Abstract
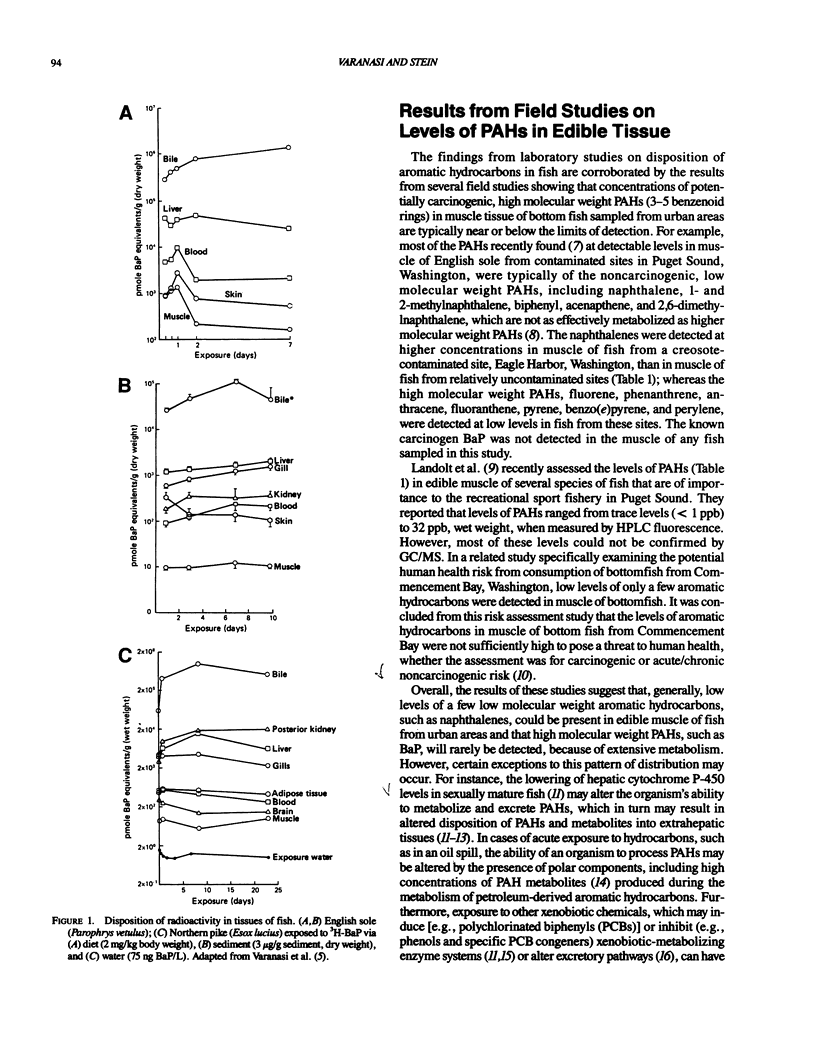
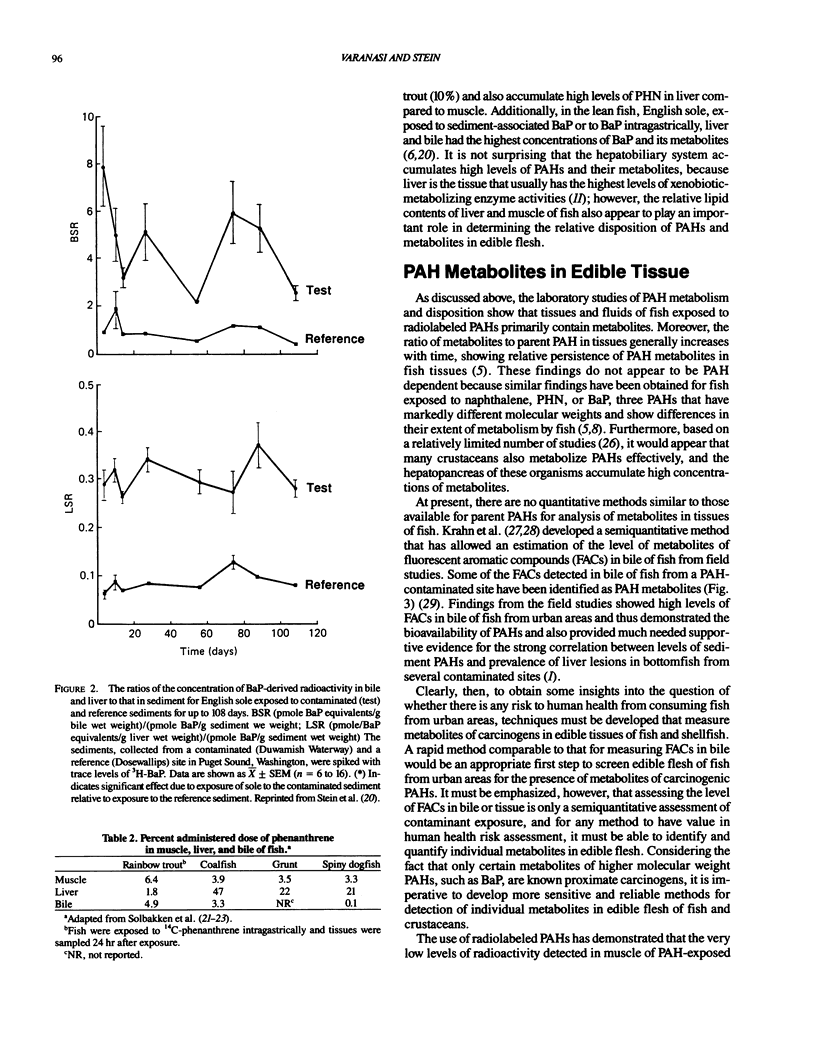
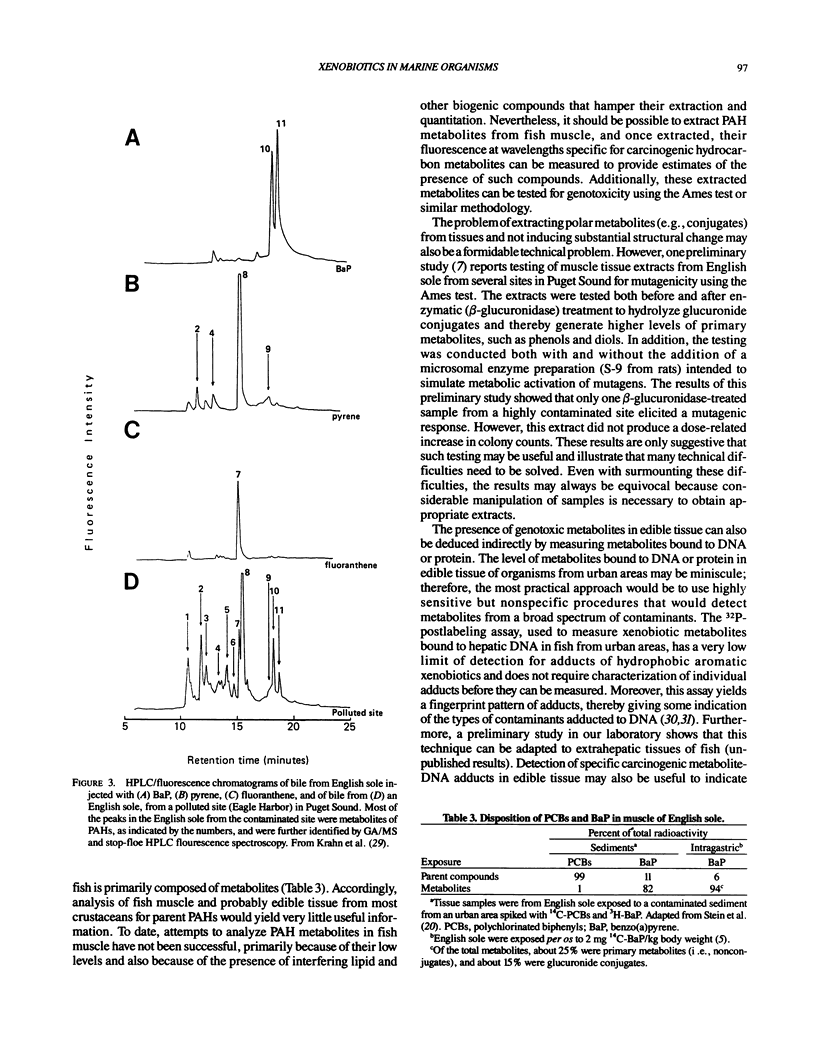
Studies with several bottom fish species from urban waterways show that of the identified xenobiotic chemicals in bottom sediments, polycylic aromatic hydrocarbons (PAHs) are the most strongly associated with the prevalence of liver lesions, including neoplasms. Accordingly, there is concern about the transfer of contaminants, such as PAHs, from aquatic species to humans. Because PAHs exert their toxicity only after being biotransformed, increasing attention has been focused on the ability of aquatic organisms to metabolize these chemicals. Overall, the results of both laboratory and field studies show that generally low levels (nanograms per gram wet weight) of a few low molecular weight PAHs may be present in edible tissue of fish from contaminated areas and that high molecular weight PAHs, such as the carcinogen benzo(a)pyrene, will rarely be detected because of extensive metabolism. Additionally, the results from a few studies suggest that even though interactions between xenobiotics can affect both biochemical and physiological systems to alter the disposition of PAHs in fish, these interactions do not markedly change the relative proportions of metabolites to parent PAH in tissues. Thus, these studies clearly demonstrate that to obtain some insight into the questions of whether there is any risk to human health from consuming fish and crustaceans from urban areas, techniques must be developed that measure metabolites of carcinogens, such as PAHs, in edible tissue. Initial attempts may focus on semiquantitative methods that permit rapid assessment of the level of metabolites in edible tissues of fish and crustaceans from many urban areas.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF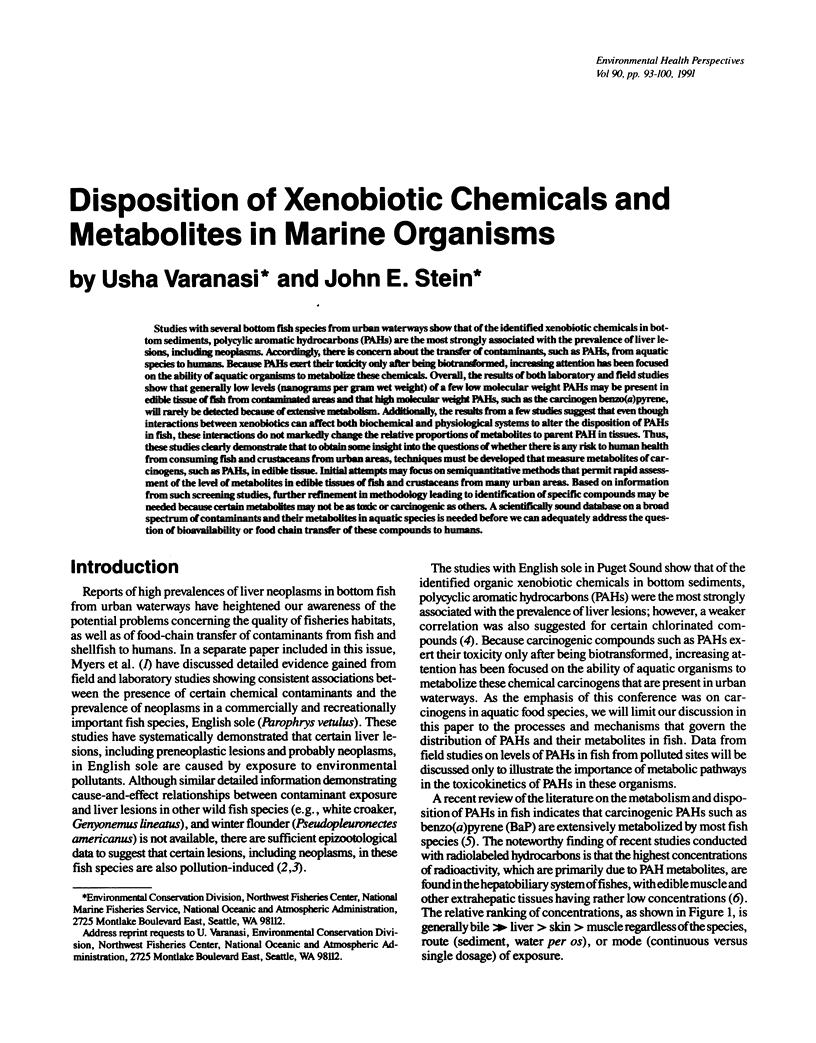



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collier T. K., Gruger E. H., Jr, Varanasi U. Effect of Aroclor 1254 on the biological fate of 2,6-dimethylnaphthalene in coho salmon (Oncorhynchus kisutch). Bull Environ Contam Toxicol. 1985 Jan;34(1):114–120. doi: 10.1007/BF01609711. [DOI] [PubMed] [Google Scholar]
- Collier T. K., Stein J. E., Wallace R. J., Varanasi U. Xenobiotic metabolizing enzymes in spawning English sole (Parophrys vetulus) exposed to organic-solvent extracts of marine sediments from contaminated and reference areas. Comp Biochem Physiol C. 1986;84(2):291–298. doi: 10.1016/0742-8413(86)90096-4. [DOI] [PubMed] [Google Scholar]
- Gooch J. W., Elskus A. A., Kloepper-Sams P. J., Hahn M. E., Stegeman J. J. Effects of ortho- and non-ortho-substituted polychlorinated biphenyl congeners on the hepatic monooxygenase system in scup (Stenotomus chrysops). Toxicol Appl Pharmacol. 1989 May;98(3):422–433. doi: 10.1016/0041-008x(89)90171-3. [DOI] [PubMed] [Google Scholar]
- Krahn M. M., Burrows D. G., MacLeod W. D., Jr, Malins D. C. Determination of individual metabolites of aromatic compounds in hydrolyzed bile of English sole (Parophrys vetulus) from polluted sites in Puget Sound, Washington. Arch Environ Contam Toxicol. 1987 Sep;16(5):511–522. doi: 10.1007/BF01055807. [DOI] [PubMed] [Google Scholar]
- Krahn M. M., Myers M. S., Burrows D. G., Malins D. C. Determination of metabolites of xenobiotics in the bile of fish from polluted waterways. Xenobiotica. 1984 Aug;14(8):633–646. doi: 10.3109/00498258409151461. [DOI] [PubMed] [Google Scholar]
- Murchelano R. A., Wolke R. E. Epizootic Carcinoma in the Winter Flounder, Pseudopleuronectes americanus. Science. 1985 May 3;228(4699):587–589. doi: 10.1126/science.228.4699.587. [DOI] [PubMed] [Google Scholar]
- Myers M. S., Landahl J. T., Krahn M. M., Johnson L. L., McCain B. B. Overview of studies on liver carcinogenesis in English sole from Puget Sound; evidence for a xenobiotic chemical etiology. I: Pathology and epizootiology. Sci Total Environ. 1990 May 1;94(1-2):33–50. doi: 10.1016/0048-9697(90)90363-y. [DOI] [PubMed] [Google Scholar]
- Plummer J. L., Smith B. R., Ball L. M., Bend J. R. Metabolism and biliary excretion of benzo[a]pyrene 4,5-oxide in the rat. Drug Metab Dispos. 1980 Mar-Apr;8(2):68–72. [PubMed] [Google Scholar]
- Pohl R. J., Bend J. R., Guarino A. M., Fouts J. R. Hepatic microsomal mixed-function oxidase activity of several marine species from coastal Maine. Drug Metab Dispos. 1974 Nov-Dec;2(6):545–555. [PubMed] [Google Scholar]
- Schnell J. V., Gruger E. H., Jr, Malins D. C. Mono-oxygenase activities of coho salmon (Oncorhynchus kisutch) liver microsomes using three polycyclic aromatic hydrocarbon substrates. Xenobiotica. 1980 Mar;10(3):229–234. doi: 10.3109/00498258009033749. [DOI] [PubMed] [Google Scholar]
- Solbakken J. E., Knap A. H., Palmork K. H. Disposition of (9-14C) phenanthrene in a subtropical marine teleost (Haemulon sciurus). Bull Environ Contam Toxicol. 1982 Mar;28(3):285–289. doi: 10.1007/BF01608509. [DOI] [PubMed] [Google Scholar]
- Solbakken J. E., Palmork K. H. Distribution of radioactivity in the chondrichthyes Squalus acanthias and the osteichthyes Salmo gairdneri following intragastric administration of (9-14C)phenanthrene. Bull Environ Contam Toxicol. 1980 Dec;25(6):902–908. doi: 10.1007/BF01985628. [DOI] [PubMed] [Google Scholar]
- Solbakken J. E., Palmork K. H., Neppelberg T., Scheline R. R. Distribution of radioactivity in coalfish (Pollachius virens) following intragastric administration of [9-14C] phenanthrene. Bull Environ Contam Toxicol. 1979 Sep;23(1-2):100–103. doi: 10.1007/BF01769924. [DOI] [PubMed] [Google Scholar]
- Statham C. N., Elcombe C. R., Szyjka S. P., Lech J. J. Effect of polycyclic aromatic hydrocarbons on hepatic microsomal enzymes and disposition of methylnaphthalene in rainbow trout in vivo. Xenobiotica. 1978 Feb;8(2):65–71. doi: 10.3109/00498257809060385. [DOI] [PubMed] [Google Scholar]
- Varanasi U., Nishimoto M., Reichert W. L., Stein J. E. Metabolism and subsequent covalent binding of benzo[a]pyrene to macromolecules in gonads and liver of ripe english sole (Parophrys vetulus). Xenobiotica. 1982 Jul;12(7):417–425. doi: 10.3109/00498258209052483. [DOI] [PubMed] [Google Scholar]
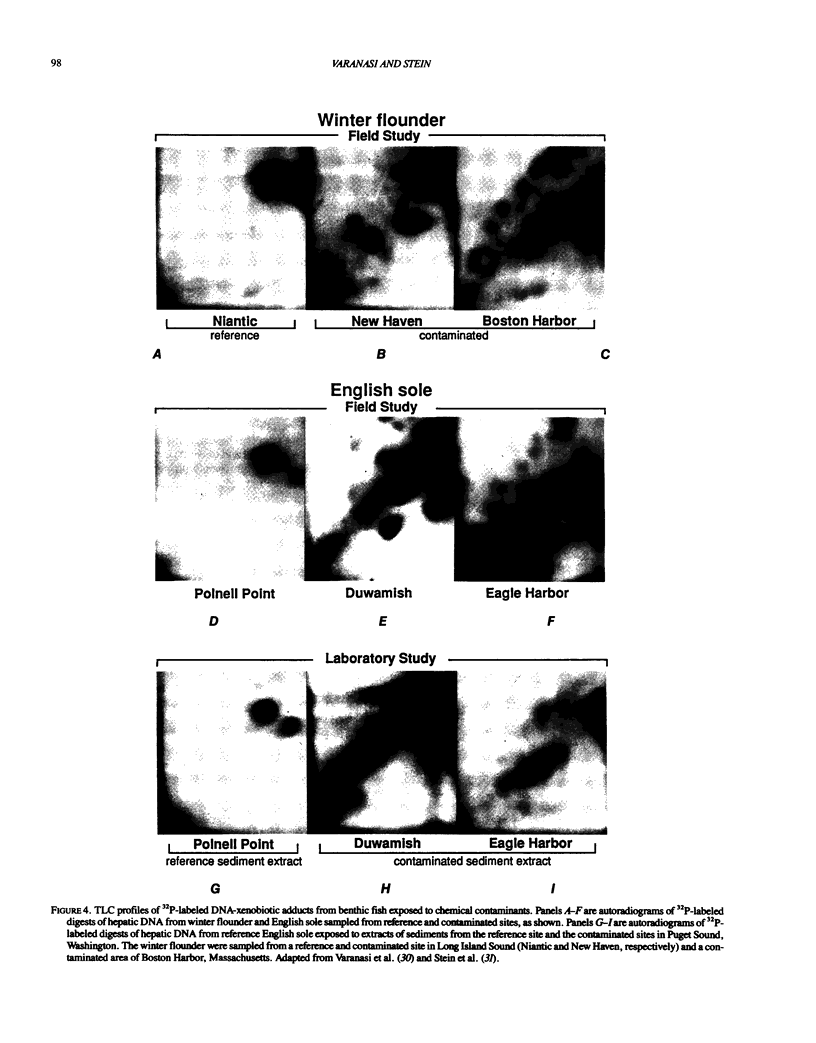
- Varanasi U., Reichert W. L., Stein J. E. 32P-postlabeling analysis of DNA adducts in liver of wild English sole (Parophrys vetulus) and winter flounder (Pseudopleuronectes americanus). Cancer Res. 1989 Mar 1;49(5):1171–1177. [PubMed] [Google Scholar]





