Abstract
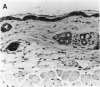
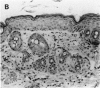
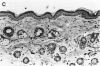
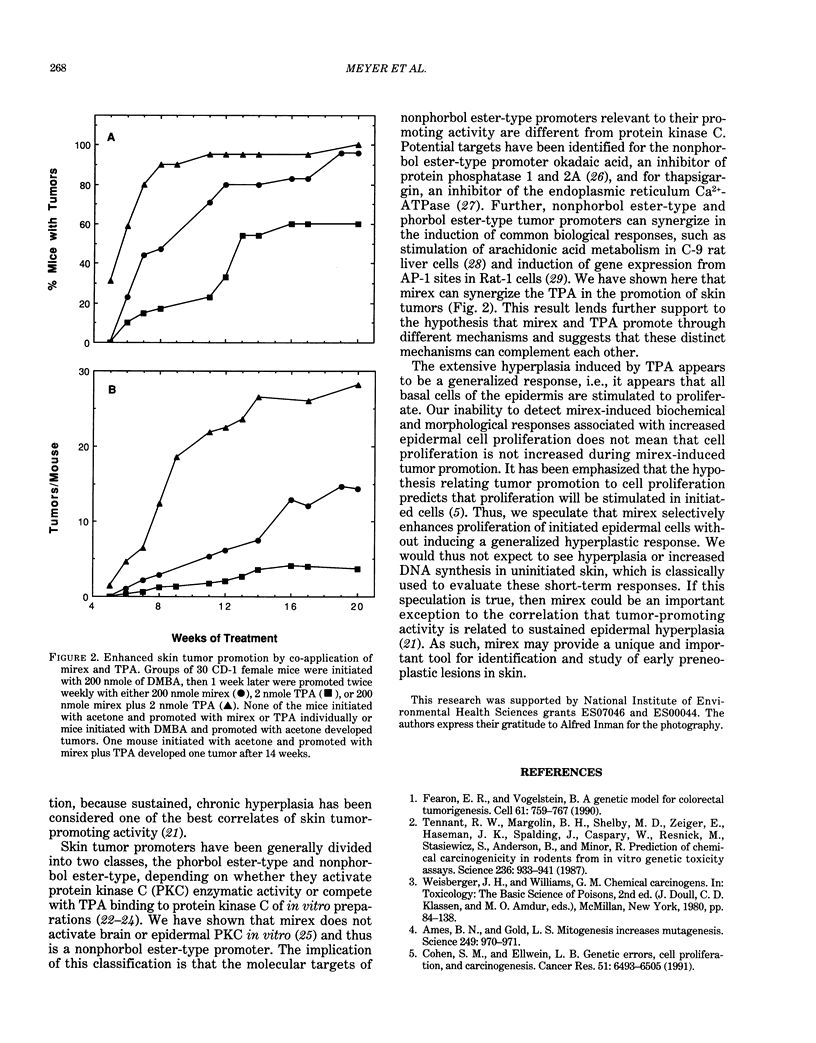
Mirex, a chlorinated hydrocarbon previously used as a systemic insecticide and flame retardant, is a nongenotoxic hepatocarcinogen in both rats and mice. In liver, mirex induced biochemical responses and hyperplasia characteristic of increased cell proliferation, which is consistent with its role as a liver tumor promoter. We have recently shown that mirex is a potent nonphorbol ester-type skin tumor promoter in 7, 12-dimethylbenz[a]anthracene (DMBA)-initiated mice. However, unlike its effect in liver, a single topical application of mirex to skin does not induce the acute biochemical responses, such as increased epidermal DNA synthesis and ornithine decarboxylase activity, indicative of increased cell proliferation. Multiple topical applications of mirex over a 1 month period induced only a minimal increase in the number of epidermal nucleated cell layers, which contrasts with definitive hyperplasia induced by a comparable tumor-promoting dose of 12-O-tetradecanoylphorbol-13-acetate (TPA). Collectively, these data indicated that mirex is promoting through a novel mechanism. Further evidence that mirex promotes tumors through a mechanism distinct from that of the prototypical skin tumor promoter, TPA, was obtained by examining the effect of their simultaneous co-treatment. The co-application of mirex and TPA yielded a tumor multiplicity greater than the sum of the responses of each promoter individually. In summary, our results demonstrate that mirex, a carcinogenic and hyperplastic agent in liver, is also a very effective tumor promoter in mouse skin, but suggest that mirex operates via a novel mechanism in skin that may involve only a minimal role for enhanced cell proliferation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argyris T. S. An analysis of the epidermal hyperplasia produced by acetic acid, a weak tumor promoter, in the skin of female mice initiated with dimethylbenzanthracene. J Invest Dermatol. 1983 May;80(5):430–435. doi: 10.1111/1523-1747.ep12555508. [DOI] [PubMed] [Google Scholar]
- Baker R. C., Coons L. B., Mailman R. B., Hodgson E. Induction of hepatic mixed function oxidases by the insecticide, mirex. Environ Res. 1972 Dec;5(4):418–424. doi: 10.1016/0013-9351(72)90043-6. [DOI] [PubMed] [Google Scholar]
- Byard J. L., Koepke U. C., Abraham R., Golberg L., Coulston F. Biochemical changes in the liver of mice fed Mirex. Toxicol Appl Pharmacol. 1975 Jul;33(1):70–77. doi: 10.1016/0041-008x(75)90245-8. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Ellwein L. B. Genetic errors, cell proliferation, and carcinogenesis. Cancer Res. 1991 Dec 15;51(24):6493–6505. [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fujiki H., Sugimura T. New classes of tumor promoters: teleocidin, aplysiatoxin, and palytoxin. Adv Cancer Res. 1987;49:223–264. doi: 10.1016/s0065-230x(08)60799-x. [DOI] [PubMed] [Google Scholar]
- Gaines T. B., Kimbrough R. D. Oral toxicity of mirex in adult and suckling rats. With notes on the ultrastructure of liver changes. Arch Environ Health. 1970 Jul;21(1):7–14. doi: 10.1080/00039896.1970.10667184. [DOI] [PubMed] [Google Scholar]
- Hakii H., Fujiki H., Suganuma M., Nakayasu M., Tahira T., Sugimura T., Scheuer P. J., Christensen S. B. Thapsigargin, a histamine secretagogue, is a non-12-O-tetradecanoylphorbol-13-acetate (TPA) type tumor promoter in two-stage mouse skin carcinogenesis. J Cancer Res Clin Oncol. 1986;111(3):177–181. doi: 10.1007/BF00389230. [DOI] [PubMed] [Google Scholar]
- Haystead T. A., Sim A. T., Carling D., Honnor R. C., Tsukitani Y., Cohen P., Hardie D. G. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989 Jan 5;337(6202):78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- Innes J. R., Ulland B. M., Valerio M. G., Petrucelli L., Fishbein L., Hart E. R., Pallotta A. J., Bates R. R., Falk H. L., Gart J. J. Bioassay of pesticides and industrial chemicals for tumorigenicity in mice: a preliminary note. J Natl Cancer Inst. 1969 Jun;42(6):1101–1114. [PubMed] [Google Scholar]
- Lenormand P., Muldoon L. L., Enslen H., Rodland K. D., Magun B. E. Two tumor promoters, 12-O-tetradecanoylphorbol-13-acetate and thapsigargin, act synergistically via distinct signaling pathways to stimulate gene expression. Cell Growth Differ. 1990 Dec;1(12):627–635. [PubMed] [Google Scholar]
- Levine L., Xiao D., Fujiki H. A combination of palytoxin with 1-oleoyl-2-acetyl-glycerol (OAG) or insulin or interleukin-1 synergistically stimulates arachidonic acid metabolism, but combinations of 12-O-tetradecanoylphorbol-13-acetate (TPA)-type tumor promoters with OAG do not. Carcinogenesis. 1986 Jan;7(1):99–103. doi: 10.1093/carcin/7.1.99. [DOI] [PubMed] [Google Scholar]
- Maslansky C. J., Williams G. M. Evidence for an epigenetic mode of action in organochlorine pesticide hepatocarcinogenicity: a lack of genotoxicity in rat, mouse, and hamster hepatocytes. J Toxicol Environ Health. 1981 Jul-Aug;8(1-2):121–130. doi: 10.1080/15287398109530056. [DOI] [PubMed] [Google Scholar]
- Mehendale H. M., Chen P. R., Fishbein L., Matthews H. B. Effect of mirex on the activities of various rat hepatic mixed-function oxidases. Arch Environ Contam Toxicol. 1973 Oct;1(3):245–254. doi: 10.1007/BF01985747. [DOI] [PubMed] [Google Scholar]
- Mitra A., Richards I., Kitchin K., Conolly R., Kulkarni A. P. Mirex induces ornithine decarboxylase in female rat liver. J Biochem Toxicol. 1990 Summer;5(2):119–124. doi: 10.1002/jbt.2570050207. [DOI] [PubMed] [Google Scholar]
- Moser G. J., Meyer S. A., Smart R. C. The chlorinated pesticide mirex is a novel nonphorbol ester-type tumor promoter in mouse skin. Cancer Res. 1992 Feb 1;52(3):631–636. [PubMed] [Google Scholar]
- Moser G. J., Smart R. C. Hepatic tumor-promoting chlorinated hydrocarbons stimulate protein kinase C activity. Carcinogenesis. 1989 May;10(5):851–856. doi: 10.1093/carcin/10.5.851. [DOI] [PubMed] [Google Scholar]
- Reuber M. D. Carcinomas and other lesions of the liver in mice ingesting organochlorine pesticides. Clin Toxicol. 1978;13(2):231–256. doi: 10.3109/15563657808988235. [DOI] [PubMed] [Google Scholar]
- Schulte-Hermann R. Initiation and promotion in hepatocarcinogenesis. Arch Toxicol. 1987;60(1-3):179–181. doi: 10.1007/BF00296976. [DOI] [PubMed] [Google Scholar]
- Suganuma M., Fujiki H., Suguri H., Yoshizawa S., Hirota M., Nakayasu M., Ojika M., Wakamatsu K., Yamada K., Sugimura T. Okadaic acid: an additional non-phorbol-12-tetradecanoate-13-acetate-type tumor promoter. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1768–1771. doi: 10.1073/pnas.85.6.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Margolin B. H., Shelby M. D., Zeiger E., Haseman J. K., Spalding J., Caspary W., Resnick M., Stasiewicz S., Anderson B. Prediction of chemical carcinogenicity in rodents from in vitro genetic toxicity assays. Science. 1987 May 22;236(4804):933–941. doi: 10.1126/science.3554512. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulland B. M., Page N. P., Squire R. A., Weisburger E. K., Cypher R. L. A carcinogenicity assay of Mirex in Charles River CD rats. J Natl Cancer Inst. 1977 Jan;58(1):133–140. doi: 10.1093/jnci/58.1.133. [DOI] [PubMed] [Google Scholar]
- Yarbrough J. D., Grimley J. M., Karl P. I. The relationship of ornithine decarboxylase and thymidine kinase to mirex-induced liver growth. Am J Physiol. 1986 Dec;251(6 Pt 1):G859–G865. doi: 10.1152/ajpgi.1986.251.6.G859. [DOI] [PubMed] [Google Scholar]
- Yarbrough J., Cunningham M., Yamanaka H., Thurman R., Badr M. Carbohydrate and oxygen metabolism during hepatocellular proliferation: a study in perfused livers from mirex-treated rats. Hepatology. 1991 Jun;13(6):1229–1234. [PubMed] [Google Scholar]