Abstract
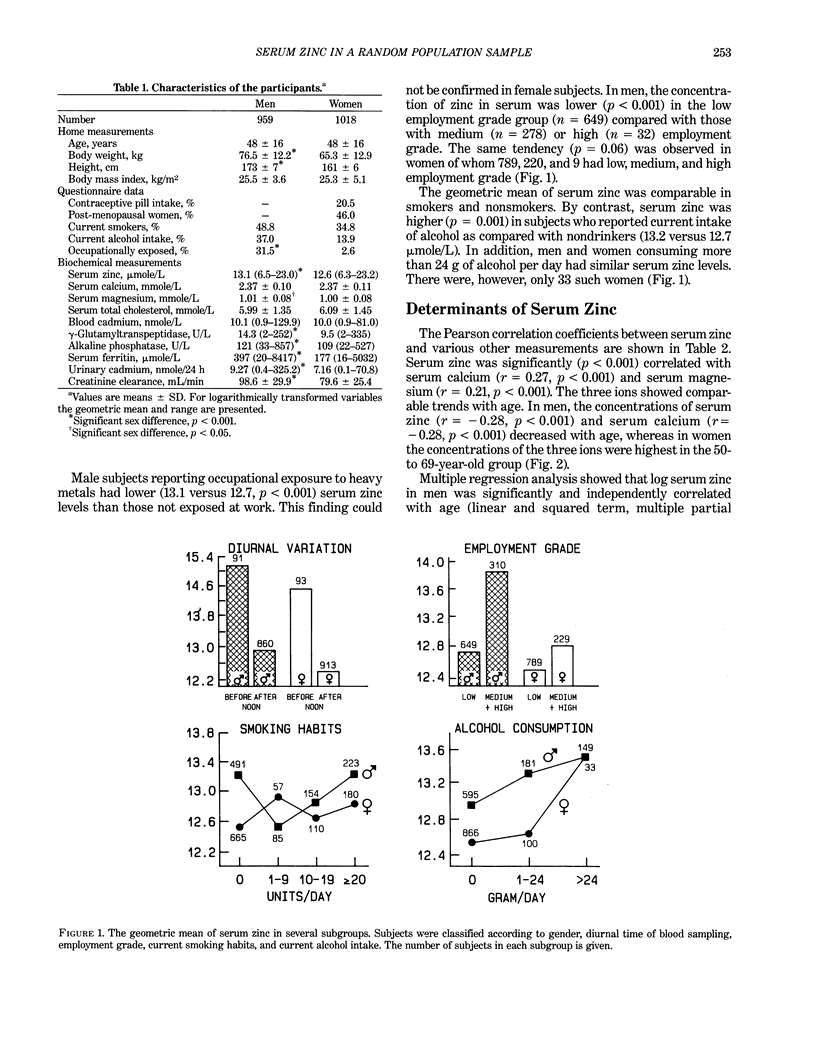
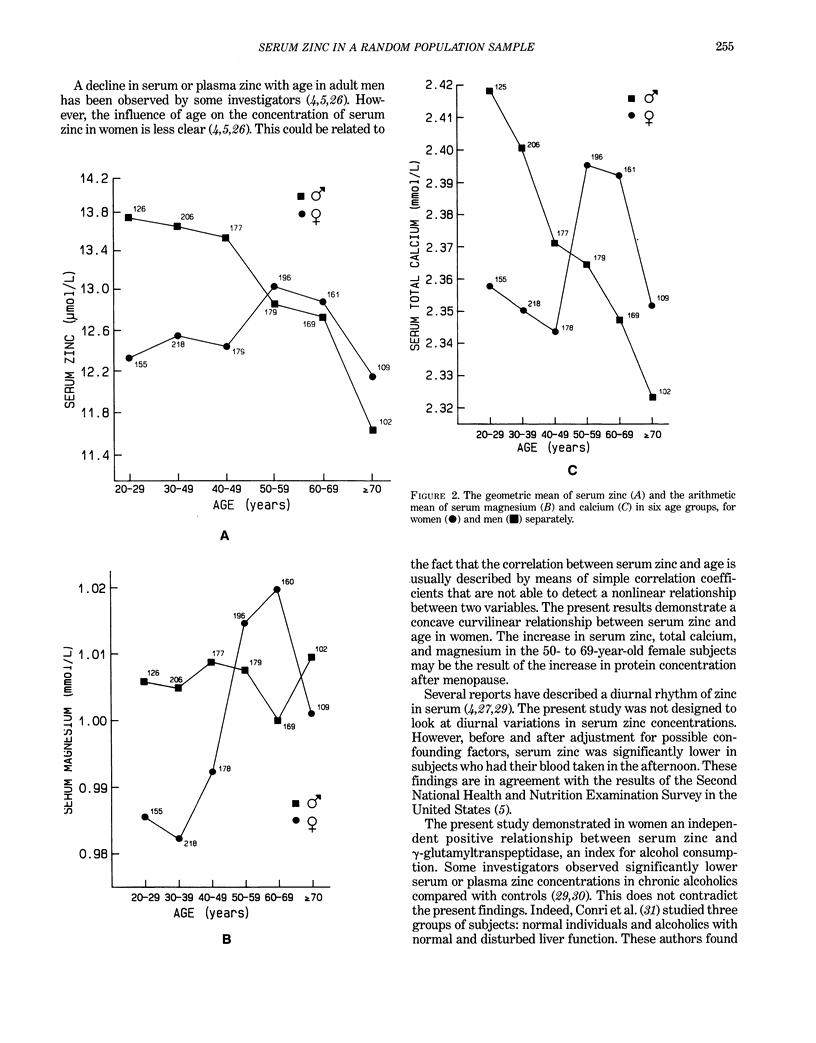
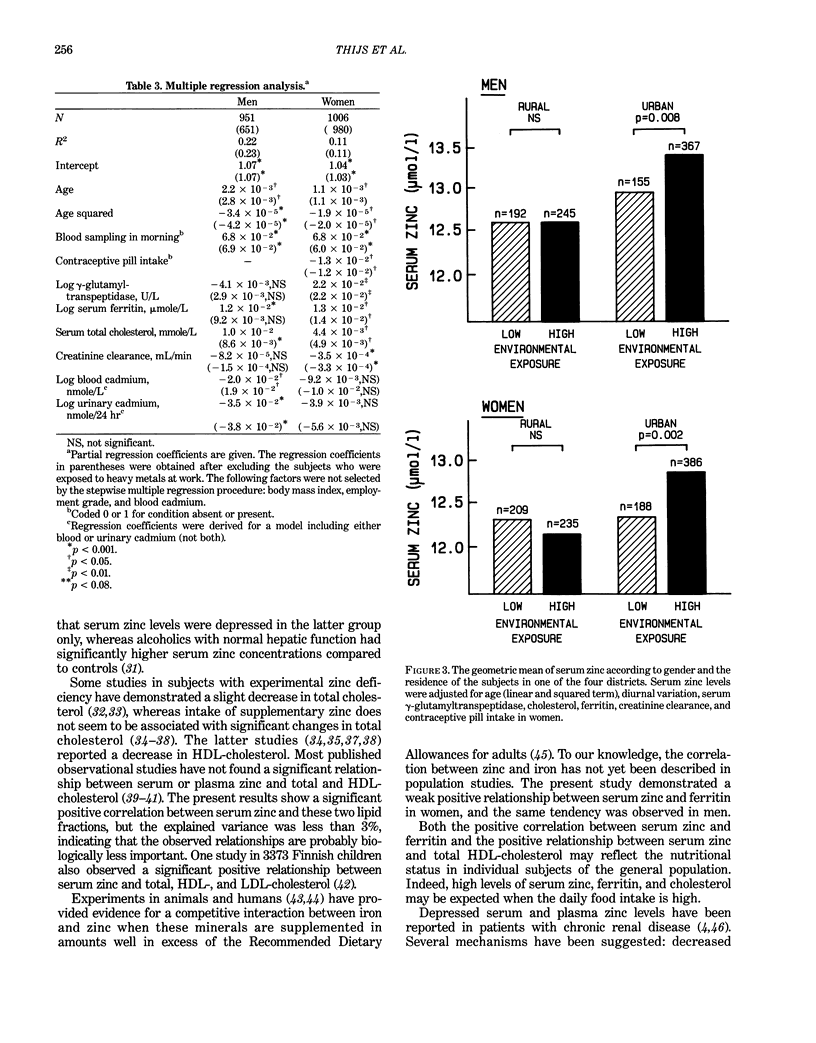
This report investigated the distribution of serum zinc and the factors determining serum zinc concentration in a large random population sample. The 1977 participants (959 men and 1018 women), 20–80 years old, constituted a stratified random sample of the population of four Belgian districts, representing two areas with low and two with high environmental exposure to cadmium. For each exposure level, a rural and an urban area were selected. The serum concentration of zinc, frequently used as an index for zinc status in human subjects, was higher in men (13.1 μmole/L, range 6.5–23.0 μmole/L) than in women (12.6 μmole/L, range 6.3–23.2 μmole/L). In men, 20% of the variance of serum zinc was explained by age (linear and squared term, R = 0.29), diurnal variation (r = 0.29), and total cholesterol (r = 0.16). After adjustment for these covariates, a negative relationship was observed between serum zinc and both blood (r = −0.10) and urinary cadmium (r = −0.14). In women, 11% of the variance could be explained by age (linear and squared term, R = 0.15), diurnal variation in serum zinc (r = 0.27), creatinine clearance (r = −0.11), log γ-glutamyltranspeptidase (r = 0.08), cholesterol (r = 0.07), contraceptive pill intake (r = −0.07), and log serum ferritin (r = 0.06). Before and after adjustment for significant covariates, serum zinc was, on average, lowest in the two districts where the body burden of cadmium, as assessed by urinary cadmium excretion, was highest. These results were not altered when subjects exposed to heavy metals at work were excluded from analysis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu-Hamdan D. K., Mahajan S. K., Migdal S. D., Prasad A. S., McDonald F. D. Zinc tolerance test in uremia. Effect of ferrous sulfate and aluminum hydroxide. Ann Intern Med. 1986 Jan;104(1):50–52. doi: 10.7326/0003-4819-104-1-50. [DOI] [PubMed] [Google Scholar]
- Antoniou L. D., Shalhoub R. J., Elliot S. Zinc tolerance tests in chronic uremia. Clin Nephrol. 1981 Oct;16(4):181–187. [PubMed] [Google Scholar]
- Baer M. T., King J. C., Tamura T., Margen S., Bradfield R. B., Weston W. L., Daugherty N. A. Nitrogen utilization, enzyme activity, glucose intolerance and leukocyte chemotaxis in human experimental zinc depletion. Am J Clin Nutr. 1985 Jun;41(6):1220–1235. doi: 10.1093/ajcn/41.6.1220. [DOI] [PubMed] [Google Scholar]
- Bartels H., Böhmer M. Eine Mikromethode zur Kreatininbestimmung. Clin Chim Acta. 1971 Mar;32(1):81–85. doi: 10.1016/0009-8981(71)90467-0. [DOI] [PubMed] [Google Scholar]
- Bernard A. M., Lauwerys R. R. Continuous-flow system for automation of latex immunoassay by particle counting. Clin Chem. 1983 Jun;29(6):1007–1011. [PubMed] [Google Scholar]
- Black M. R., Medeiros D. M., Brunett E., Welke R. Zinc supplements and serum lipids in young adult white males. Am J Clin Nutr. 1988 Jun;47(6):970–975. doi: 10.1093/ajcn/47.6.970. [DOI] [PubMed] [Google Scholar]
- Briggs M. H., Briggs M., Austin J. Effects of steroid pharmaceuticals on plasma zinc. Nature. 1971 Aug 13;232(5311):480–481. doi: 10.1038/232480a0. [DOI] [PubMed] [Google Scholar]
- Burstein M., Scholnick H. R., Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res. 1970 Nov;11(6):583–595. [PubMed] [Google Scholar]
- Buxaderas S. C., Farré-Rovira R. Whole blood and serum zinc levels in relation to sex and age. Rev Esp Fisiol. 1985 Dec;41(4):463–470. [PubMed] [Google Scholar]
- Chandra R. K. Excessive intake of zinc impairs immune responses. JAMA. 1984 Sep 21;252(11):1443–1446. [PubMed] [Google Scholar]
- Condon C. J., Freeman R. M. Zinc metabolism in renal failure. Ann Intern Med. 1970 Oct;73(4):531–536. doi: 10.7326/0003-4819-73-4-531. [DOI] [PubMed] [Google Scholar]
- Conri C., Fleury B., Simonoff M., Ducloux G., Berdeu B., Moretto P. Sélénium, zinc et cuivre sériques au cours de l'alcoolisme. Ann Med Interne (Paris) 1988;139(2):138–139. [PubMed] [Google Scholar]
- Cousins R. J. Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin. Physiol Rev. 1985 Apr;65(2):238–309. doi: 10.1152/physrev.1985.65.2.238. [DOI] [PubMed] [Google Scholar]
- Fischer P. W., Collins M. W. Relationship between serum zinc and copper and risk factors associated with cardiovascular disease. Am J Clin Nutr. 1981 Apr;34(4):595–597. doi: 10.1093/ajcn/34.4.595. [DOI] [PubMed] [Google Scholar]
- Guillard O., Piriou A., Gombert J., Reiss D. Diurnal variations of zinc, copper and magnesium in the serum of normal fasting adults. Biomedicine. 1979 Nov;31(7):193–194. [PubMed] [Google Scholar]
- Hooper P. L., Visconti L., Garry P. J., Johnson G. E. Zinc lowers high-density lipoprotein-cholesterol levels. JAMA. 1980 Oct 24;244(17):1960–1961. [PubMed] [Google Scholar]
- Kelson J. R., Shamberger R. J. Methods compared for determining zinc in serum by flame atomic absorption spectroscopy. Clin Chem. 1978 Feb;24(2):240–244. [PubMed] [Google Scholar]
- Lauwerys R., Amery A., Bernard A., Bruaux P., Buchet J. P., Claeys F., De Plaen P., Ducoffre G., Fagard R., Lijnen P. Health effects of environmental exposure to cadmium: objectives, design and organization of the Cadmibel Study: a cross-sectional morbidity study carried out in Belgium from 1985 to 1989. Environ Health Perspect. 1990 Jul;87:283–289. doi: 10.1289/ehp.9087283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeman R. D., Clark M. L., Colmore J. P. Influence of age and sex on plasma and red-cell zinc concentrations. J Gerontol. 1971 Jul;26(3):358–363. doi: 10.1093/geronj/26.3.358. [DOI] [PubMed] [Google Scholar]
- Mahajan S. K. Zinc in kidney disease. J Am Coll Nutr. 1989 Aug;8(4):296–304. doi: 10.1080/07315724.1989.10720305. [DOI] [PubMed] [Google Scholar]
- Markowitz M. E., Rosen J. F., Mizruchi M. Circadian variations in serum zinc (Zn) concentrations: correlation with blood ionized calcium, serum total calcium and phosphate in humans. Am J Clin Nutr. 1985 Apr;41(4):689–696. doi: 10.1093/ajcn/41.4.689. [DOI] [PubMed] [Google Scholar]
- Medeiros D., Pellum L., Brown B. Serum lipids and glucose as associated with hemoglobin levels and copper and zinc intake in young adults. Life Sci. 1983 Apr 18;32(16):1897–1904. doi: 10.1016/0024-3205(83)90069-3. [DOI] [PubMed] [Google Scholar]
- Persijn J. P., van der Slik W. A new method for the determination of gamma-glutamyltransferase in serum. J Clin Chem Clin Biochem. 1976 Sep;14(9):421–427. doi: 10.1515/cclm.1976.14.1-12.421. [DOI] [PubMed] [Google Scholar]
- Pilch S. M., Senti F. R. Analysis of zinc data from the second National Health and Nutrition Examination Survey (NHANES II). J Nutr. 1985 Nov;115(11):1393–1397. doi: 10.1093/jn/115.11.1393. [DOI] [PubMed] [Google Scholar]
- Prasad A. S., Oberleas D., Moghissi K. S., Lei K. Y., Stryker J. C. Effect of oral contraceptive agents on nutrients: I. Minerals. Am J Clin Nutr. 1975 Apr;28(4):377–384. doi: 10.1093/ajcn/28.4.377. [DOI] [PubMed] [Google Scholar]
- Reimold E. W. Zinc changes after renal allotransplantation. South Med J. 1980 Nov;73(11):1457–1460. doi: 10.1097/00007611-198011000-00011. [DOI] [PubMed] [Google Scholar]
- Roels H. A., Lauwerys R. R., Buchet J. P., Bernard A. Environmental exposure to cadmium and renal function of aged women in three areas of Belgium. Environ Res. 1981 Feb;24(1):117–130. doi: 10.1016/0013-9351(81)90138-9. [DOI] [PubMed] [Google Scholar]
- Solomons N. W. On the assessment of zinc and copper nutriture in man. Am J Clin Nutr. 1979 Apr;32(4):856–871. doi: 10.1093/ajcn/32.4.856. [DOI] [PubMed] [Google Scholar]
- Staessen J., Bulpitt C. J., Fagard R., Joossens J. V., Lijnen P., Amery A. Salt intake and blood pressure in the general population: a controlled intervention trial in two towns. J Hypertens. 1988 Dec;6(12):965–973. doi: 10.1097/00004872-198812000-00003. [DOI] [PubMed] [Google Scholar]
- Sullivan J. F., Blotcky A. J., Jetton M. M., Hahn H. K., Burch R. E. Serum levels of selenium, calcium, copper magnesium, manganese and zinc in various human diseases. J Nutr. 1979 Aug;109(8):1432–1437. doi: 10.1093/jn/109.8.1432. [DOI] [PubMed] [Google Scholar]
- Tiber A. M., Sakhaii M., Joffe C. D., Ratnaparkhi M. V. Relative value of plasma copper, zinc, lipids and lipoproteins as markers for coronary artery disease. Atherosclerosis. 1986 Nov;62(2):105–110. doi: 10.1016/0021-9150(86)90054-7. [DOI] [PubMed] [Google Scholar]
- Verschoor M., Herber R., van Hemmen J., Wibowo A., Zielhuis R. Renal function of workers with low-level cadmium exposure. Scand J Work Environ Health. 1987 Jun;13(3):232–238. doi: 10.5271/sjweh.2065. [DOI] [PubMed] [Google Scholar]
- Yadrick M. K., Kenney M. A., Winterfeldt E. A. Iron, copper, and zinc status: response to supplementation with zinc or zinc and iron in adult females. Am J Clin Nutr. 1989 Jan;49(1):145–150. doi: 10.1093/ajcn/49.1.145. [DOI] [PubMed] [Google Scholar]
- Zarski J. P., Arnaud J., Dumolard L., Favier A., Rachail M. Oligo-éléments (zinc, cuivre, manganèse) dans la cirrhose alcoolique: influence de l'alcoolisme chronique. Gastroenterol Clin Biol. 1985 Oct;9(10):664–669. [PubMed] [Google Scholar]


