Abstract
A new pathway for aerobic benzoate oxidation has been postulated for Azoarcus evansii and for a Bacillus stearothermophilus-like strain. Benzoate is first transformed into benzoyl coenzyme A (benzoyl-CoA), which subsequently is oxidized to 3-hydroxyadipyl-CoA and then to 3-ketoadipyl-CoA; all intermediates are CoA thioesters. The genes coding for this benzoate-induced pathway were investigated in the β-proteobacterium A. evansii. They were identified on the basis of N-terminal amino acid sequences of purified benzoate metabolic enzymes and of benzoate-induced proteins identified on two-dimensional gels. Fifteen genes probably coding for the benzoate pathway were found to be clustered on the chromosome. These genes code for the following functions: a putative ATP-dependent benzoate transport system, benzoate-CoA ligase, a putative benzoyl-CoA oxygenase, a putative isomerizing enzyme, a putative ring-opening enzyme, enzymes for β-oxidation of CoA-activated intermediates, thioesterase, and lactone hydrolase, as well as completely unknown enzymes belonging to new protein families. An unusual putative regulator protein consists of a regulator protein and a shikimate kinase I-type domain. A deletion mutant with a deletion in one gene (boxA) was unable to grow with benzoate as the sole organic substrate, but it was able to grow with 3-hydroxybenzoate and adipate. The data support the proposed pathway, which postulates operation of a new type of ring-hydroxylating dioxygenase acting on benzoyl-CoA and nonoxygenolytic ring cleavage. A β-oxidation-like metabolism of the ring cleavage product is thought to lead to 3-ketoadipyl-CoA, which finally is cleaved into succinyl-CoA and acetyl-CoA.
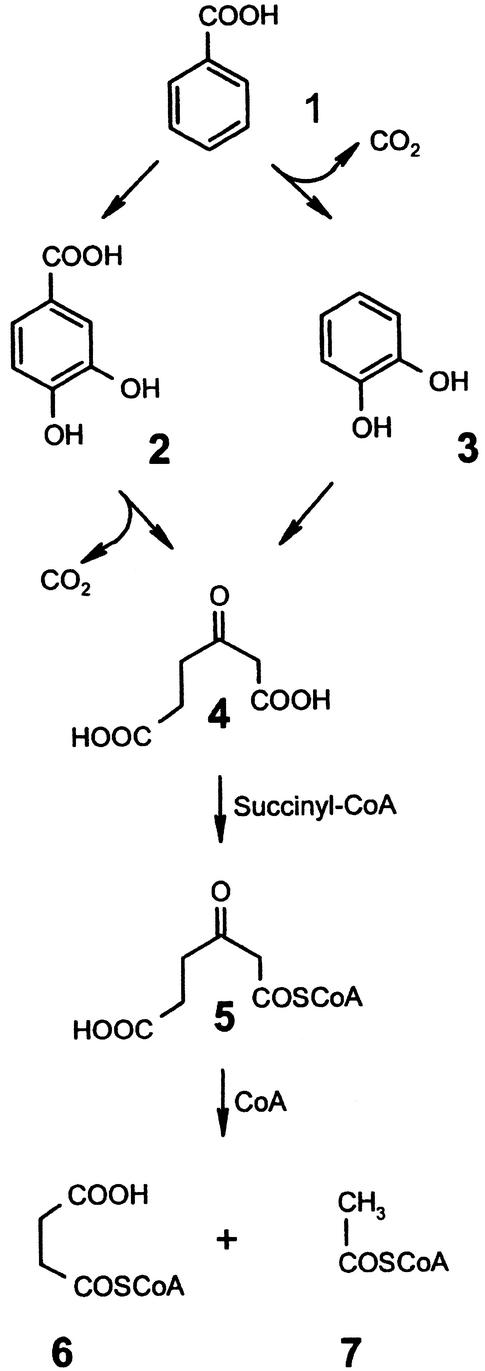
Aerobic metabolism of aromatic compounds, such as benzoate, has been studied in considerable detail in various microorganisms (45a; for a recent review, see reference 23). Catechol (1,2-dihydroxybenzene) and protocatechuate (3,4-dihydroxybenzoate) were identified as early intermediates, depending on the initial oxygenases catalyzing benzoate hydroxylation. Benzoate metabolism via 4-hydroxybenzoate and protocatechuate is common in fungi, whereas in bacteria the catechol pathway has been established and the protocatechuate route (45a) is uncertain. Both compounds serve as substrates for ring-cleaving dioxygenases, which in the case of the ortho-cleavage pathway cleave the aromatic ring between the hydroxyl groups. Catechol and protocatechuate ortho cleavage and the subsequent reactions lead to 3-ketoadipate, which is converted into succinyl coenzyme A (succinyl-CoA) and acetyl-CoA via 3-ketoadipyl-CoA (Fig. 1).
FIG. 1.
Conventional routes of microbial benzoate oxidation via ortho cleavage of catechol (in bacteria) or protocatechuate (in fungi) by the β-ketoadipate pathway (23, 45a). 1, benzoate; 2, protocatechuate; 3, catechol; 4, 3-oxoadipate (β-ketoadipate); 5, 3-oxoadipyl-CoA; 6, succinyl-CoA; 7, acetyl-CoA. Not all intermediates are shown.
However, some observations could not be explained by the established mechanisms. Thus, cell extracts of some Bacillus spp. grown on benzoate or 3-hydroxybenzoate utilized gentisate but not catechol or protocatechuate. In an attempt to explain these findings, hydroxylation of benzoate resulting in 3-hydroxybenzoate and gentisate (2,5-dihydroxybenzoate) was proposed (9, 10, 12). However, direct evidence for the suggested intermediates and reactions has not been obtained so far.
More recently, it was shown that a gram-positive Bacillus stearothermophilus-like strain (26) and the facultatively denitrifying gram-negative bacterium Azoarcus evansii, belonging to the β-group of the Proteobacteria (2, 6), are able to utilize benzoate, 3-hydroxybenzoate, and gentisate aerobically as sole sources of carbon and energy (1, 26, 32). 2-Hydroxy- and 4-hydroxybenzoates, protocatechuate, catechol, and 2,3-dihydroxybenzoate did not support aerobic growth (1, 26). In conjunction with the presence of an aerobically inducible benzoate-CoA ligase (AMP forming) and gentisate 1,2-dioxygenase (1, 42), the degradation of benzoate was proposed to proceed via benzoyl-CoA and either 2-hydroxybenzoyl-CoA or 3-hydroxybenzoyl-CoA as intermediates (1, 26, 32). Further hydroxylation of either compound hypothetically could yield gentisyl-CoA, which might undergo thioester hydrolysis to gentisate (1, 26). However, the enzymatic reactions catalyzing the proposed pathway to gentisate have remained elusive. 3-Hydroxybenzoate was shown to be metabolized via 6-hydroxylation of 3-hydroxybenzoate to gentisate, and gentisate is cleaved by gentisate 1,2-dioxygenase to maleylpyruvate. Maleylpyruvate is isomerized to fumarylpyruvate, which is cleaved into fumarate and pyruvate (1).
A study of the conversion of 13C-labeled benzoyl-CoA by cell extracts of A. evansii and the B. stearothermophilus-like strain under aerobic conditions revealed unexpected intermediates. In contrast to earlier proposals, benzoate was not converted into hydroxybenzoate or gentisate. Under aerobic conditions benzoyl-CoA was an in vivo product of benzoate catabolism in both microbial species and was converted into various CoA thioesters by cell extracts in oxygen- and NADPH-dependent reactions. By using [13C]benzoyl-CoA as a substrate, cis-3,4-dehydroadipyl-CoA, trans-2,3-dehydroadipyl-CoA, the 3,6-lactone of 3-hydroxyadipyl-CoA, and 3-hydroxyadipyl-CoA were identified as products by nuclear magnetic resonance spectroscopy. A protein mixture from A. evansii transformed benzoyl-CoA in an NADPH- and oxygen-dependent reaction into 6-hydroxy-3-hexenoyl-CoA (48). The data suggested that there is a novel aerobic pathway of benzoate catabolism via CoA intermediates leading to β-ketoadipyl-CoA, an intermediate of the known β-ketoadipate pathway (23) (Fig. 2).
FIG. 2.
Proposed new aerobic benzoate oxidation pathway in A. evansii and a B. stearothermophilus-like strain. Experimentally documented compounds are enclosed in boxes. The suggested pathway leads to β-ketoadipyl-CoA, a known intermediate of the β-ketoadipate pathway (see Fig. 1).
Hence, the committed first step in the new benzoate pathway is the activation of benzoate to benzoyl-CoA by a specifically induced benzoate-CoA ligase (AMP forming) that catalyzes the following reaction: benzoate + CoA + Mg-ATP → benzoyl-CoA + Mg-AMP + pyrophosphate. This enzyme was purified and was shown to differ from an isoenzyme that catalyzes the same reaction under anaerobic conditions (29). The second step is postulated to involve the hydroxylation of benzoyl-CoA to an unknown product by a novel benzoyl-CoA oxygenase, presumably a multicomponent enzyme system. A benzoate-induced iron-sulfur flavoprotein, BoxA, which may be a component of this system, was purified and characterized (29). This protein has a native molecular mass of 98 kDa (homodimer of 50-kDa subunits) and contains (per mole of native protein) 0.72 mol of flavin adenine dinucleotide (FAD), 10.4 to 18.4 mol of Fe, and 13.3 to 17.9 mol of acid-labile sulfur, depending on the method of protein determination. This enzyme catalyzes a benzoyl-CoA-, FAD-, and O2-dependent NADPH oxidation, surprisingly without hydroxylation of the aromatic ring. However, H2O2 is formed as follows: NADPH + H+ + O2 → NADP+ + H2O2.
The gene coding for this enzyme (boxA, for benzoyl-CoA oxidizing) was cloned and sequenced (29). This gene codes for a 46-kDa protein (414 amino acids) with two consensus amino acid sequences for two [4Fe-4S] centers at the N terminus. The deduced amino acid sequence shows homology with subunits of ferredoxin-NADP+ oxidoreductase, nitric oxide synthase, NADPH-cytochrome P450 oxidoreductase, and phenol hydroxylase. Upstream of the boxA gene another benzoate-induced gene, boxB, encoding a 55-kDa protein (473 amino acids), was found. The boxB gene exhibits the highest level of homology to an open reading frame (ORF) in Sulfolobus solfataricus (44) which probably codes for a component of a putative aerobic phenylacetyl-CoA-oxidizing system (15). In the present work we tried to find and study the missing genes involved in this novel aerobic benzoate oxidation pathway in A. evansii. Here we show that up to 15 genes are involved, some of which belong to new enzyme families.
MATERIALS AND METHODS
Materials and bacterial strains.
Chemicals were obtained from Sigma-Aldrich (Deisenhofen, Germany), Merck (Darmstadt, Germany), Biomol (Hamburg, Germany), or Roth (Karlsruhe, Germany); biochemicals were obtained from Roche Diagnostics (Mannheim, Germany) or Gerbu (Craiberg, Germany). High-pressure liquid chromatography equipment was obtained from Waters (Eschborn, Germany), Grom (Herrenberg-Kayh, Germany), and Raytest (Straubenhardt, Germany). Enzymes used for cloning experiments were purchased from MBI Fermentas (St.Leon-Rot, Germany), Roche Diagnostics, and Amersham Pharmacia Biotech (Freiburg, Germany).
A. evansii KB 740 (= DSMZ6869) (2) (formerly designated Pseudomonas sp. strain KB 740) (6) has been deposited in the Deutsche Sammlung für Mikroorganismen und Zellkulturen (Braunschweig, Germany). Escherichia coli strains XL1-blue, MRF′ {Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac [F′ proAB lac IqZΔM15 Tn10 (Tetr)]}, and SURE {e14− (mcrA) Δ(mcrCB-hsdSMR-mrr)171 endA1 supE44 thi-1 gyrA96 relA1 lac recB recJ sbcC umuC::Tn5 (kan) uvrC [F′ proAB lacIqZΔM15 Tn10 (Tetr)]} from Stratagene (Heidelberg, Germany) and strain S17-1 (45) were used for boxA gene replacement experiments with A. evansii. E. coli strains XL1-blue, SURE, and XLOLR {Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 thi-1 recA1 gyrA96 relA1 lac [F′ proAB lac IqZΔM15 Tn10 (Tetr)] Su− λr} were used for cloning and subcloning experiments. The DNA clones and vectors used are summarized in Table 1.
TABLE 1.
DNA clones and vectors used
| Clone or vector | Genes | Source or reference |
|---|---|---|
| pGEX 6P-1 | amp lacIq | Amersham Pharmacia Biotech, Freiburg, Germany |
| Supercos1 | amp neo | Stratagene, Amsterdam, The Netherlands |
| pJQ200SK | Gm, sacB traJ | 38 |
| pUC4-KSAC vector | kan ampR | Amersham Pharmacia Biotech, Freiburg, Germany |
| pBK-CMV | kan | Stratagene, Amsterdam, The Netherlands |
| pBK-CMVe5 | Includes bp 1 to 4370 of benzoate gene cluster | This study |
| pBK-CMVw17 | Includes bp 2028 to 6862 of benzoate gene cluster | This study |
| pBK-CMVcII | Includes bp 4115 to 9506 of benzoate gene cluster | This study |
| SupercosIGD11 | Includes bp 7131 to 19189 of benzoate gene cluster | This study |
Bacterial cultures.
A. evansii was grown aerobically at 37°C with benzoate as the sole source of carbon and energy (32, 46) in a 200-liter fermentor (airflow, 100 liter/min; 200 rpm). Benzoate was added continuously when the substrate added initially (5 mM) was almost consumed. Cells were harvested in the exponential growth phase at an optical density at 578 nm of 2.3, which corresponded to 0.6 g (dry weight) of cells/liter. The culture was cooled to 15°C, and cells were harvested by continuous-flow centrifugation. The yield was 200 g (wet weight) of cells/mol of benzoate. For comparative experiments with the wild type and the boxA mutant, cells were grown in shaker flasks containing 400 ml of medium. Cells were harvested by centrifugation in the exponential growth phase at an optical density at 578 nm of 0.3 to 0.4. The substrates used were benzoate (5 mM), 3-hydroxybenzoate (5 mM), phenylacetate (5 mM), adipate (10 mM), and acetate (5 mM). The combinations of substrates used were benzoate (5 mM) plus 3-hydroxybenzoate (5 mM), benzoate (5 mM) plus phenylacetate (5 mM), benzoate (5 mM) plus adipate (10 mM), and benzoate (5 mM) plus acetate (5 mM). For a negative control of immunodetection of BoxA, A. evansii was grown on malate (5 mM).
E. coli strains were grown at 37°C in Luria-Bertani medium (3, 40). Antibiotics were added to E. coli cultures as follows (final concentrations): ampicillin, 50 μg ml−1; kanamycin, 50 μg ml−1; and gentamicin, 15 μg ml−1.
Preparation of cell extracts.
All steps used for preparation of cell extracts were performed at 4°C. Frozen cells were suspended in an equal volume of water containing 0.1 mg of DNase I ml−1. The suspensions were passed through a French pressure cell at 132 MPa and then centrifuged (100,000 × g). For comparative experiments with the wild type and the boxA mutant, 0.6 ml of cold cell suspension was disrupted by grinding with 1.2 g of glass beads (diameter, 0.1 to 0.25 mm) in a mixer mill (MM 200; Retsch, Haan, Germany) for 7 min at 30 Hz.
Construction of A. evansii gene banks.
A cosmid library of A. evansii was constructed as described by Redenbach et al. (39). A λZAP Express gene library was constructed as described in the ZAP Express cloning kit instruction manual (Stratagene).
Screening of cosmid and λZAP Express gene banks.
PCR products used as probes in screening analyses were labeled with digoxigenin-11-dUTP by PCR. They were detected by using anti-digoxigenin-AP Fab fragments, nitroblue tetrazolium chloride, and 5-chloro-4-bromo-3-indoylphosphate (toluidine salt) (Biomol, Hamburg, Germany). Probes were amplified with primers MoI and MorI, Probelilyfor and Probelilyrev, ABC-probefor and ABC-proberev, and hbahfor and hbahrev (Table 2).
TABLE 2.
Primers used
| Primer | Sequence | Usage |
|---|---|---|
| RT5′ Enoyl-for | GACACGGCGGGATCGCGGTC | RT-PCR, fragment 0 |
| Thio-for | CGACTTTTTCGCACCGAGCATC | RT-PCR, fragment 0 |
| RT-Thioesterase-for | CTCATCGCCGTGCATGCTCG | RT-PCR, fragment 1 |
| Hydro-rev | GATCCAGGGCGAGGACGAG | RT-PCR, fragment 1 |
| RT-Hydro-for | CTGCGGGAAGTCGCGCCAC | RT-PCR, fragment 2 |
| hbah-rev | GCCACATCCTGCACGGCG | RT-PCR, fragment 2 |
| RTligase-rev | GCGTTGCCGGAAGACATCGG | RT-PCR, fragment 3 |
| bindeprot-for | CTGCTCCTGCACGTACTGCTTG | RT-PCR, fragment 3 |
| RT-ligase-for | CGTGGGCGGACTCGTGCTG | RT-PCR, fragment 4 |
| ORF3-rev | TGCGCGCGCTGGCGATGTATC | RT-PCR, fragment 4 |
| paaZ-for | GTTTCGCGGCGCGCTCCTCG | RT-PCR, fragment 5 |
| Regulator-rev | CGCTTGCCGAGCGTCGGCAG | RT-PCR, fragment 5 |
| Shiki-Fus-for | GCGACAACCCGCGCGCCGTG | RT-PCR, fragment 6 |
| Shiki-Fus-rev | GTTGAGCTTCAGCTTGTAGC | RT-PCR, fragment 6 |
| Fus-boxB-for | CTGGATCTTCAACCGCCCCA | RT-PCR, fragment 7 |
| Fus-boxB-rev | GATGATGCGGCGCAGGGTCG | RT-PCR, fragment 7 |
| box b-boxA-for | GTTCGGCTTCGACTTCCGCTTC | RT-PCR, fragment 8 |
| box b-boxA-rev | GCTCGGTGGTGTCGGGCAGG | RT-PCR, fragment 8 |
| boxA-Thio-for | GCTACATCTACATCTGCGGC | RT-PCR, fragment 9 |
| NMO4 | GCGCCAGCCGATGGTGGT | RT-PCR, fragment 9 |
| Thio-Hypo-for | GCCAGGGCATCGCGCTCGCG | RT-PCR, fragment 10 |
| Thio-Hypo-rev | CTGATCGCGAGGTGCTCGTC | RT-PCR, fragment 10 |
| MoI | ATGAACGCSCCSGCSGARCA | Screening probe 1 |
| MorI | CCGAAGTASGGSAGYTCYTC | Screening probe 1 |
| Probelilyfor | CGCCGCGAGTTCATCGTAGC | Screening probe 2 |
| Probelilyrev | ACGGCTTCAGCGTCGACGTC | Screening probe 2 |
| ABC-probefor | CAGCAGCACCTTGCCGAAGG | Screening probe 3 |
| ABC-proberev | GATCCAGAAGACGCGCCTC | Screening probe 3 |
| hbahfor | GGATCAGGTTCTCCCGGAC | Screening probe 4 |
| hbahfor | GCCACATCCTGCACGGCG | Screening probe 4 |
| BoxABamHI-forw | CAGCGGATCCCGCCATCACCCGCTC | boxA mutant |
| BoxANotI-rev | ATAAGAATGCGGCCGCCGACGCATTGCGCGCGACG | boxA mutant |
RT-PCR.
Total RNA from A. evansii cells grown aerobically on benzoate and from cells grown aerobically on malate was used for reverse transcription-PCR (RT-PCR). RNA was isolated with an RNeasy total RNA kit (Qiagen, Hilden, Germany) and was separated from contaminating DNA by treatment with fast protein liquid chromatography-purified DNase I (1 U per μg of total RNA; Amersham Pharmacia Biotech) for 30 min at 37°C. Complete removal of DNA from RNA preparations was verified by amplifying the intergenic region between two benzoate catabolic genes coding in different directions with cDNA as the template. One microgram of purified total RNA was used to prepare cDNA by using avian myeloblastosis virus reverse transcriptase (20 U/μg of RNA; Amersham Pharmacia Biotech) and a mixture of completely random hexanucleotides (3.2 μg/20 μl) for random priming. Gene expression was studied by amplification of intergenic regions between ORFs. The sequences of the primers used are shown in Table 2.
DNA techniques and purification of nucleic acids.
Standard protocols were used for DNA cloning, transformation, amplification, purification, and sequencing (3, 40). Plasmid DNA was purified by the method of Birnboim and Doly (5). Both strands of the cloned chromosomal DNA containing the gene cluster were sequenced.
Computer analysis.
DNA and amino acid sequences were analyzed by using the BLAST network service at the National Center for Biotechnology Information. Alignments were generated by using the CLUSTLW program contained in the DNAman software package (Lynnon, Montreal, Canada).
Synthesis of CoA esters.
Benzoyl-CoA was prepared by previously described procedures (19, 41). The yield was 65%.
Purification of component A of benzoyl-CoA oxygenase (BoxA).
BoxA was purified by the method of Mohamed et al. (29).
Enzyme assay for BoxA.
BoxA enzyme activity was monitored spectrophotometrically at 365 nm by determining benzoyl-CoA-, oxygen-, and FAD-dependent oxidation of NADPH at 37°C. The standard assay mixture (0.5 ml) contained 100 mM Tris-HCl (pH 8.0), 0.1 mM FAD, 0.3 mM NADPH, and cell extract (10 μl of a 100,000-×-g supernatant). The test was started by adding 0.1 mM benzoyl-CoA.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Polyacrylamide (11.5%) gel electrophoresis was performed by the Laemmli method (11). Proteins were visualized by Coomassie blue staining (49).
Two-dimensional gel electrophoresis.
For two-dimensional gel electrophoresis the first dimension (isoelectric focusing) was performed with cell extract (120 μg of protein) as described by Görg et al. (17) by using the Immobiline Dry Strip system (linear gradient from pH 3 to 10; Amersham Pharmacia Biotech) according to the manufacturer's protocol. The second dimension (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) was performed as described above. A comparison of extracts of cells grown on benzoate and 3-hydroxybenzoate allowed identification of benzoate-induced proteins.
Electrophoretic transfer of protein and determination of N-terminal amino acid sequences.
Proteins of benzoate-grown cells were separated by two-dimensional gel electrophoresis and transblotted onto an Immobilon-Psq transfer membrane (Millipore, Bedford, Mass.) by using the Nova Blot system (Multiphor II; Pharmacia LKB, Freiburg, Germany) (11). Transblotted proteins were detected by Commassie blue staining (49). Benzoate-induced proteins were excised and sequenced by using an Applied Biosystems 473A sequencer.
Immunodetection.
Cell extracts of benzoate-grown and malate-grown cells were separated by two-dimensional gel electrophoresis and transblotted onto nitrocellulose filters (pore size, 0.45 μm) (11). The transblotted proteins were detected by Ponceau S staining (11). Polyclonal antibodies raised against BoxA of A. evansii were used to test for the presence of BoxA. BoxA was detected by using the Amersham enhanced chemoluminescence system.
Construction of boxA mutant.
The boxA mutant was constructed by replacement of previously cloned boxA that had been manipulated by insertion of a kanamycin resistance Geneblock (Amersham Pharmacia Biotech). Two primers were amplified upstream and downstream of the gene coding for BoxA (ORF 13); these primers carried restriction sites for BamHI and NotI, respectively (BoxABamHI-forw and BoxANotI-rev [Table 2]). The boxA gene was amplified by PCR and cloned into the pGex vector (Amersham Pharmacia Biotech) (Table 1) by using E. coli XL1-blue as the host. The kanamycin resistance cassette was cut out of the pUC4-KSAC vector (Amersham Pharmacia Biotech) with SacI and inserted into a SacI restriction site of the boxA gene in the recombinant plasmid. A DNA fragment carrying the boxA gene with the kanamycin cassette was cut out with NotI and BamHI and cloned into the sacB-containing suicide vector pJQ200SK (38) (Table 1). The resulting plasmid was transformed into E. coli S17-1 and transferred by conjugation into A. evansii (35). The mating mixture was plated on Gelrite (0.8%, wt/vol) minimal medium containing sucrose (5 mM) and kanamycin (50 μg ml−1). Exconjugants which had lost the sacB-containing vector due to double recombination were selected by screening for sucrose resistance. The presence of the desired boxA mutation was confirmed by colony PCR performed with the primers mentioned above and by Western immunoblotting, which showed that the mutant failed to produce BoxA.
Protein contents.
Protein contents were determined by the method of Bradford (11) by using bovine serum albumin as the standard.
Nucleotide sequence accession number.
The sequence data reported here have been deposited in the EMBL database under accession no. AF548005.
RESULTS AND DISCUSSION
Proteins induced by benzoate.
The ability to metabolize benzoate aerobically is induced by the substrate benzoate. Benzoate-grown cells contained 20-fold-higher specific activities of benzoate-CoA ligase and BoxA enzyme activity than 3-hydroxybenzoate-grown cells, and in acetate- or malate-grown cells these enzyme activities were hardly detectable. In contrast, the 3-hydroxybenzoate pathway was induced to similar extents in 3-hydroxybenzoate-grown cells and in benzoate-grown cells. This is consistent with similar specific activities of characteristic enzymes of 3-hydroxybenzoate metabolism in the two types of cells (i.e., 3-hydroxybenzoate 6-hydroxylase and gentisate 1,2-dioxygenase activities) (32). We therefore used 3-hydroxybenzoate-grown cells as a reference for comparison of the protein patterns. Benzoate-grown cells should differ only by additional, benzoate-induced proteins when they are compared to 3-hydroxybenzoate-grown cells.
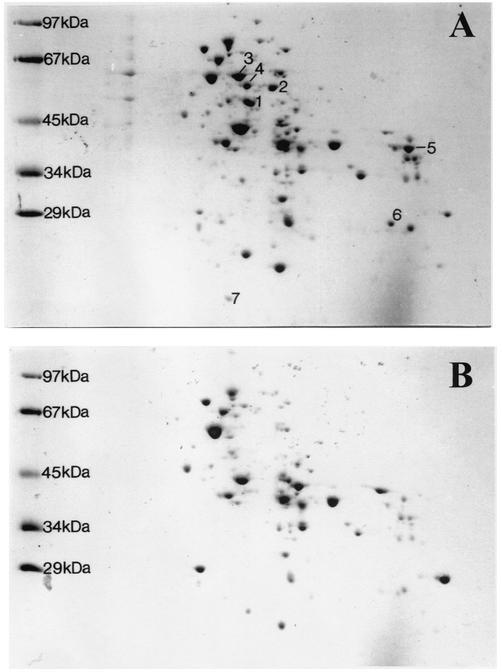
Two-dimensional gel electrophoresis of soluble cell extracts (100,000-×-g supernatant) revealed at least seven strongly benzoate-induced proteins in benzoate-grown cells, five of which were N terminally sequenced (Fig. 3). The induced protein spots were labeled protein spots 1 to 7. The proteins of three of the spots, protein spots 1, 3, and 5, could be sequenced, and two others (protein spots 2 and 4) gave no sequence, possibly due to an N-terminal block. In addition, the N-terminal sequences of two purified proteins, benzoate-CoA ligase and BoxA, were known (see above). All N-terminal amino acid sequences obtained, as well as the estimated sizes and isoelectric points of benzoate-induced proteins, are summarized in Table 3. Antibodies raised against BoxA were used to determine by Western blotting whether one of the benzoate-induced proteins was BoxA. Cells grown on malate were used as a negative control. Malate-grown cells instead of 3-hydroxybenzoate-grown cells were used in this experiment since malate-grown cells contained virtually no BoxA activity, whereas 3-hydroxybenzoate-grown cells contained approximately 5% of the fully benzoate-induced enzyme activity. This low level was still detected by the sensitive immunoassay. A faint protein spot next to benzoate-induced protein spot 1, which was not identical to protein spot 1, specifically reacted with the serum. This protein spot did not appear when cells were grown on malate. This confirmed that BoxA is another benzoate-induced protein which is present at only a low concentration.
FIG. 3.
Patterns after two-dimensional gel electrophoresis of soluble proteins of benzoate-grown cells (A) and 3-hydroxybenzoate-grown cells (B). Strongly benzoate-induced spots are indicated by numbers. See Table 3 for properties of proteins.
TABLE 3.
Properties of purified and/or benzoate-induced proteins thought to play a role in benzoate oxidationa
| Purified and/or induced protein (PRF) | Estimated molecular mass (kDa) | Estimated isoelectric point | N-terminal amino acid sequenceb | Deduced N-terminal amino acid sequence |
|---|---|---|---|---|
| Benzoate-CoA ligase (ORF 7) | 56 | 5.7 | MTTLSAADHSTSPPTItLPRQYNAAD | MTTLSAADHSTSPPTITLPRQYNAAD |
| BoxA (ORF 13) | 46 | 5.7 | MNAPAEHANLARQHLIDPE | MNAPAEHANLARQHLIDPEICIRCNT |
| Protein 1 (BoxB; ORF 12) | 50 | 5.8 | XINYSERIPN | MINYSERIPNNVNL |
| Protein 2 | 55 | 6.2 | No sequence | |
| Protein 3 (ORF 11) | 60 | 5.6 | XqAVANKPVAELvDYRtEPs | MQAVANKPVAELVDY RTEPS |
| Protein 4 | 57 | 5.7 | No sequence | |
| Protein 5 (ORF 6) | 40 | 8.7 | AEKIKVGLMLPYTGTYAALG | MKNARMNRRTLMQAMLGVIAGALVPLGAAQA AEKIKVGLMLPYTGTYAALG |
| Protein 6 (possibly ORF 3) | 28 | 8.6 | NDc | |
| Protein 7 (possibly ORF 1 or ORF 8) | 19 | 5.5 | ND |
The experimentally determined N-terminal amino acid sequences are compared to the N-terminal amino acid sequences deduced from the presumed genes of the benzoate oxidation pathway. X indicates an unidentified amino acid, lowercase letters indicate uncertain amino acids, and underlining indicates a leader peptide.
The N-terminal amino acid sequences of the ORF 7 and 13 products were reported in references 1 and 29 and are included for comparison.
ND, not determined.
Cloning and sequencing of the genes coding for benzoate-induced proteins.
Before this study, only the genes for BoxA and BoxB had been sequenced; the order of these genes is boxBA. As determined by inference from the high number of benzoate-induced soluble proteins, the complexity of the pathway is much greater, and several genes must be considered still missing. A cosmid gene library of chromosomal DNA was generated, and DNA probes derived from boxA were used for screening. The cloning strategy is summarized in Fig. 4.
FIG. 4.
Structure of benzoate metabolism gene cluster. ORF 7 encodes benzoate-CoA ligase, ORF 12 encodes BoxA, and ORF 13 encodes BoxB. Genes are indicated by arrows. The numbers in the arrows are ORF numbers. The lengths of ORFs are indicated under the arrows. The lines at the top indicate the positions and lengths of inserts in λZAP and cosmid clones. The genomic DNA of A. evansii used for construction of gene libraries was cut either by Sau3AI or EcoRI. Possible secondary structures are indicated by hairpin symbols. The numbers under these symbols indicate the positions of secondary structures within the gene cluster. The double-ended arrows under the ORFs show the regions which were amplified by RT-PCR (see Fig. 5 and Table 5). Note that intergenic regions between ORFs are always parts of the fragments.
One positive clone was obtained, which contained a 37-kb insert carrying boxBA. Upstream of boxBA the DNA sequence contained two additional ORFs which were oriented in the same direction as boxBA. The N-terminal sequence deduced from the 5′ end of one of these ORFs (ORF 11) was identical to the N-terminal sequence of induced protein 3. Further upstream, three ORFs oriented in the opposite direction were found, and the last of these ORFs was incomplete. The N-terminal amino acid sequence deduced from the 5′ end of this incomplete ORF was identical to the N-terminal amino acid sequence of benzoate-CoA ligase. This suggested that all these ORFs are likely to be involved in benzoate metabolism. Sequencing of the ORFs downstream of boxBA revealed two other ORFs oriented in the same direction as boxBA, which were separated from each other by only short intergenic regions. Then 74 bp farther downstream there was a putative ORF without a ribosome-binding site, which exhibited similarity only to putative ORFs in the database. Hence, the whole 11.8-kbp DNA sequence contained one incomplete and eight complete putative genes.
Further screening of a λZAP Express gene bank led to the discovery of a total of 15 ORFs which were found to be clustered and which are likely to be involved in benzoate metabolism; 126 bp downstream of ORF 1 an incomplete ORF coding for another putative enoyl-CoA hydratase was found. The order of these ORFs, their orientations, their sizes, and their secondary DNA structures are shown in Fig. 4. Table 4 summarizes the properties of these ORFs.
TABLE 4.
Properties of genes and gene products assumed or proven to be involved in benzoate oxidationa
| Gene | G + C content (mol %) | Length of intergenic region to next ORF (bp) | Putative function of gene product | Molecular mass (kDa) | Isoelectric point (pH) | Putative cellular localization | Similar proteins in databases | % Identity | % Similarity | E value | Accession no. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ORF 1 | 65.1 | 6 | Thioesterase | 16 | 5.72 | Cytosolic | Putative 4-hydroxybenzoyl-CoA thioesterase (Ralstonia solanacearum) | 41 | 60 | 7e-20 | NC_003296 |
| 4-Hydroxy-benzoyl-CoA thioesterase (Pseudomonas sp. strain Cbs-3) | 43 | 77 | 7.7 | P56653 | |||||||
| ORF 2 | 68.5 | 1 | Lactonase | 28 | 5.22 | Cytosolic | Putative hydrolase-related protein (Ralstonia solanacearum) | 46 | 59 | 6e-48 | NC_003295 |
| Hydrolase-related protein (Deinococcus radiodurans) | 42 | 60 | 2e-41 | NC_001263 | |||||||
| ORF 3 | 66.9 | 2 | ABC transporter, ATP binding | 28 | 9.56 | Cytosolic | Putative ABC transporter subunit HbaH (Rhodopseudomonas palustris) | 52 | 68 | 1e-60 | AAC13363 |
| Probable ABC transporter subunit (Pseudomonas sp. strain CA10) | 43 | 58 | 3e-45 | BAB32462 | |||||||
| ORF 4 | 67.2 | 17 | ABC transporter, ATP-binding membrane-spanning protein | 62 | 6.88 | Membrane | Putative ABC transporter subunit HbaG (Rhodopseudomonas palustris) | 39 | 54 | 1e-104 | AAC13364 |
| Branched-chain amino acid ABC transporter, ATP-binding protein (Deinococcus radiodurans) | 27 | 41 | 2e-43 | NP_285584 | |||||||
| ORF 5 | 65.1 | 77 | ABC transporter, membrane-spanning protein | 31 | 6.18 | Membrane | Putative ABC transporter subunit HbaF (Rhodopseudomonas palustris) | 45 | 59 | 1e-53 | AAC13365 |
| Putative membrane-spanning protein (Pseudomonas aeruginosa) | 37 | 51 | 1e-29 | AAC69487 | |||||||
| ORF 6 | 65.2 | 140 | ABC transporter, substrate-binding protein | 41 (with leader peptide), 39 (without leader peptide) | 9.03 (with leader peptide), 8.52 (without leader peptide) | Periplasm | Putative substrate-binding protein (Azoarcus evansii) | 80 | 86 | 1e-171 | AAL02070 |
| Putative ABC transporter subunit (Azoarcus evansii) | 71 | 81 | 1e-150 | CAD21641 | |||||||
| ORF 7 | 66.6 | 218 | Benzoate-CoA ligase | 58 | 5.61 | Cytosolic | Benzoate-CoA ligase (Thauera aromatica) | 76 | 84 | <1e-170 | CAD21683/PICK> |
| Benzoate-CoA ligase (Azoarcus evansii) | 69 | 79 | <le-170 | CAD21640 | |||||||
| ORF 8 | 66.0 | 98 | Unknown | 17 | 4.97 | Cytosolic | p-Hydroxylaminobenzoate lyase (Pseudomonas sp. strain YH102) | 31 | 44 | 1e-12 | AAF01448 |
| PnbB (Pseudomonas putida) | 30 | 43 | 1e-11 | AAG01543 | |||||||
| ORF 9 | 70.4 | 327 | Aldehyde dehydrogenase | 54 | 8.62 | Cytosolic | MaoC-like protein (phenylacetic acid degradation protein PaaZ) (Escherichia coli) | 41 | 59 | 2e-97 | P77455 |
| PhaL (Pseudomonas putida) | 42 | 52 | 1e-95 | AAC24340 | |||||||
| ORF 10 | 66.5 | 40 | Unknown regulatory two-domain protein | 34 | 6.37 | Cytosolic | Helix-turn-helix protein (Pyrobaculum aerophilum) | 37 | 61 | 3e-4 | A65134 |
| Shikimate kinase I (EC 2.7.1.71) (Escherichia coli) | 31 | 52 | 3e-12 | NP_560573 | |||||||
| ORF 11 | 68.1 | 159 | Enoyl-CoA-hydratase/isomerase and possible ring-cleaving enzyme | 61 | 5.44 | Cytosolic | Enoyl-CoA hydratase (Fad-1) (Archaeoglobus fulgidus) | 34 | 48 | 9e-6 | NP_069271 |
| Enoyl-CoA hydratase (Bacillus halodurans) | 36 | 48 | 2e-5 | NP_242001 | |||||||
| ORF 12 | 64.3 | 163 | Benzoyl-CoA oxygenase component B | 55 | 5.62 | Cytosolic | Ring oxidation complex, phenylacetic acid degradation-related protein (Sulfolobus solfataricus) | 30 | 47 | 4e-14 | NP_342758 |
| ORF 13 | 66.9 | 245 | Benzoyl-CoA oxygenase component A | 46 | 5.59 | Cytosolic | Ferredoxin-NADP+ reductase (EC 1.18.1.2) precursor (Cyanophora paradoxa) | 42 | 56 | 8e-54 | A56664 |
| Ferredoxin-NADP+ oxidoreductase (Synechococcus elongatus) | 38 | 53 | 6e-48 | BAB61060 | |||||||
| ORF 14 | 71.0 | 179 | β-Ketoadipyl-CoA thiolase | 42 | 5.68 | Cytosolic | Beta-ketoadipyl-CoA thiolase (Burkholderia pseudomallei) | 75 | 85 | 1e-157 | AAG12159 |
| Beta-ketoadipyl-CoA thiolase (Acinetobacter calcoaceticus) | 68 | 82 | 1e-154 | Q43974 | |||||||
| ORF 15 | 67.2 | Unknown | 21 | 9.51 | Cytosolic | Hypothetical protein (Thauera aromatica) | 46 | 52 | 4e-40 | Schühler and Fuchs, unpublished | |
| Hypothetical protein (Vibrio cholerae) | 35 | 42 | 0.016 | NP_231988 |
Similarity searches were done with the the program blastp (http://www.ncbi.nlm.nih.gov/BLAST/). The percentage of identity was defined as the percentage of amino acids that are identical in two proteins. The percentage of similarity was defined as the percentage of amino acids that are identical or conserved in two proteins. The E value is an estimate of the statistical significance of the match, which specifies the number of matches with a given score that is expected in a search of a database of this size absolutely by chance.
Gene organization and induction.
The orientation and organization of the 15 ORFs indicate that the benzoate metabolic genes are organized in at least two different operons. Several DNA duplex structures appear to be present (Fig. 4); the function of these secondary structures is unknown. The lengths of the intergenic regions vary (Table 4); the largest intergenic region is between ORFs 9 and 10 and is the presumed promoter and operator region of two divergently transcribed operons. This region contains several direct and inverted repeats that are more than 7 bp long. All ORFs but ORF 1 contain ribosome-binding sites which are very similar to the consensus sequence (AGGAGG).
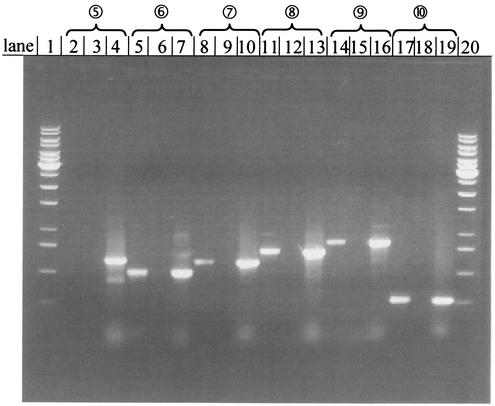
Induction of ORFs during growth on benzoate was studied by performing RT-PCR experiments with mRNA from benzoate-grown cells and comparing the results to the results obtained with cells grown on malate (Fig. 5 and Table 5). The amplified DNA fragments contained the intergenic regions between adjacent genes. The data indicate that there was induction of all ORFs but ORF 1. Clearly, as expected, no transcript between ORFs 9 and 10 could be obtained. ORFs 2 to 9 and 11 to 13 were cotranscribed and detected even in highly (100- to 1,000-fold) diluted samples. At high dilutions there was no amplification of the intergenic region upstream of ORF 1 and of the regions between ORFs 1 and 2, ORFs 10 and 11, ORFs 13 and 14, and ORFs 14 and 15. Basal expression of ORFs 2 to 9 and ORFs 11 to 13 could be part of a global regulation strategy because ORFs 3 to 6 code for putative benzoate transport and ORFs 7, 11, 12, and 13 code for the proven or putative first steps in benzoate metabolism (see below). A basal level of expression of these genes could help if there were a change in carbon source. Cells grown on malate did not show any BoxA in immunodetection experiments, although RT-PCR detected boxA transcription. This may indicate that BoxA production is also regulated at the translation stage. In summary, these data suggest that ORFs 2 to 13 function in benzoate metabolism. The putative regulator gene ORF 10 may be transcribed separately. The role of ORFs 1 and 15 is questionable.
FIG. 5.
Study of the organization and induction of genes of the benzoate degradation gene cluster performed with RT-PCR and agarose gel electrophoresis. cDNA from A. evansii cells grown on benzoate was compared with cDNA from cells grown on malate. Standard PCRs were carried out with these cDNAs and with genomic DNA of A. evansii as a positive control. The cDNA templates used in these reactions were not diluted and were diluted 10-, 100-, and 1,000-fold. The numbers of the amplified fragments are indicated by numbers in circles. The positions of these fragments are indicated in Fig. 4. The agarose gel shows the results of the experiments with fragments 5 to 10 (experiments with the first cluster are not shown). Lanes 1 and 20 contained a 1-kb ladder; lanes 2, 5, 8, 11, 14, and 17 contained cDNA from benzoate-grown cells; lanes 3, 6, 9, 12, 15, and 18 contained cDNA from malate-grown cells; and lanes 4, 7, 10, 13, 16, and 19 contained genomic DNA (positive control). Lanes 2, 3, 5, 6, 14, 15, undiluted cDNA; lanes 17 and 18, cDNA diluted 10-fold; lanes 8, 9, 11, and 12, cDNA diluted 100-fold.
TABLE 5.
Induction of genes of the benzoate degradation gene cluster, as determined by using cDNA from cells grown on benzoate and from cells grown on malate
| Amplified fragmenta | Fragment length (bp) | Templates
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Undiluted cDNA
|
cDNA diluted 10-fold
|
cDNA diluted 100-fold
|
cDNA diluted 1,000-fold
|
||||||
| Benzoate-grown cells | Malate-grown cells | Benzoate-grown cells | Malate-grown cells | Benzoate-grown cells | Malate-grown cells | Benzoate-grown cells | Malate-grown cells | ||
| 0 | 500 | +b | + | + | − | − | − | − | − |
| 1 | 402 | + | + | − | − | − | − | − | − |
| 2 | 801 | + | + | + | − | + | − | − | − |
| 3 | 1,008 | + | + | + | − | + | − | − | − |
| 4 | 862 | + | − | + | − | + | − | − | − |
| 5 | 618 | − | − | − | − | − | − | − | − |
| 6 | 500 | + | − | − | − | − | − | − | − |
| 7 | 593 | + | + | + | − | + | − | + | − |
| 8 | 721 | + | + | + | − | + | − | + | − |
| 9 | 836 | + | − | + | − | − | − | − | − |
| 10 | 259 | + | − | + | − | − | − | − | − |
See Fig. 5.
+, DNA fragment detected; −, no fragment detected.
Of the products of the 15 ORFs of the putative benzoate degradation gene cluster, 5 proteins were directly identified as being part of this functional unit (Table 3). The products of ORFs 7 and 13 were purified as benzoate-induced enzymes or proteins, and their N-terminal amino acid sequences agreed well with the deduced sequences. The products of ORFs 6, 11, and 12 were identified as benzoate-induced proteins by N-terminal sequencing. In addition, the product of ORF 13 was identified as a benzoate-induced protein by Western blotting performed with antibodies raised against BoxA.
Characterization of the genes of benzoate metabolism and their putative functions.
The putative functions of the sequenced genes and deduced gene products are summarized in Table 4. The first gene cluster consists of the following nine ORFs.
ORF 1 encodes a protein that has similarity to thioesterases, notably 4-hydroxybenzoyl-CoA thioesterase involved in the conversion of 4-chlorobenzoate to 4-hydroxybenzoate (4). The enzyme may function in benzoate metabolism as a security valve, cleaving CoA thioesters (or derived dead-end products) that accumulate and trap CoA if subsequent steps (e.g., oxygen-dependent steps) become limiting. This may easily occur in bacteria that profit from their ability to change quickly from an oxic life style to an anoxic, denitrifying life style, which is characteristic of A. evansii. Otherwise, CoA trapping would be life threatening. The missing ribosome-binding site could then be part of a downregulation strategy for this ORF, with the aim of securing just a low level of the corresponding protein.
The ORF 2 product has similarity to lactone hydrolases. Its secondary structure is similar to the conserved structure of α/β-hydrolase fold enzymes (31), and the amino acid sequence 103GHSDGG108 fits well with the consensus motif (Sm-X-Nu-X-Sm-Sm, where Sm is a small amino acid, X is any amino, and Nu is a nucleophilic amino acid) for the nucleophile member (the nucleophile elbow) of the catalytic triad (34). Since the lactone of 3-hydroxyadipyl-CoA was observed to be product of benzoate transformation by cell extracts, this enzyme may function in hydrolyzing this lactone.
The product of ORF 3 has similarity to a putative ATP-binding subunit of an ABC transporter system. It has the typical Walker motifs of ATP-binding proteins (Walker A, 61GRNGMGKTT69; Walker B, 179LLILDE184) (24). The ORF 4 product has similarity to a membrane-spanning subunit of an ABC transporter system (24). A prediction for transmembrane helices indicates that there are nine possible membrane-spanning α-helices. Furthermore, this protein has Walker consensus motifs for an ATP-binding site (Walker A, 358GPNGAGKST366; Walker B, 488VLLLDE494). Therefore, this protein seems to be a two-domain protein. The protein encoded by ORF 5 has similarity to a putative membrane-spanning protein of an ABC transporter system. It seems to contain six transmembrane helices. The ORF 6 product has similarity to a putative substrate-binding protein of an ATP transporter system. This protein corresponds to benzoate-induced protein 5 (Table 3). It contains a 31-amino-acid leader peptide. The sequence of this leader peptide is typical of the sequences found in the Sec transport system (37). It contains an N domain with a net positive charge (amino acids 1 to 10) and a hydrophobic H domain (amino acids 11 to 24). In summary, the products of ORFs 3 to 6 are likely to represent the four components of an ATP transporter system responsible for the highly efficient uptake of benzoate.
ORF 7 codes for the aerobically induced benzoate-CoA ligase, which differs from the corresponding isoenzyme induced during anoxic growth on aromatic substrates. The deduced N-terminal amino acid sequence is identical to the 20-amino-acid N-terminal sequence determined for the purified enzyme. The purified enzyme is a homodimer which has a native molecular mass of 130 kDa and subunits with molecular masses of approximately 56 kDa (deduced molecular mass, 58 kDa). The enzyme acts on benzoate but not on 3-hydroxybenzoate (1).
The protein encoded by ORF 8 shows low similarity to the enzyme 4-hydroxylaminobenzoate lyase, which catalyzes an odd reaction, the hydrolytic transformation of 4-hydroxylaminobenzoate to protocatechuate (3,4-dihydroxybenzoate) and ammonia during 4-nitrobenzoate degradation in Pseudomonas putida TW3 (25). There are similar entries in the database (18) which show only very low similarity to other gene products, indicating that these proteins may form a new family. The role of the ORF 8 product in benzoate metabolism is enigmatic. In any case, the substrate of this enzyme seems to be a benzoate derivative in both types of metabolism (i.e., 4-nitrobenzoate degradation and benzoate degradation).
The ORF 9 product has similarity to proteins which are assumed to play a role in aerobic phenylacetate metabolism in E. coli (PaaZ) (14, 15), P. putida (PhaL) (33), A. evansii (PaaZ) (30), and presumably other bacteria (30). Phenylacetate metabolism also proceeds via CoA thioesters, and the products of the ORFs related to ORF 9 are thought to be involved in hydrolytic or acyloin (or aldol) cleavage of the ring and/or in aldehyde oxidation. The hypothetical substrate for this kind of ring cleavage is a nonaromatic product (a cis-dihydrodiol) formed by a dioxygenase/reductase acting on phenylacetyl-CoA and benzoyl-CoA. Ring cleavage may be preceded by isomerization of the double bonds (see below). The protein encoded by ORF 9 may be involved in further oxidation of the intermediate formed after C—C bond cleavage (see below). The N-terminal amino acid sequence of the ORF 9 product (amino acids 10 to 434) shows similarity to the sequences of aldehyde dehydrogenases. The conserved domains 254IEADSVN260 and 291GQKCTAIR298 probably contain the glutamate-268 and cysteine-302 homologues (underlined), which in mammal aldehyde dehydrogenases are involved in the active center (14).
The second gene cluster, which is oriented in a different direction, consists of six genes with the following properties. The product of ORF 10 seems to represent a two-domain protein. A similar ORF was found in a gene cluster of the β-proteobacterium Thauera aromatica, which contains genes for BoxA, BoxB, and benzoate-CoA ligase (K. Schühle and G. Fuchs, unpublished data). The N-terminal domain (amino acids 32 to 87) has similarity to various regulatory proteins of the HTH family and seems to have the typical primary and secondary structures of helix-turn-helix proteins (7, 47). The first putative helix is between amino acids 44 and 51; it is separated from the second helix by a typical turn sequence (52GVS54). The second helix is between amino acids 55 and 62. The C-terminal domain (amino acids 137 to 285) has similarity to shikimate kinase I of E. coli. Shikimate kinase I does not function in shikimate phosphorylation and in the biosynthesis of aromatic amino acids, although it phosphorylates shikimate in vitro, but with very low affinity for its substrate (Km, 20 mM). The actual shikimate kinase II has a 100-fold-lower Km (13). The ORF 10 product may play a role as an unprecedented regulator protein that becomes activated or inactivated by ATP-dependent phosphorylation in response to benzoate.
The protein encoded by ORF 11 corresponds to benzoate-induced protein spot 3. It has a mosaic structure. An N-terminal domain (amino acids 47 to 162) and a C-terminal domain (amino acids 387 to 471) together form a 199-amino-acid domain found in the enoyl-CoA hydratase/isomerase (crotonase) protein family. Members of this enzyme family catalyze a variety of different reactions, including enoyl-CoA hydration, enoyl-CoA isomerization, and C—C bond cleavage (20), all based on abstraction or addition of the alpha-proton of the carboxylic acid. The majority of the rest of the ORF 11 product shows no similarity to other proteins in the databases. This may indicate that the protein encoded by ORF 11 catalyzes a complex reaction in which enoyl-CoA hydration and/or isomerization or even C—C bond cleavage is involved. The ORF 11 product seems to be a novel type of enzyme whose catalyzed reaction will be interesting to understand.
The product of ORF 12 is identical to BoxB and corresponds to benzoate-induced protein spot 1. This protein has similarities to a component of a putative multicomponent phenylacetyl-CoA oxygenase in E. coli (PaaA) (15) and P. putida (PhaF) (33). The phenylacetyl-CoA oxygenase is thought to consist of four subunits (PaaABCD) and to require a one-subunit NAD(P)H oxidoreductase component (PaaE). Similar ORFs are found in phenylacetate metabolism gene clusters of other bacteria, including A. evansii (30) and S. solfataricus (44). The primary structure of BoxB shows the two repeats of residues EX2H separated by 86 amino acids (150EGRH153 and 239EEAH242) that characterize the dinuclear iron-binding site of the large oxygenase subunit of methane, phenol, and toluene diiron monooxygenase (16, 36). We postulate that BoxB functions as the oxygenase part of benzoyl-CoA oxygenase in conjunction with BoxA, the reductase component.
The ORF 13 product is identical to the benzoate-induced enzyme BoxA. A similar ORF was found in the related organism T. aromatica next to the gene for BoxB (Schühle and Fuchs, unpublished). The similarity of BoxA to ferredoxin-NADP+ oxidoreductases and the benzoyl-CoA-dependent oxidation of NADPH catalyzed by the enzyme suggest that BoxA functions as the reducing component of benzoyl-CoA oxygenase, which, upon binding of benzoyl-CoA, transfers two electrons to the ring in the course of dioxygenation.
The protein encoded by ORF 14 has strong similarity to 3-ketoadipyl-CoA thiolase. This is probably also the function of the protein in benzoate metabolism, which is assumed to lead to 3-ketoadipyl-CoA. This implies that acetyl-CoA, succinyl-CoA, and CO2 are the products of the benzoate oxidation pathway.
ORF 15 is the last ORF of the cluster. Its product has no similarity to proteins in the database. Interestingly, a similar ORF was found in the gene cluster of T. aromatica mentioned above, which may code for benzoate oxidation in this related bacterium. The occurrence in similar gene clusters is an argument for a role for the ORF 15 product in the benzoate pathway, although no function can be ascribed to this protein yet.
Genes encoding a homologue of β-hydroxyacyl-CoA dehydrogenase and possibly another enoyl-CoA hydratase/isomerase are missing in the gene cluster sequenced. A β-oxidation dehydrogenase is needed to catalyze dehydrogenation of the detected intermediate 3-hydroxyadipyl-CoA to 3-oxoadipyl-CoA. Possibly the benzoyl-CoA pathway enzyme(s) is supplemented through the homologous protein(s) of primary metabolism.
Two-dimensional gel electrophoresis revealed at least seven benzoate-induced soluble proteins, and Western blotting revealed another protein. Of the proteins encoded by the 15 ORFs, 2 are likely to be membrane-bound components of an ABC transport system and therefore are not soluble; only small amounts of the regulator protein and the thioesterase are likely to be present. Hence, one would expect there to be 11 soluble benzoate-induced proteins. Thus, the complex protein induction pattern corresponds to the complex set of benzoate metabolic genes.
BoxA− mutant and expression of BoxA under different growth conditions.
A boxA mutant was constructed by homologous recombination between the wild-type chromosome and an insertionally inactivated version of the gene carried on plasmid pJQ200MK. The boxA mutant failed to grow on benzoate as a sole carbon source. This shows that BoxA is essential for growth on benzoate.
To find a growth-supporting substrate (for further mutant analysis and biochemical studies of the pathway) which allowed expression of the benzoate pathway, regulation of boxA expression and therefore induction of the benzoate genes were tested with different substrates. Wild-type cells were grown on different substrates and combinations of substrates along with benzoate as an inducer for the benzoate genes. Benzoate gene expression was assessed by spectrophotometric measurement of BoxA activity (i.e., NADPH oxidation in the presence of benzoyl-CoA, FAD, and O2 under H2O2 production conditions). The substrates used, alone and in combination, included benzoate, 3-hydroxybenzoate, phenylacetate, adipate, malate, and acetate. No BoxA activity was observed with extracts of cells grown on acetate, malate, and adipate, while some activity was detected with cells grown on phenylacetate (10%) and 3-hydroxybenzoate (5%). Aromatic compounds resembling the aromatic substrate benzoate, like phenylacetate and 3-hydroxybenzoate, may act as poor gratuitous inducers. A combination of benzoate and 3-hydroxybenzoate resulted in 12% BoxA activity, a combination of benzoate and phenylactetate resulted in 50% BoxA activity, and a combination of benzoate and adipate resulted in 82% BoxA activity. No activity was observed with benzoate and acetate. This suggests that acetate acts as a strong catabolite repressor, whereas adipate does not. Also, 3-hydroxybenzoate and phenylacetate seemed to repress activity.
Proposal for the benzoate pathway and putative role of ORFs.
We propose a benzoate pathway (Fig. 6) which is induced by benzoate and may be regulated by the product of ORF 10, a two-domain protein. An ABC transporter system consisting of the products of ORFs 3 to 6 may be responsible for effective benzoate uptake. The ORF 7 product activates benzoate to benzoyl-CoA. A protein mixture from A. evansii transformed benzoyl-CoA in an NADPH- and oxygen-dependent reaction into 6-hydroxy-3-hexenoyl-CoA. The proteins encoded by ORFs 9, 11, 12, and 13 may be involved in this complex reaction. Benzoyl-CoA is attacked by a putative 2,3-dioxygenase to obtain the cis-dihydrodiol product (unpublished results); BoxA (encoded by ORF 13) is thought to deliver electrons from NADPH to BoxB (encoded by ORF 12), which interacts with substrate and oxygen. BoxA has erroneously been reported to be benzoyl-CoA 3-monooxygenase (32), but a detailed study of the purified protein revealed that FAD-dependent oxidation of NADPH occurs in the presence of benzoyl-CoA without hydroxylase activity (29). The ORF 1 product may act as a thioesterase. This protein may be required to release CoA from the intermediates of the pathway if enzymes downstream of benzoyl-CoA become limiting, which would lead to trapping of CoA.
FIG. 6.
Proposed benzoate pathway and putative function of gene products. Experimentally documented compounds are enclosed in boxes. The numbers in boxes indicate the ORFs that encode proteins which might catalyze the individual steps.
The next steps are different from what one would expect from conventional pathways involving a non-CoA-activated free cis-dihydrodiol intermediate. In conventional pathways, the cis-diol undergoes oxidation and rearomatization to a dihydroxy aromatic product (Fig. 1). Yet a putative cis-diol dehydrogenase gene could not be found in the gene cluster. We propose that the CoA thioester grouping of the activated diol allows isomerization of the conjugated double-bond system of the cis-diol, leading to an enol which tautomerizes to the more stable unsaturated cyclic 3-ketoacyl-CoA. The postulated intermediate formed contains an α-hydroxycarbonyl (acyloin) group. Two types of hydrolytic C—C cleavage reactions can be envisaged (Fig. 6), followed by decarboxylation. These reactions may be catalyzed by the ORF 11 product, a complex protein which contains an enoyl-CoA hydratase/isomerase domain.
Oxidation of the alcohol group of 6-hydroxy-3-hexenoyl-CoA to the carboxyl group results in cis-3,4-dehydroadipyl-CoA, which was also detected. The protein encoded by ORF 9 may be involved in this four-electron oxidation. The order of events involved in transformation of cis-3,4-dehydroadipyl-CoA, possibly via trans-2,3-dehydroadipyl-CoA and/or the 3,6-lactone of 3-hydroxyadipyl-CoA, to 3-hydroxyadipyl-CoA cannot be determined on theoretical grounds; all these intermediates were observed, but the order of formation could not be inferred. The lactone may form spontaneously and/or be a preparation artifact. The product of ORF 2 may be involved in lactone hydrolysis. In any case, it seems logical that 3-hydroxyadipyl-CoA is oxidized to 3-ketoadipyl-CoA, which is thiolytically cleaved by the ORF 14 product into succinyl-CoA and acetyl-CoA. Hence, the pathway converges with the classical β-ketoadipate pathway of benzoate oxidation at the last intermediate, 3-ketoadipyl-CoA. So far, the roles of the proteins encoded by ORF 8 and ORF 15 are completely unknown.
Similar genes in other bacteria.
Genes similar to those described here are found in very recent database entries for proteobacterial genomes, but their roles are unknown (Fig. 7). The bacteria examined include Burkholderia fungorum LB400, which contains two very similar sets of genes, Ralstonia metallidurans CH34, Magnetospirillum magnetotacticum MS-1, and Rhodopseudomonas palustris CGA009. Similar genes have also been found in T. aromatica K172 (Schühle and Fuchs, unpublished).
FIG. 7.
Gene cluster for aerobic benzoate metabolism in A. evansii and similar genes or gene clusters in other organisms. Corresponding ORFs are indicated by the same color. Putative functions: dark blue, benzoate-CoA ligase; medium blue, regulatory protein; green, enoyl-CoA hydratase/isomerase; light blue, BoxB; yellow, BoxA; white, aldehyde dehydrogenase; red, lactonase; green stripes, PnbB-type protein; grey stripes, thioesterase; black, ORFs which show no similarity to ORFs 1 to 15 of A. evansii; grey, ORFs which are present only in the A. evansii gene cluster so far. The numbers in the arrows indicate the ORF numbers in the A. evansii gene cluster and the deduced numbers of amino acids in the gene products. The percentages under the ORFs indicate the percentages of similar amino acids in an ORF product and the corresponding ORF product in A. evansii. An asterisk indicates that an ORF could not be fully translated into an amino acid sequence, either because of possible frameshifts in sequence or because of unsequenced regions.
Interestingly, in most cases homologs of ORFs 11 to 13 occur in the same order as in A. evansii. In T. aromatica sequencing is incomplete, and the ORF 11 homologue is expected to be upstream of the ORF 12 homologue. In R. palustris, ORF 13 is still missing. We postulate that the products of ORFs 11 to 13 in these cases represent the core enzymes that act on benzoyl-CoA and its first product, the 2,3-dihydrodiol. In most cases the regulator protein (encoded by ORF 10) and the putative lactone hydrolase (encoded by ORF 2) are also present. The ABC transporter (encoded by ORFs 3 to 6) is generally missing. The putative thioesterase (encoded by ORF 1) could be found only in M. magnetotacticum. The product of ORF 15, whose function is not known, could be found only in T. aromatica. This may suggest that ORFs 1, 3 to 6, and 15 are not crucial for the new benzoate pathway. The thiolase (encoded by ORF 14) is missing in all other organisms. The function of this enzyme may be performed by common β-ketothiolases; this argument also holds true for 3-hydroxyacyl-CoA dehydrogenase.
The R. palustris strain which has been sequenced may grow aerobically on benzoate, although other strains reportedly do not do this (22). In this case the benzoate-CoA ligase of the anaerobic benzoate degradation cluster could function in both anaerobic and aerobic metabolism of benzoate, although there would be two completely different strategies for dearomatization under the two conditions. There are indications that the benzoate-CoA ligase (encoded by an ORF 7 homologue) in T. aromatica not only is involved in benzoate transformation to benzoyl-CoA under aerobic conditions but also is involved in activation of benzoate and 2-aminobenzoate under anoxic, denitrifying conditions (Schühle and Fuchs, unpublished).
Comparison with other aromatic degradation pathways that proceed via CoA thioesters and working hypothesis for future experiments.
In A. evansii, aerobic metabolism of 2-aminobenzoate, benzoate, and phenylacetate proceeds via the CoA esters of the individual substrates. While the pathway for 2-aminobenzoate has been studied so far only in A. evansii (8, 21, 27, 28, 43, 50), the new benzoate pathway has also been found in the related organism T. aromatica (unpublished results) and in a gram-positive thermophilic B. stearothermophilus-like strain (48). The phenylacetate pathway is widely distributed (reviewed in reference 30). The three metabolic pathways seem to use a novel principle. Our working hypothesis is schematically presented in Fig. 8. The assumptions are as follows. (i) The pathways start with activation of the substrates to CoA thioesters, and the intermediates are further processed in this form. (ii) The aryl-CoA thioesters are attacked by dioxygenases and reductases (benzoate, phenylacetate) to obtain the corresponding cis-dihydrodiol, which is not rearomatized by oxidation but is isomerized to a dihydroxylated nonaromatic product with an acyloin structure. In the case of anthranilate, a monooxygenase/reductase forms a monohydroxylated nonaromatic product. The CoA thioester allows reactions that would not be feasible in the case of nonactivated aromatic acids and their intermediates. (iii) The early reactive intermediates are labile and may rearomatize spontaneously, in the case of 2-aminobenzoate to 5-hydroxy-2-aminobenzoyl-CoA (by isomerization), in the case of benzoyl-CoA to 3-hydroxybenzoyl-CoA (by water elimination), and in the case of phenylacetyl-CoA to 2-hydroxyphenylacetyl-CoA (by water elimination). Thioesterases cleave the thioesters of these dead-end products, and the corresponding acids (5-hydroxy-2-aminobenzoate, 3-hydroxybenzoate, 2-hydroxyphenylacetate) appear in the medium; they cannot be metabolized further by the induced pathways. (iv) The ring is opened nonoxygenolytically, and the resulting noncyclic product is further oxidized via β-oxidation to presumably β-ketoadipyl-CoA. Thiolytic cleavage yields succinyl-CoA and acetyl-CoA.
FIG. 8.
Working hypothesis showing the proposed initial steps in the pathways of metabolism of 2-aminobenzoate, benzoate, and phenylacetate in A. evansii. The reactions involved in further metabolism of the CoA-activated aromatic acids remain to be identified. The compounds in brackets are assumed to be enzyme bound or rapidly transformed to more stable nonaromatic intermediates. The C—C bonds which are thought to be cleaved nonoxygenolytically are indicated. The dead-end products of rearomatization reactions are indicated in side reactions. These dead-end products are formed if the intermediates (in brackets) accumulate in the cell.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft, Bonn, Germany, and by the Fonds der chemischen Industrie, Frankfurt/Main, Germany.
We thank Caroline S. Harwood (University of Iowa) for the kind gift of the suicide vector and J. Alt-Mörbe (Labor für DNA-Analytik, Freiburg, Germany) for DNA sequencing. We especially thank J. Heider (Freiburg, Germany) for helpful suggestions and critical comments.
REFERENCES
- 1.Altenschmidt, U., B. Oswald, E. Steiner, H. Herrmann, and G. Fuchs. 1993. New aerobic benzoate oxidation pathway via benzoyl-coenzyme A and 3-hydroxybenzoyl-coenzyme A in a denitrifying Pseudomonas sp. J. Bacteriol. 175:4851-4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anders, J. H., A. Kaetzke, P. Kämpfer, W. Ludwig, and G. Fuchs. 1995. Taxonomic position of aromatic-degrading denitrifying pseudomonad strains K172 and KB740 and their description as new members of genera Thauera, as Thauera aromatica sp. nov., and Azoarcus, as Azoarcus evansii sp. nov., respectively, members of the beta subclass of Proteobacteria. Int. J. Syst. Bacteriol. 45:327-333. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. John Wiley and Sons, New York, N.Y.
- 4.Benning, M. M., G. Wesenberg, R. Liu, K. L. Taylor, D. Dunaway-Mariano, and H. M. Holden. 1998. The three-dimensional structure of 4-hydroxybenzoyl-CoA thioesterase from Pseudomonas sp. strain CBS-3. J. Biol. Chem. 273:33572-33579. [DOI] [PubMed] [Google Scholar]
- 5.Birnboim, H., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun, K., and D. T. Gibson. 1984. Anaerobic degradation of 2-aminobenzoate (anthranilic acid) by denitrifying bacteria. Appl. Environ. Microbiol. 48:102-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan, R. G., and B. W. Matthews. 1989. The helix-turn-helix DNA binding motif. J. Biol. Chem. 264:1903-1906. [PubMed] [Google Scholar]
- 8.Buder, R., K. Ziegler, G. Fuchs, B. Langkau, and S. Ghisla. 1989. 2-Aminobenzoyl-CoA monooxygenase/reductase, a novel type of flavoenzyme. Studies on the stoichiometry and the course of reaction. Eur. J. Biochem. 185:637-643. [DOI] [PubMed] [Google Scholar]
- 9.Buswell, J. A., and J. S. Clark. 1976. Oxidation of aromatic acids by a facultative thermophilic Bacillus sp. J. Gen. Microbiol. 96:209-213. [DOI] [PubMed] [Google Scholar]
- 10.Clark, J. S., and J. A. Buswell. 1979. Catabolism of gentisic acid by two strains of Bacillus stearothermophilus. J. Gen. Microbiol. 112:191-195. [Google Scholar]
- 11.Coligan, J. E., B. M. Dunn, H. L. Ploegh, D. W. Speicher, and P. T. Wingfield. 1995. Current protocols in protein science. John Wiley & Sons, Inc., New York, N.Y.
- 12.Crawford, R. L. 1976. Pathways of 4-hydroxybenzoate degradation among species of Bacillus. J. Bacteriol. 127:204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeFeyter, R. C., and J. Pittard. 1986. Genetic and molecular analysis of aroL, the gene for shikimate kinase II in Escherichia coli K-12. J. Bacteriol. 165:226-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrandez, A., M. A. Prieto, J. L. Garcia, and E. Diaz. 1997. Molecular characterization of PadA, a phenylacetaldehyde dehydrogenase from Escherichia coli, FEBS Lett. 406:23-27. [DOI] [PubMed] [Google Scholar]
- 15.Ferrandez, A., B. Minambres, B. Garcia, E. R. Olivera, J. M. Luengo, J. L. Garcia, and E. Diaz. 1998. Catabolism of phenylacetic acid in Escherichia coli. Characterization of a new aerobic hybrid pathway. J. Biol. Chem. 273:25974-25986. [DOI] [PubMed] [Google Scholar]
- 16.Fox, B. G., J. Shanklin, J. Ai, T. M. Loehr, and J. Sanders-Loehr. 1994. Resonance Raman evidence for an Fe-O-Fe center in stearoyl-ACP desaturase. Primary sequence identity with other diiron-oxo proteins. Biochemistry. 33:12776-12786. [DOI] [PubMed] [Google Scholar]
- 17.Görg, A., W. Postel, and S. Günther. 1988. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis 9:531-546. [DOI] [PubMed] [Google Scholar]
- 18.Groenewegen, P. E., P. Breeuwer, J. M. van Helvoort, A. A. Langenhoff, F. P. de Vries, and J. A. de Bont. 1992. Novel degradative pathway of 4-nitrobenzoate in Comamonas acidovorans NBA-10. J. Gen. Microbiol. 138:1599-1605. [DOI] [PubMed] [Google Scholar]
- 19.Gross, G. G., and M. H. Zenk. 1966. Darstellung und Eigenschaften von Coenzym A-Thioestern substituierter Zimtsäuren. Z. Naturforsch. Teil B 21:683-690. [Google Scholar]
- 20.Haller, T., T. Buckel, J. Retey, and J. A. Gerlt. 2000. Discovering new enzymes and metabolic pathways: conversion of succinate to propionate by Escherichia coli. Biochemistry 39:4622-4629. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann, S., C. Hultschig, W. Eisenreich, A. Bacher, and S. Ghisla. 1999. NIH shift in flavin-dependent monooxygenation: mechanistic studies with 2-aminobenzoyl-CoA monooxygenase/reductase. Proc. Natl. Acad. Sci. USA 96:7831-7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harwood, C. S., and J. Gibson. 1988. Anaerobic and aerobic metabolism of diverse aromatic compounds by the photosynthetic bacterium Rhodopseudomonas palustris. Appl. Environ. Microbiol. 54:712-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harwood, C. S., and R. E. Parales. 1996. The beta-ketoadipate pathway and the biology of self-identity. Annu. Rev. Microbiol. 50:553-590. [DOI] [PubMed] [Google Scholar]
- 24.Higgins, C. F. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67-113. [DOI] [PubMed] [Google Scholar]
- 25.Hughes, M. A., and P. A. Williams. 2001. Cloning and characterization of the pnb genes, encoding enzymes for 4-nitrobenzoate catabolism in Pseudomonas putida TW3. J. Bacteriol. 183:1225-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiemer, P., B. Tshisuaka, S. Fetzner, and F. Lingens. 1996. Degradation of benzoate via benzoyl-coenzyme A and gentisate by Bacillus stearothermophilus PK1, and purification of gentisate 1,2-dioxygenase. Biol. Fertil. Soils 23:307-313. [Google Scholar]
- 27.Langkau, B., S. Ghisla, R. Buder, K. Ziegler, and G. Fuchs. 1990. 2-Aminobenzoyl-CoA monoxygenase/reductase, a novel type of flavoenzyme. Identification of the products. Eur. J. Biochem. 191:365-371. [DOI] [PubMed] [Google Scholar]
- 28.Langkau, B., P. Vock, V. Massey, G. Fuchs, and S. Ghisla. 1995. 2-Aminobenzoyl-CoA monooxygenase/reductase. Evidence for two distinct loci catalyzing substrate monooxygenation and hydrogenation. Eur. J. Biochem. 230:676-685. [PubMed] [Google Scholar]
- 29.Mohamed, M. E., C. Ebenau-Jehle, A. Zaar, and G. Fuchs. 2001. Reinvestigation of a new type of aerobic benzoate metabolism in the proteobacterium Azoarcus evansii. J. Bacteriol. 183:1899-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohamed, M. E., W. Ismail, J. Heider, and G. Fuchs. 2002. Aerobic metabolism of phenylacetic acids in Azoarcus evansii. Arch. Microbiol. 178:180-192. [DOI] [PubMed]
- 31.Nardini, M., and B. W. Dijkstra. 1999. Alpha/beta hydrolase fold enzymes: the family keeps growing. Curr. Opin. Struct. Biol. 9:732-737. [DOI] [PubMed] [Google Scholar]
- 32.Niemetz, R., U. Altenschmidt, S. Brucker, and G. Fuchs. 1995. Benzoyl-coenzyme A 3-monooxygenase, a flavin-dependent hydroxylase. Purification, some properties and its role in aerobic benzoate oxidation via gentisate in a denitrifying bacterium. Eur. J. Biochem. 227:161-168. [DOI] [PubMed] [Google Scholar]
- 33.Olivera, E. R., B. Minambres, B. Garcia, C. Muniz, M. A. Moreno, A. Ferrandez, E. Diaz, J. L. Garcia, and J. M. Luengo. 1998. Molecular characterization of the phenylacetic catabolic pathway in Pseudomonas putida U: the phenylacetyl-CoA catabolon. Proc. Natl. Acad. Sci. USA 95:6419-6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ollis, D. L., E. Cheah, M. Cygler, B. Dijkstra, F. Frolow, S. M. Franken, M. Harel, S. J. Remington, I. Silman, and J. Schrag. 1992. The alpha/beta hydrolase fold. Protein Eng. 5:197-211. [DOI] [PubMed] [Google Scholar]
- 35.Parales, R. E., and C. S. Harwood. 1993. Construction and use of a new broad-host-range lacZ transcriptional fusion vector, pHRP309, for gram− bacteria. Gene 133:23-30. [DOI] [PubMed] [Google Scholar]
- 36.Pikus, J. D., J. M. Studts, C. Achim, K. E. Kauffmann, E. Munck, R. J. Steffan, K. McClay, and B. G. Fox. 1996. Recombinant toluene-4-monooxygenase: catalytic and Moessbauer studies of the purified diiron and Rieske components of a four-protein complex. Biochemistry 35:9106-9119. [DOI] [PubMed] [Google Scholar]
- 37.Pugsley, A. P. 1993. The complete general secretory pathway in gram-negative bacteria. Microbiol. Rev. 57:50-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 39.Redenbach, M., H. M. Kieser, D. Denapaite, A. Eichner, J. Cullum, H. Kinashi, and D. A. Hopwood. 1996. A set of ordered cosmids and a detailed genetic and physical map for the 8 Mb Streptomyces coelicolor A3(2) chromosome. Mol. Microbiol. 21:77-96 [DOI] [PubMed] [Google Scholar]
- 40.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 41.Schachter, D., and J. V. Taggart. 1976. Benzoyl coenzyme A and hippurate synthesis. J. Biol. Chem. 203:925-933. [PubMed] [Google Scholar]
- 42.Schennen, U., K. Braun, and H. J. Knackmuss. 1985. Anaerobic degradation of 2-fluorobenzoate by benzoate-degrading, denitrifying bacteria. J. Bacteriol. 161:321-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schühle, K., M. Jahn, S. Ghisla, and G. Fuchs. 2001. Two similar gene clusters coding for enzymes of a new type of aerobic 2-aminobenzoate (anthranilate) metabolism in the bacterium Azoarcus evansii. J. Bacteriol. 183:5268-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.She, Q., R. K., Singh, F. Confalonieri, Y. Zivanovic, G. Allard, M. J. Awayez, C. C. Chan-Weiher, I. G. Clausen, B. A. Curtis, A. De Moors, G. Erauso, C. Fletcher, P. M. Gordon, I. Heikamp-de Jong, A. C. Jeffries, C. J. Kozera, N. Medina, X. Peng, H. P. Thi-Ngoc, P. Redder, M. E. Schenk, C. Theriault, N. Tolstrup, R. L. Charlebois, W. F. Doolittle, M. Duguet, T. Gaasterland, R. A. Garrett, M. A. Ragan, C. W. Sensen, and J. Van der Oost. 2001. The complete genome of the crenarchaeon Sulfolobus solfataricus P2. Proc. Natl. Acad. Sci. USA 98:7835-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simon, R., U., Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering. Transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 45a.Stanier, R. Y., and L. N. Ornston. 1973. The β-ketoadipate pathway. Adv. Microb. Physiol. 9:89-151. [PubMed] [Google Scholar]
- 46.Tschech, A., and G. Fuchs. 1987. Anaerobic degradation of phenol by pure cultures of newly isolated denitrifying pseudomonads. Arch. Microbiol. 148:213-217. [DOI] [PubMed] [Google Scholar]
- 47.Wintjens, R., and M. Rooman. 1996. Structural classification of HTH DNA-binding domains and protein-DNA interaction modes. J. Mol. Biol. 262:294-313. [DOI] [PubMed] [Google Scholar]
- 48.Zaar, A., W. Eisenreich, A. Bacher, and G. Fuchs. 2001. Intermediates of a new aerobic benzoate metabolic pathway in the bacteria Azoarcus evansii and Bacillus stearothermophilus. J. Biol. Chem. 276:24997-25004. [DOI] [PubMed] [Google Scholar]
- 49.Zehr, B. D., T. J. Savin, and R. E. Hall. 1989. A one-step, low background Coomassie staining procedure for polyacrylamide gels. Anal. Biochem. 182:157-159. [DOI] [PubMed] [Google Scholar]
- 50.Ziegler, K., R. Buder, J. Winter, and G. Fuchs. 1989. Activation of aromatic acids and aerobic 2-aminobenzoate metabolism in a denitrifying Pseudomonas strain. Arch. Microbiol. 151:171-176. [Google Scholar]