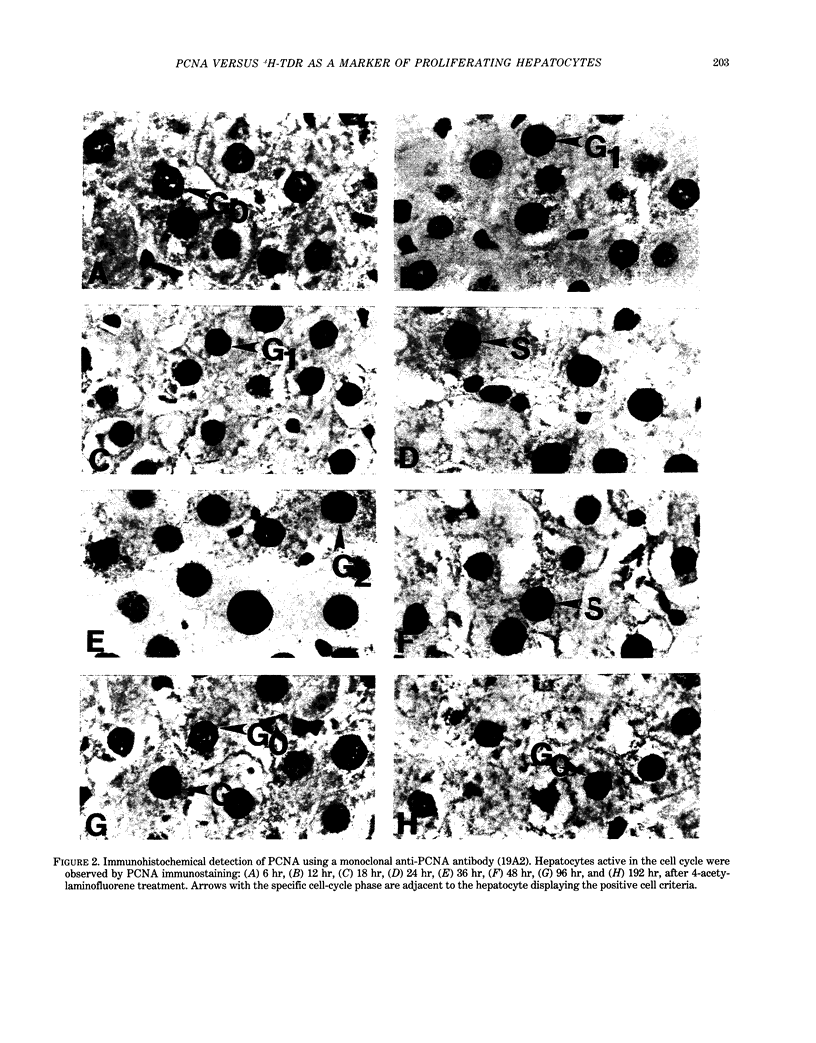
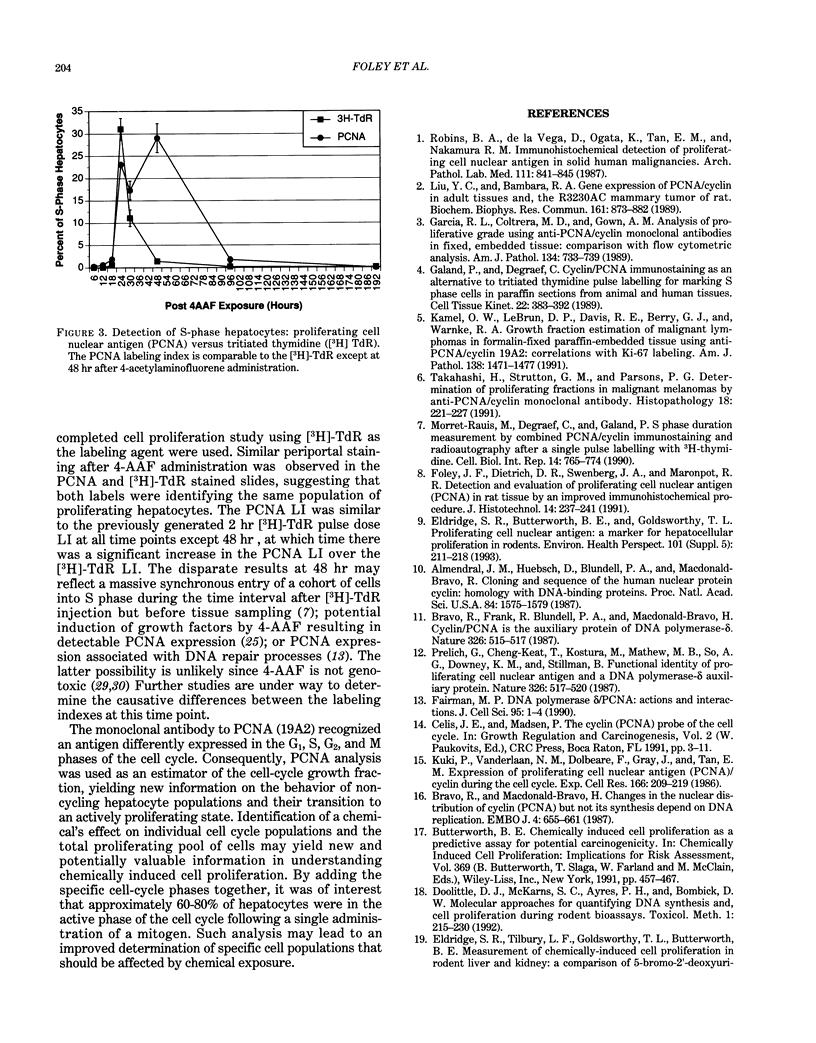
Proliferating cell nuclear antigen (PCNA), an endogenous nuclear protein, has recently been used to identify replicating cells. PCNA was compared to tritiated thymidine ([3H]-TdR), a reliable and accurate exogenous labeling agent, to ascertain if PCNA gives comparable results for quantitative cell proliferation. Male F344 rats were treated with a single dose of 500 mg/kg 4-acetylaminofluorene (4-AAF), a known liver mitogen. Rats (n = 5) were euthanized and necropsied at 6, 12, 18, 24, 36, 48, 96, or 192 hr after treatment. Two hours before necropsy, rats were pulsed-dosed with [3H]-TdR (2 mCi/kg body weight). Livers were sectioned, autoradiography performed, and labeling indexes (LI), a measurement of the percentage of S-phase hepatocytes, determined. One and a half years after the completion of this study, the archival paraffin blocks of the liver tissue were sectioned and stained for PCNA by an immunohistochemical procedure. Immunocytochemical staining patterns of proliferating cell nuclear antigen antigen expression permitted the recognition of G1, S, G2, M, and quiescent cells. PCNA LI, generated by scoring only cells exhibiting S-phase staining patterns, was compared to the pulse [3H]-TdR LI for each animal. Similar periportal staining patterns of S-phase nuclei were detected by both markers. The [3H]-TdR LI and the PCNA LI exhibited a peak at 24 hr of approximately the same magnitude. However, while the [3H]-TdR LI had returned to near baseline at the 48-hr time point, the PCNA LI remained elevated until the 96-hr time point. This sustained elevation of the PCNA index cannot be explained at this time.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Huebsch D., Blundell P. A., Macdonald-Bravo H., Bravo R. Cloning and sequence of the human nuclear protein cyclin: homology with DNA-binding proteins. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1575–1579. doi: 10.1073/pnas.84.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Bravo R., Macdonald-Bravo H. Changes in the nuclear distribution of cyclin (PCNA) but not its synthesis depend on DNA replication. EMBO J. 1985 Mar;4(3):655–661. doi: 10.1002/j.1460-2075.1985.tb03679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltrera M. D., Gown A. M. PCNA/cyclin expression and BrdU uptake define different subpopulations in different cell lines. J Histochem Cytochem. 1991 Jan;39(1):23–30. doi: 10.1177/39.1.1670579. [DOI] [PubMed] [Google Scholar]
- Eldrige S. R., Butterworth B. E., Goldsworthy T. L. Proliferating cell nuclear antigen: a marker for hepatocellular proliferation in rodents. Environ Health Perspect. 1993 Dec;101 (Suppl 5):211–218. doi: 10.1289/ehp.93101s5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairman M. P. DNA polymerase delta/PCNA: actions and interactions. J Cell Sci. 1990 Jan;95(Pt 1):1–4. doi: 10.1242/jcs.95.3.1. [DOI] [PubMed] [Google Scholar]
- Galand P., Degraef C. Cyclin/PCNA immunostaining as an alternative to tritiated thymidine pulse labelling for marking S phase cells in paraffin sections from animal and human tissues. Cell Tissue Kinet. 1989 Sep;22(5):383–392. doi: 10.1111/j.1365-2184.1989.tb00223.x. [DOI] [PubMed] [Google Scholar]
- Garcia R. L., Coltrera M. D., Gown A. M. Analysis of proliferative grade using anti-PCNA/cyclin monoclonal antibodies in fixed, embedded tissues. Comparison with flow cytometric analysis. Am J Pathol. 1989 Apr;134(4):733–739. [PMC free article] [PubMed] [Google Scholar]
- Greenwell A., Foley J. F., Maronpot R. R. An enhancement method for immunohistochemical staining of proliferating cell nuclear antigen in archival rodent tissues. Cancer Lett. 1991 Sep;59(3):251–256. doi: 10.1016/0304-3835(91)90149-c. [DOI] [PubMed] [Google Scholar]
- Hall P. A. Cell proliferation. J Pathol. 1991 Dec;165(4):349–354. doi: 10.1002/path.1711650412. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Woods A. L. Immunohistochemical markers of cellular proliferation: achievements, problems and prospects. Cell Tissue Kinet. 1990 Nov;23(6):505–522. doi: 10.1111/j.1365-2184.1990.tb01343.x. [DOI] [PubMed] [Google Scholar]
- Kamel O. W., LeBrun D. P., Davis R. E., Berry G. J., Warnke R. A. Growth fraction estimation of malignant lymphomas in formalin-fixed paraffin-embedded tissue using anti-PCNA/Cyclin 19A2. Correlation with Ki-67 labeling. Am J Pathol. 1991 Jun;138(6):1471–1477. [PMC free article] [PubMed] [Google Scholar]
- Kurki P., Vanderlaan M., Dolbeare F., Gray J., Tan E. M. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp Cell Res. 1986 Sep;166(1):209–219. doi: 10.1016/0014-4827(86)90520-3. [DOI] [PubMed] [Google Scholar]
- Morret-Rauis M., Degraef C., Galand P. S phase duration measurement by combined PCNA/cyclin immunostaining and radioautography after a single pulse-labelling with 3H-thymidine. Cell Biol Int Rep. 1990 Sep;14(9):765–774. doi: 10.1016/0309-1651(90)90003-h. [DOI] [PubMed] [Google Scholar]
- Nashed N., Chandra P. An in vivo-in vitro short term carcinogenicity test using rat peritoneal cells. Cancer Lett. 1980 Aug;10(2):95–107. doi: 10.1016/0304-3835(80)90032-4. [DOI] [PubMed] [Google Scholar]
- Prelich G., Tan C. K., Kostura M., Mathews M. B., So A. G., Downey K. M., Stillman B. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987 Apr 2;326(6112):517–520. doi: 10.1038/326517a0. [DOI] [PubMed] [Google Scholar]
- Robbins B. A., de la Vega D., Ogata K., Tan E. M., Nakamura R. M. Immunohistochemical detection of proliferating cell nuclear antigen in solid human malignancies. Arch Pathol Lab Med. 1987 Sep;111(9):841–845. [PubMed] [Google Scholar]
- Rowlands D. C., Brown H. E., Barber P. C., Jones E. L. The effect of tissue fixation on immunostaining for proliferating cell nuclear antigen with the monoclonal antibody PC10. J Pathol. 1991 Dec;165(4):356–357. doi: 10.1002/path.1711650415. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Strutton G. M., Parsons P. G. Determination of proliferating fractions in malignant melanomas by anti-PCNA/cyclin monoclonal antibody. Histopathology. 1991 Mar;18(3):221–227. doi: 10.1111/j.1365-2559.1991.tb00829.x. [DOI] [PubMed] [Google Scholar]
- Tong C., Telang S., Williams G. M. Differences in responses of 4 adult rat-liver epithelial cell lines to a spectrum of chemical mutagens. Mutat Res. 1984 Feb;130(1):53–61. doi: 10.1016/0165-1161(84)90006-2. [DOI] [PubMed] [Google Scholar]
- Waseem N. H., Lane D. P. Monoclonal antibody analysis of the proliferating cell nuclear antigen (PCNA). Structural conservation and the detection of a nucleolar form. J Cell Sci. 1990 May;96(Pt 1):121–129. doi: 10.1242/jcs.96.1.121. [DOI] [PubMed] [Google Scholar]
- van Dierendonck J. H., Wijsman J. H., Keijzer R., van de Velde C. J., Cornelisse C. J. Cell-cycle-related staining patterns of anti-proliferating cell nuclear antigen monoclonal antibodies. Comparison with BrdUrd labeling and Ki-67 staining. Am J Pathol. 1991 May;138(5):1165–1172. [PMC free article] [PubMed] [Google Scholar]