Abstract
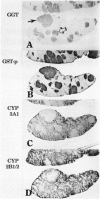
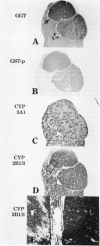
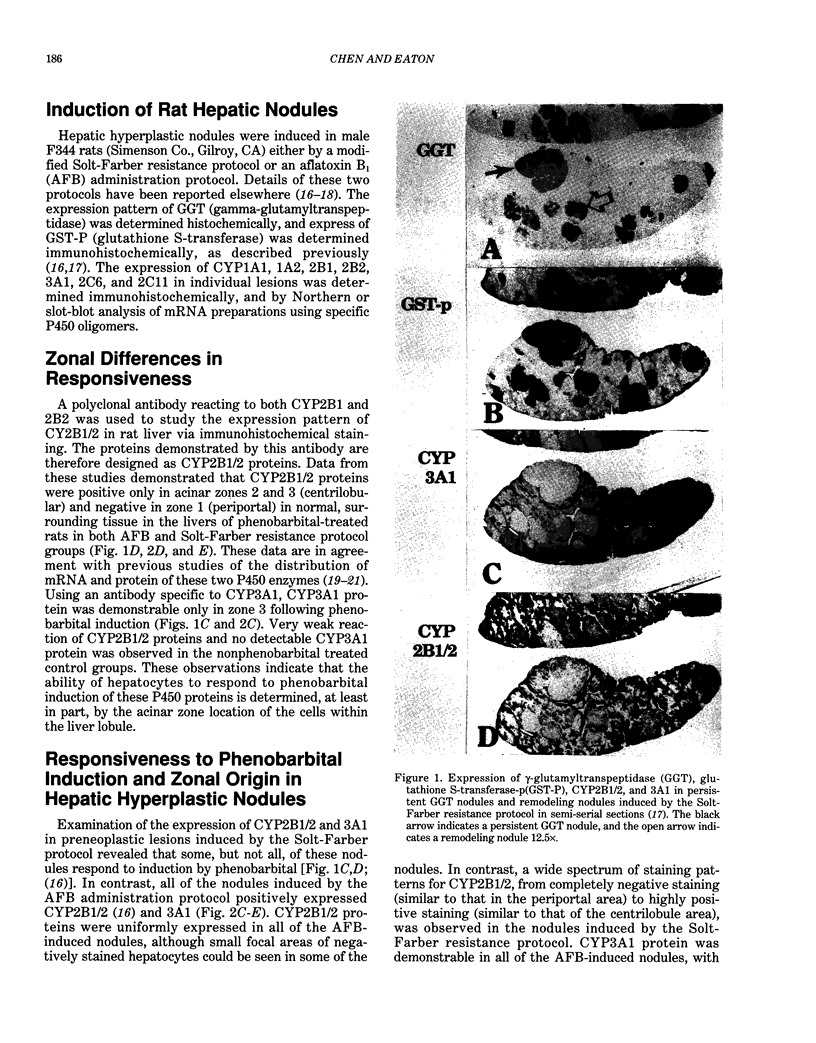
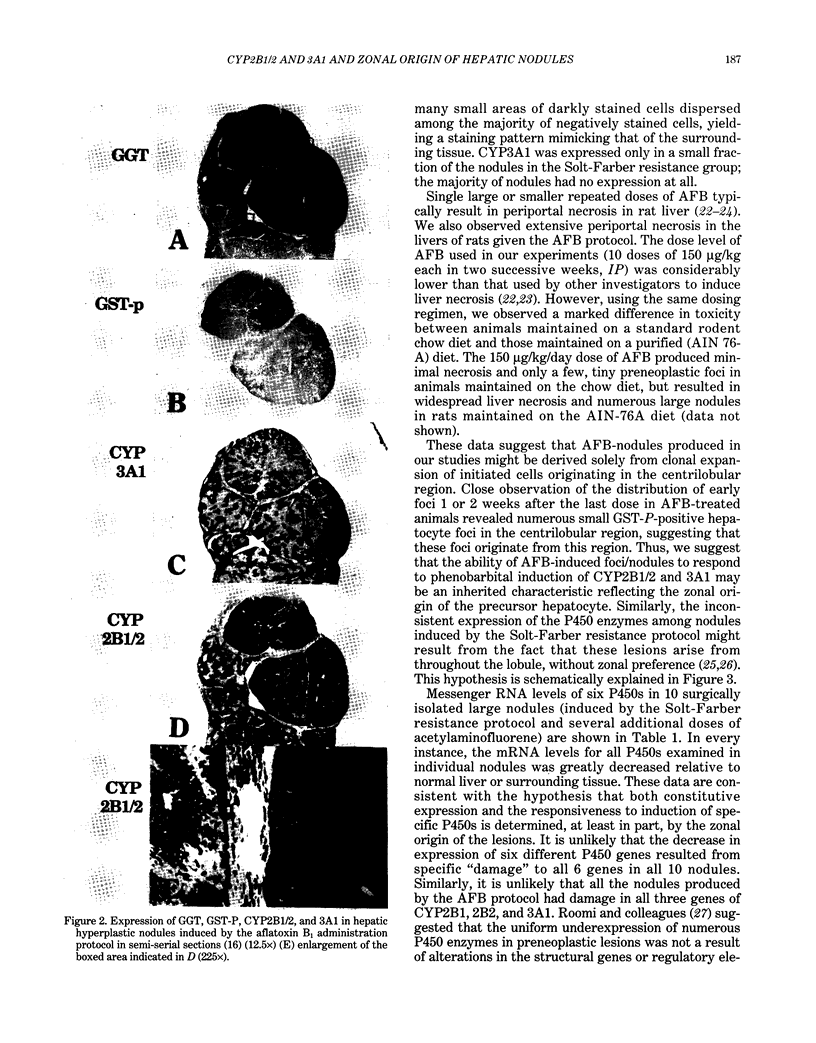
To explore further the mechanism underlying the alteration in expression of P450 enzymes in hepatic preneoplastic lesions, expression of CYP2B1/2 and 3A1 in individual hepatic hyperplastic nodules induced by an aflatoxin B1 (AFB) administration protocol and the Solt-Farber resistance protocol in male F344 rats was examined via immunohistology. In nodules induced by the resistance protocol, expression of both CYP2B1/2 and 3A1 proteins was highly variable among different nodules, whereas these P450 proteins were expressed in all nodules induced by the AFB protocol. Nodules induced by the resistance protocol have been shown previously to arise from throughout the acinar lobule. In contrast to the resistance protocol, the AFB protocol causes extensive periportal necrosis, potentially resulting in a heavy selection pressure for clonal expansion of initiated cells arising from the centrilobular area. As phenobarbital-induced expression of both CYP2B1/2 and 3A1 in normal liver is heavily localized to the centrilobular zone, these observations suggest that the ability of preneoplastic nodules to respond to induction of these P450 proteins is determined primarily from the zonal origin of the precursor hepatocytes. Thus, the nodules from the resistance protocol that express little or no CYP2B1/2 and 3A1 may have been derived from the periportal hepatocytes, whereas all the nodules in the AFB group and some of the nodules from the resistance protocol which expressed these P450 proteins in response to phenobarbital induction may have been derived from centrilobular hepatocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber N., Zajicek G., Ariel I. The streaming liver. II. Hepatocyte life history. Liver. 1988 Apr;8(2):80–87. doi: 10.1111/j.1600-0676.1988.tb00972.x. [DOI] [PubMed] [Google Scholar]
- Asamoto M., Tsuda H., Kato T., Ito N., Masuko T., Hashimoto Y., Nagase S. Strain differences in susceptibility to 2-acetylaminofluorene and phenobarbital promotion of hepatocarcinogenesis: immunohistochemical analysis of cytochrome P-450 isozyme induction by 2-acetylaminofluorene and phenobarbital. Jpn J Cancer Res. 1989 Nov;80(11):1041–1046. doi: 10.1111/j.1349-7006.1989.tb02256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTLER W. H. ACUTE TOXICITY OF AFLATOXIN B-1 IN RATS. Br J Cancer. 1964 Dec;18:756–762. doi: 10.1038/bjc.1964.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann A., Schwarz M., Schmitt R., Wolf C. R., Oesch F., Kunz W. Development of cytochrome P-450-altered preneoplastic and neoplastic lesions during nitrosamine-induced hepatocarcinogenesis in the rat. Cancer Res. 1987 Jun 1;47(11):2911–2918. [PubMed] [Google Scholar]
- Cameron R. G. Identification of the putative first cellular step of chemical hepatocarcinogenesis. Cancer Lett. 1989 Oct;47(3):163–167. doi: 10.1016/0304-3835(89)90086-4. [DOI] [PubMed] [Google Scholar]
- Cameron R., Sweeney G. D., Jones K., Lee G., Farber E. A relative deficiency of cytochrome P-450 and aryl hydrocarbon [benzo(a)pyrene] hydroxylase in hyperplastic nodules induced by 2-acetylaminofluorene in rat liver. Cancer Res. 1976 Nov;36(11 Pt 1):3888–3893. [PubMed] [Google Scholar]
- Chen Z. Y., Eaton D. L. Differential regulation of cytochrome(s) P450 2B1/2 by phenobarbital in hepatic hyperplastic nodules induced by aflatoxin B1 or diethylnitrosamine plus 2-acetylaminofluorene in male F344 rats. Toxicol Appl Pharmacol. 1991 Oct;111(1):132–144. doi: 10.1016/0041-008x(91)90142-2. [DOI] [PubMed] [Google Scholar]
- Chen Z. Y., Farin F., Omiecinski C. J., Eaton D. L. Association between growth stimulation by phenobarbital and expression of cytochromes P450 1A1, 1A2, 2B1/2 and 3A1 in hepatic hyperplastic nodules in male F344 rats. Carcinogenesis. 1992 Apr;13(4):675–682. doi: 10.1093/carcin/13.4.675. [DOI] [PubMed] [Google Scholar]
- Diwan B. A., Rice J. M., Nims R. W., Lubet R. A., Hu H., Ward J. M. P-450 enzyme induction by 5-ethyl-5-phenylhydantoin and 5,5-diethylhydantoin, analogues of barbiturate tumor promoters phenobarbital and barbital, and promotion of liver and thyroid carcinogenesis initiated by N-nitrosodiethylamine in rats. Cancer Res. 1988 May 1;48(9):2492–2497. [PubMed] [Google Scholar]
- Farber E. Cellular biochemistry of the stepwise development of cancer with chemicals: G. H. A. Clowes memorial lecture. Cancer Res. 1984 Dec;44(12 Pt 1):5463–5474. [PubMed] [Google Scholar]
- Farber E. The biochemistry of preneoplastic liver: a common metabolic pattern in hepatocyte nodules. Can J Biochem Cell Biol. 1984 Jun;62(6):486–494. doi: 10.1139/o84-066. [DOI] [PubMed] [Google Scholar]
- Gebhardt R., Tanaka T., Williams G. M. Glutamine synthetase heterogeneous expression as a marker for the cellular lineage of preneoplastic and neoplastic liver populations. Carcinogenesis. 1989 Oct;10(10):1917–1923. doi: 10.1093/carcin/10.10.1917. [DOI] [PubMed] [Google Scholar]
- Jungermann K., Katz N. Functional specialization of different hepatocyte populations. Physiol Rev. 1989 Jul;69(3):708–764. doi: 10.1152/physrev.1989.69.3.708. [DOI] [PubMed] [Google Scholar]
- Liu Y. L., Roebuck B. D., Yager J. D., Groopman J. D., Kensler T. W. Protection by 5-(2-pyrazinyl)-4-methyl-1,2-dithiol-3-thione (oltipraz) against the hepatotoxicity of aflatoxin B1 in the rat. Toxicol Appl Pharmacol. 1988 May;93(3):442–451. doi: 10.1016/0041-008x(88)90047-6. [DOI] [PubMed] [Google Scholar]
- Newberne P. M., Butler W. H. Acute and chronic effects of aflatoxin on the liver of domestic and laboratory animals: a review. Cancer Res. 1969 Jan;29(1):236–250. [PubMed] [Google Scholar]
- Okita K., Noda K., Fukumoto Y., Takemoto T. Cytochrome P-450 in hyperplastic liver nodules during hepatocarcinogenesis with N-2-fluorenylacetamide in rats. Gan. 1976 Dec;67(6):899–902. [PubMed] [Google Scholar]
- Omiecinski C. J., Walz F. G., Jr, Vlasuk G. P. Phenobarbital induction of rat liver cytochromes P-450b and P-450e. Quantitation of specific RNAs by hybridization to synthetic oligodeoxyribonucleotide probes. J Biol Chem. 1985 Mar 25;260(6):3247–3250. [PubMed] [Google Scholar]
- Peraino C., Fry R. J., Staffeldt E., Christopher J. P. Comparative enhancing effects of phenobarbital, amobarbital, diphenylhydantoin, and dichlorodiphenyltrichloroethane on 2-acetylaminofluorene-induced hepatic tumorigenesis in the rat. Cancer Res. 1975 Oct;35(10):2884–2890. [PubMed] [Google Scholar]
- Roomi M. W., Ho R. K., Sarma D. S., Farber E. A common biochemical pattern in preneoplastic hepatocyte nodules generated in four different models in the rat. Cancer Res. 1985 Feb;45(2):564–571. [PubMed] [Google Scholar]
- Schulte-Hermann R., Timmermann-Trosiener I., Schuppler J. Facilitated expression of adaptive responses to phenobarbital in putative pre-stages of liver cancer. Carcinogenesis. 1986 Oct;7(10):1651–1655. doi: 10.1093/carcin/7.10.1651. [DOI] [PubMed] [Google Scholar]
- Schulte-Hermann R. Tumor promotion in the liver. Arch Toxicol. 1985 Aug;57(3):147–158. doi: 10.1007/BF00290879. [DOI] [PubMed] [Google Scholar]
- Schwarz M., Peres G., Buchmann A., Friedberg T., Waxman D. J., Kunz W. Phenobarbital induction of cytochrome P-450 in normal and preneoplastic rat liver: comparison of enzyme and mRNA expression as detected by immunohistochemistry and in situ hybridization. Carcinogenesis. 1987 Sep;8(9):1355–1357. doi: 10.1093/carcin/8.9.1355. [DOI] [PubMed] [Google Scholar]
- Semple-Roberts E., Hayes M. A., Armstrong D., Becker R. A., Racz W. J., Farber E. Alternative methods of selecting rat hepatocellular nodules resistant to 2-acetylaminofluorene. Int J Cancer. 1987 Nov 15;40(5):643–645. doi: 10.1002/ijc.2910400512. [DOI] [PubMed] [Google Scholar]
- Solt D. B., Medline A., Farber E. Rapid emergence of carcinogen-induced hyperplastic lesions in a new model for the sequential analysis of liver carcinogenesis. Am J Pathol. 1977 Sep;88(3):595–618. [PMC free article] [PubMed] [Google Scholar]
- Tsuda H., Moore M. A., Asamoto M., Inoue T., Ito N., Satoh K., Ichihara A., Nakamura T., Amelizad Z., Oesch F. Effect of modifying agents on the phenotypic expression of cytochrome P-450, glutathione S-transferase molecular forms, microsomal epoxide hydrolase, glucose-6-phosphate dehydrogenase and gamma-glutamyltranspeptidase in rat liver preneoplastic lesions. Carcinogenesis. 1988 Apr;9(4):547–554. doi: 10.1093/carcin/9.4.547. [DOI] [PubMed] [Google Scholar]
- Wojcik E., Dvorak C., Chianale J., Traber P. G., Keren D., Gumucio J. J. Demonstration by in situ hybridization of the zonal modulation of rat liver cytochrome P-450b and P-450e gene expression after phenobarbital. J Clin Invest. 1988 Aug;82(2):658–666. doi: 10.1172/JCI113645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff G. L., Leakey J. E., Bazare J. J., Harmon J. R., Webb P. J., Law M. G. Susceptibility to phenobarbital promotion of hepatotumorigenesis: correlation with differential expression and induction of hepatic drug metabolizing enzymes in heavy and light male (C3H x VY) F1 hybrid mice. Carcinogenesis. 1991 May;12(5):911–915. doi: 10.1093/carcin/12.5.911. [DOI] [PubMed] [Google Scholar]
- Zajicek G., Oren R., Weinreb M., Jr The streaming liver. Liver. 1985 Dec;5(6):293–300. doi: 10.1111/j.1600-0676.1985.tb00252.x. [DOI] [PubMed] [Google Scholar]