Abstract
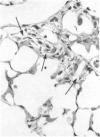
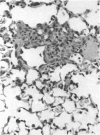
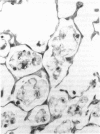
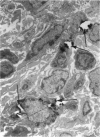
The effects of whole body irradiation (WBR) on particle clearance and the development of pulmonary fibrosis have been investigated. Using carbon, clearance is accomplished by polymorphonuclear leukocytes (PMN) and alveolar macrophages (AM), and only a few particles reach the interstitum. However, in preirradiated mice, the usual eflux of inflammatory cells is much delayed so that more free carbon remains in the alveoli, and by 1 week, many particles cross the epithelium to be phagocytized by interstitial macrophages. Carbon is found in the peribronchiolar interstitium 6 months later with no evidence of fibrosis. In the present study, mice received 1 mg silica intratracheally 2 days after 6.5 Gy WBR when the white blood cell count was low. A much-reduced AM and PMN response was found in the following 2 weeks compared to the reaction to silica alone, and many silica particles reached interstitial macrophages. In this case, macrophage activation by silica was associated with fibroblast proliferation, and by 16 weeks, much more pulmonary fibrosis was produced than after silica or irradiation only. This was measured biochemically and correlated with a large increase in retained silica in the irradiation-silica group. The results indicate that radiation inhibits the inflammatory response to particle instillation, resulting in greater translocation of free particles to the pulmonary interstitium. In the case of silica, the greater, prolonged interaction with interstitial macrophages leads to a much exaggerated fibrotic reaction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. Dose response of the pulmonary macrophagic system to various particulates and its relationship to transepithelial passage of free particles. Exp Lung Res. 1981 Aug;2(3):165–175. doi: 10.3109/01902148109052312. [DOI] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. Role of monocytes and interstitial cells in the generation of alveolar macrophages II. Kinetic studies after carbon loading. Lab Invest. 1980 May;42(5):518–524. [PubMed] [Google Scholar]
- Adamson I. Y., Letourneau H. L., Bowden D. H. Enhanced macrophage-fibroblast interactions in the pulmonary interstitium increases fibrosis after silica injection to monocyte-depleted mice. Am J Pathol. 1989 Feb;134(2):411–418. [PMC free article] [PubMed] [Google Scholar]
- Bitterman P. B., Adelberg S., Crystal R. G. Mechanisms of pulmonary fibrosis. Spontaneous release of the alveolar macrophage-derived growth factor in the interstitial lung disorders. J Clin Invest. 1983 Nov;72(5):1801–1813. doi: 10.1172/JCI111140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. Alveolar macrophage response to carbon in monocyte-depleted mice. Am Rev Respir Dis. 1982 Oct;126(4):708–711. doi: 10.1164/arrd.1982.126.4.708. [DOI] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. Role of monocytes and interstitial cells in the generation of alveolar macrophages I. Kinetic studies of normal mice. Lab Invest. 1980 May;42(5):511–517. [PubMed] [Google Scholar]
- Brody A. R., Hill L. H. Interstitial accumulation of inhaled chrysotile asbestos fibers and consequent formation of microcalcifications. Am J Pathol. 1982 Oct;109(1):107–114. [PMC free article] [PubMed] [Google Scholar]
- Dauber J. H., Daniele R. P. Secretion of chemotaxins by guinea pig lung macrophages. I. The spectrum of inflammatory cell responses. Exp Lung Res. 1980 Mar;1(1):23–32. doi: 10.3109/01902148009057510. [DOI] [PubMed] [Google Scholar]
- Dethloff L. A., Lehnert B. E. Pulmonary interstitial macrophages: isolation and flow cytometric comparisons with alveolar macrophages and blood monocytes. J Leukoc Biol. 1988 Jan;43(1):80–90. doi: 10.1002/jlb.43.1.80. [DOI] [PubMed] [Google Scholar]
- Goldstein R. H., Fine A. Fibrotic reactions in the lung: the activation of the lung fibroblast. Exp Lung Res. 1986;11(4):245–261. doi: 10.3109/01902148609062828. [DOI] [PubMed] [Google Scholar]
- Heppleston A. G., Young A. E. Uptake of inert particulate matter by alveolar cells: an ultrastructural study. J Pathol. 1973 Nov;111(3):159–164. doi: 10.1002/path.1711110303. [DOI] [PubMed] [Google Scholar]
- Sawyer R. T., Strausbauch P. H., Volkman A. Resident macrophage proliferation in mice depleted of blood monocytes by strontium-89. Lab Invest. 1982 Feb;46(2):165–170. [PubMed] [Google Scholar]
- Sawyer R. T. The significance of local resident pulmonary alveolar macrophage proliferation to population renewal. J Leukoc Biol. 1986 Jan;39(1):77–87. doi: 10.1002/jlb.39.1.77. [DOI] [PubMed] [Google Scholar]
- Shellito J., Esparza C., Armstrong C. Maintenance of the normal rat alveolar macrophage cell population. The roles of monocyte influx and alveolar macrophage proliferation in situ. Am Rev Respir Dis. 1987 Jan;135(1):78–82. doi: 10.1164/arrd.1987.135.1.78. [DOI] [PubMed] [Google Scholar]
- Sorokin S. P., Hoyt R. F., Jr Pure population of nonmonocyte derived macrophages arising in organ cultures of embryonic rat lungs. Anat Rec. 1987 Jan;217(1):35–52. doi: 10.1002/ar.1092170107. [DOI] [PubMed] [Google Scholar]
- Tarling J. D., Coggle J. E. The absence of effect on pulmonary alveolar macrophage numbers during prolonged periods of monocytopenia. J Reticuloendothel Soc. 1982 Mar;31(3):221–224. [PubMed] [Google Scholar]