Abstract
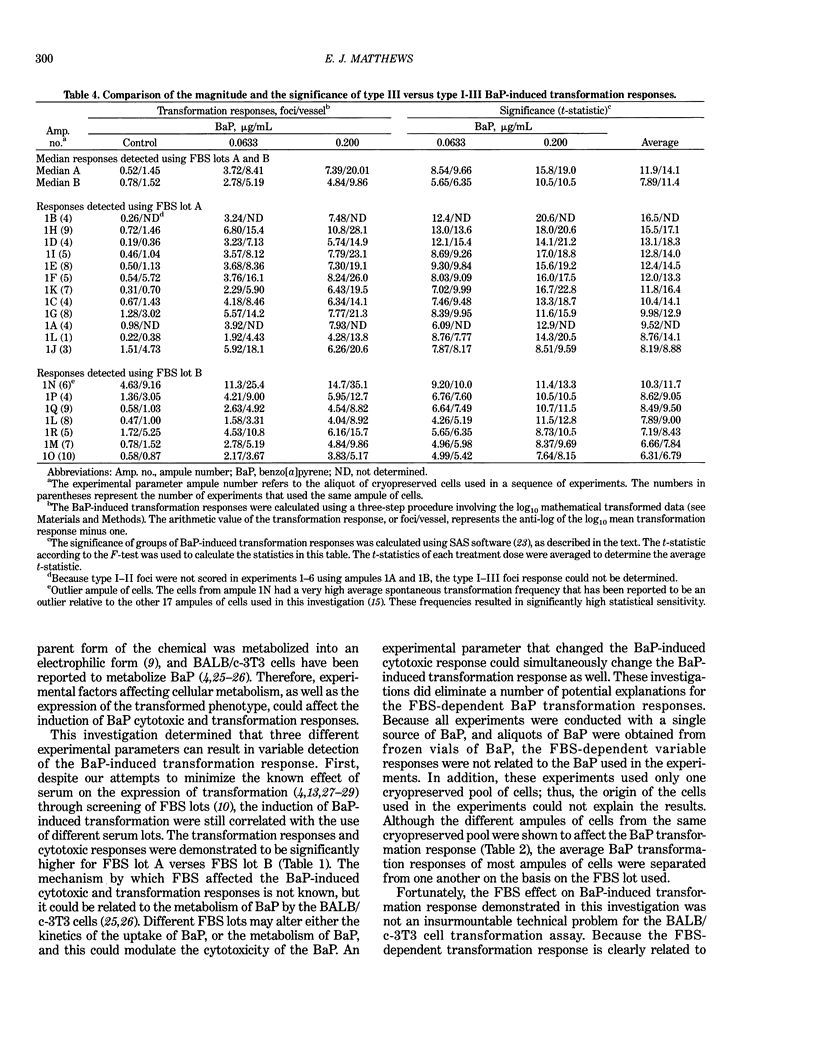
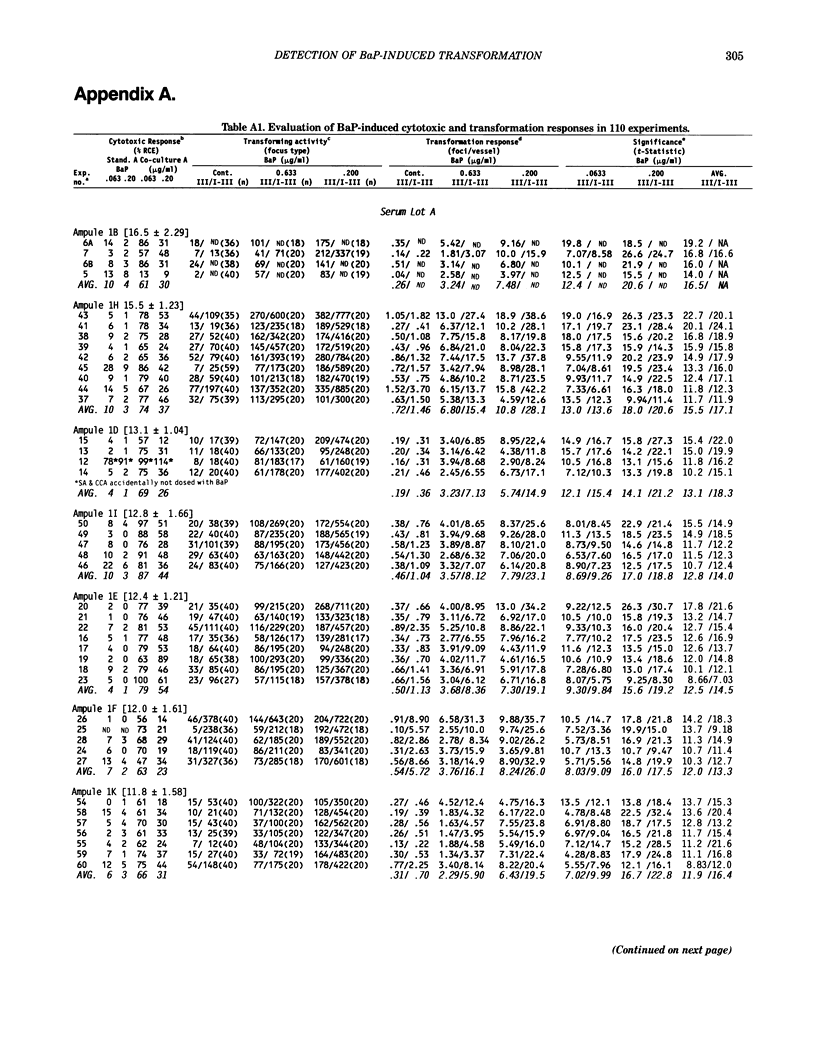
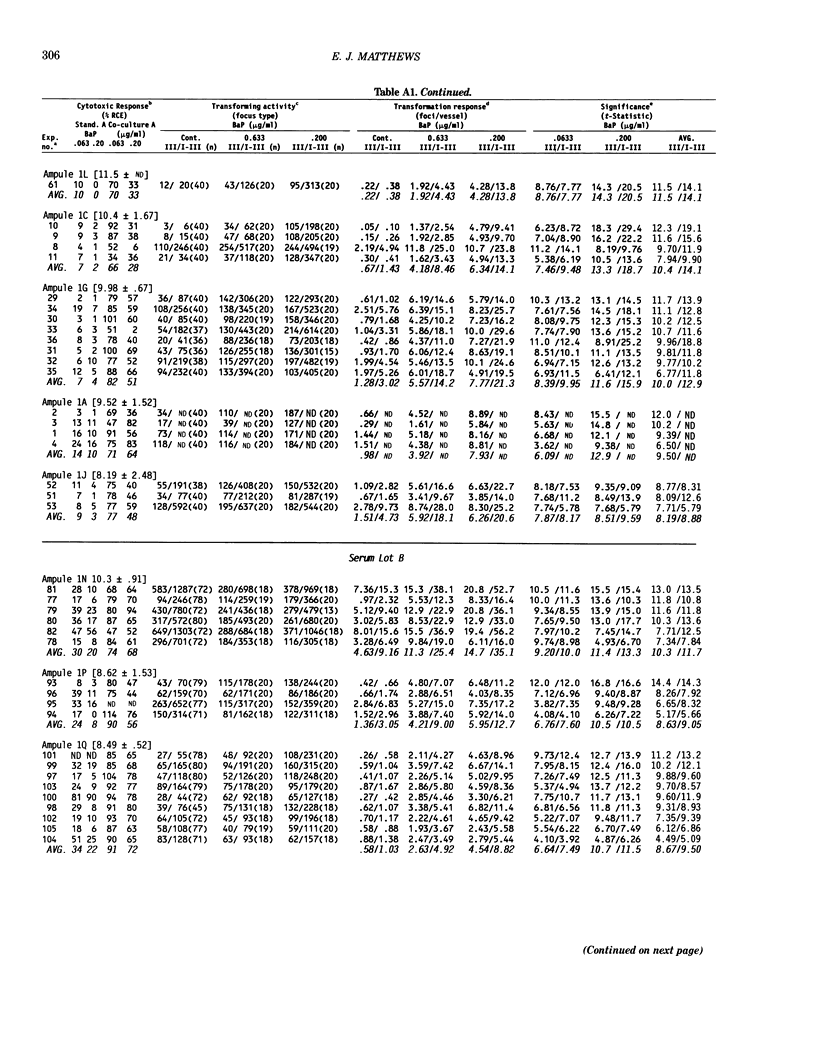
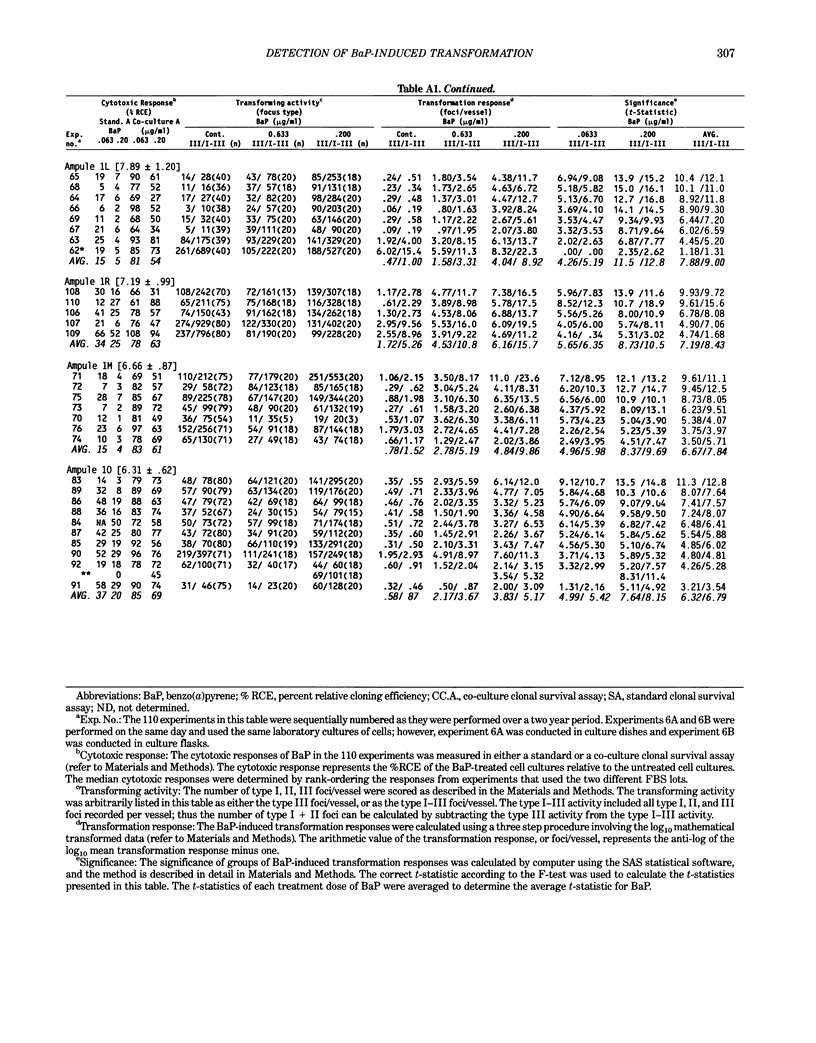
Benzo[a]pyrene (BaP) induced significant morphological transformation of clone A31-1-13 BALB/c-3T3 cells without exogenous activation. Therefore, BaP was selected as a model to determine the internal consistency of detection of chemical-induced transformation. BaP induced a continuum of type I-III foci of different sizes, and the ratio of type I-III to type III foci/vessel was usually about 2-fold. The major finding was that BaP induced highly significant transformation responses, and the magnitude of these responses were inversely correlated with the cytotoxicity of the treatment doses. Thus, the induction of BaP-induced transformation behaved as though it was caused by a mutational event. Variability among responses were shown to depend on the serum lot and the cryopreserved ampule of cells. In addition, experiments with low spontaneous transformation responses had an impaired ability to detect BaP; however, experiments with high or normal spontaneous responses had a normal ability to detect BaP. Because the expression of BaP-induced transformation depended on both the cytotoxicity of the treatment and the cumulative number of mitoses, the frequency of BaP-induced transformation should be reported as the number of foci/vessel, but not expressed as the number of foci/viable cell surviving the chemical treatment. These conclusions are important because the same 110 experiments described in this report were also used to evaluate the transformation responses of many different carcinogenic and noncarcinogenic chemicals. These data are being reported separately.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby J., Tennant R. W. Chemical structure, Salmonella mutagenicity and extent of carcinogenicity as indicators of genotoxic carcinogenesis among 222 chemicals tested in rodents by the U.S. NCI/NTP. Mutat Res. 1988 Jan;204(1):17–115. doi: 10.1016/0165-1218(88)90114-0. [DOI] [PubMed] [Google Scholar]
- Bertram J. S. Effects of serum concentration on the expression of carcinogen-induced transformation in the C3H/10T1/2 CL8 cell line. Cancer Res. 1977 Feb;37(2):514–523. [PubMed] [Google Scholar]
- Dunkel V. C., Pienta R. J., Sivak A., Traul K. A. Comparative neoplastic transformation responses of Balb/3T3 cells, Syrian hamster embryo cells, and Rauscher murine leukemia virus-infected Fischer 344 rat embryo cells to chemical compounds. J Natl Cancer Inst. 1981 Dec;67(6):1303–1312. [PubMed] [Google Scholar]
- Frazelle J. H., Abernethy D. J., Boreiko C. J. Factors influencing the promotion of transformation in chemically-initiated C3H/10T1/2 Cl 8 mouse embryo fibroblasts. Carcinogenesis. 1983;4(6):709–715. doi: 10.1093/carcin/4.6.709. [DOI] [PubMed] [Google Scholar]
- Grisham J. W., Smith G. J., Lee L. W., Bentley K. S., Fatteh M. V. Induction of foci of morphologically transformed cells in synchronized populations of 10T1/2 cells by N-methyl-N'-nitro-N-nitrosoguanidine and the effect of spontaneous transformation on calculated transformation frequency. Cancer Res. 1988 Nov 1;48(21):5977–5983. [PubMed] [Google Scholar]
- Grisham J. W., Smith G. J., Lee L. W., Bentley K. S., Fatteh M. V. Spontaneous formation of foci of morphologically transformed cells in populations of C3H 10T1/2 (clone 8) cells. Cancer Res. 1988 Nov 1;48(21):5969–5976. [PubMed] [Google Scholar]
- Heidelberger C., Freeman A. E., Pienta R. J., Sivak A., Bertram J. S., Casto B. C., Dunkel V. C., Francis M. W., Kakunaga T., Little J. B. Cell transformation by chemical agents--a review and analysis of the literature. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat Res. 1983 Apr;114(3):283–385. doi: 10.1016/0165-1110(83)90036-2. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Casciano D. A., Couch D. B., Krahn D. F., O'Neill J. P., Whitfield B. L. The use of Chinese hamster ovary cells to quantify specific locus mutation and to determine mutagenicity of chemicals. A report of the gene-tox program. Mutat Res. 1981 Mar;86(2):193–214. doi: 10.1016/0165-1110(81)90024-5. [DOI] [PubMed] [Google Scholar]
- Kakunaga T. A quantitative system for assay of malignant transformation by chemical carcinogens using a clone derived from BALB-3T3. Int J Cancer. 1973 Sep 15;12(2):463–473. doi: 10.1002/ijc.2910120217. [DOI] [PubMed] [Google Scholar]
- Kakunaga T., Crow J. D. Cell variants showing differential susceptibility to ultraviolet light--induced transformation. Science. 1980 Jul 25;209(4455):505–507. doi: 10.1126/science.7394516. [DOI] [PubMed] [Google Scholar]
- Kuroki T., Sasaki K. Relationship between in-vitro cell transformation and in-vivo carcinogenesis based on available data on the effects of chemicals. IARC Sci Publ. 1985;67:93–118. [PubMed] [Google Scholar]
- Lo K. Y., Kakunaga T. Similarities in the formation and removal of covalent DNA adducts in benzo(a)pyrene-treated BALB/3T3 variant cells with different induced transformation frequencies. Cancer Res. 1982 Jul;42(7):2644–2650. [PubMed] [Google Scholar]
- Matthews E. J., Spalding J. W., Tennant R. W. Transformation of BALB/c-3T3 cells: IV. Rank-ordered potency of 24 chemical responses detected in a sensitive new assay procedure. Environ Health Perspect. 1993 Jul;101 (Suppl 2):319–345. doi: 10.1289/ehp.93101s2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. J. Transformation of BALB/c-3T3 cells: I. Investigation of experimental parameters that influence detection of spontaneous transformation. Environ Health Perspect. 1993 Jul;101 (Suppl 2):277–291. doi: 10.1289/ehp.93101s2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. J. Transformation of BALB/c-3T3 cells: III. Development of a co-culture clonal survival assay for quantification of chemical cytotoxicity in high-density cell cultures. Environ Health Perspect. 1993 Jul;101 (Suppl 2):311–318. doi: 10.1289/ehp.93101s2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. B., Brown A., Cattanach P., Edwards I., McBride D., Riach C., Caspary W. J. Responses of the L5178Y tk+/tk- mouse lymphoma cell forward mutation assay: III. 72 coded chemicals. Environ Mol Mutagen. 1988;12(1):85–154. doi: 10.1002/em.2860120111. [DOI] [PubMed] [Google Scholar]
- Oshiro Y., Balwierz P. S., Piper C. E. Selection of fetal bovine serum for use in the C3H/10T 1/2 CL8 cell transformation assay system. Environ Mutagen. 1982;4(5):569–574. doi: 10.1002/em.2860040508. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Schechtman L. M. Metabolic activation of procarcinogens by subcellular enzyme fractions in the C3H 10T1/2 and BALB/c 3T3 cell transformation systems. IARC Sci Publ. 1985;67:137–162. [PubMed] [Google Scholar]
- Sivak A., Van Duuren B. L. Phenotypic expression of transformation: induction in cell culture by a phorbol ester. Science. 1967 Sep 22;157(3795):1443–1444. doi: 10.1126/science.157.3795.1443. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Margolin B. H., Shelby M. D., Zeiger E., Haseman J. K., Spalding J., Caspary W., Resnick M., Stasiewicz S., Anderson B. Prediction of chemical carcinogenicity in rodents from in vitro genetic toxicity assays. Science. 1987 May 22;236(4804):933–941. doi: 10.1126/science.3554512. [DOI] [PubMed] [Google Scholar]
- Whorton E. B., Jr, Ward J. B., Jr, Morris D. L. Experimental design and statistical analysis considerations for in vitro mammalian cell transformation assays with BALB/3T3 cells. Environ Mutagen. 1982;4(5):595–603. doi: 10.1002/em.2860040511. [DOI] [PubMed] [Google Scholar]


