Abstract
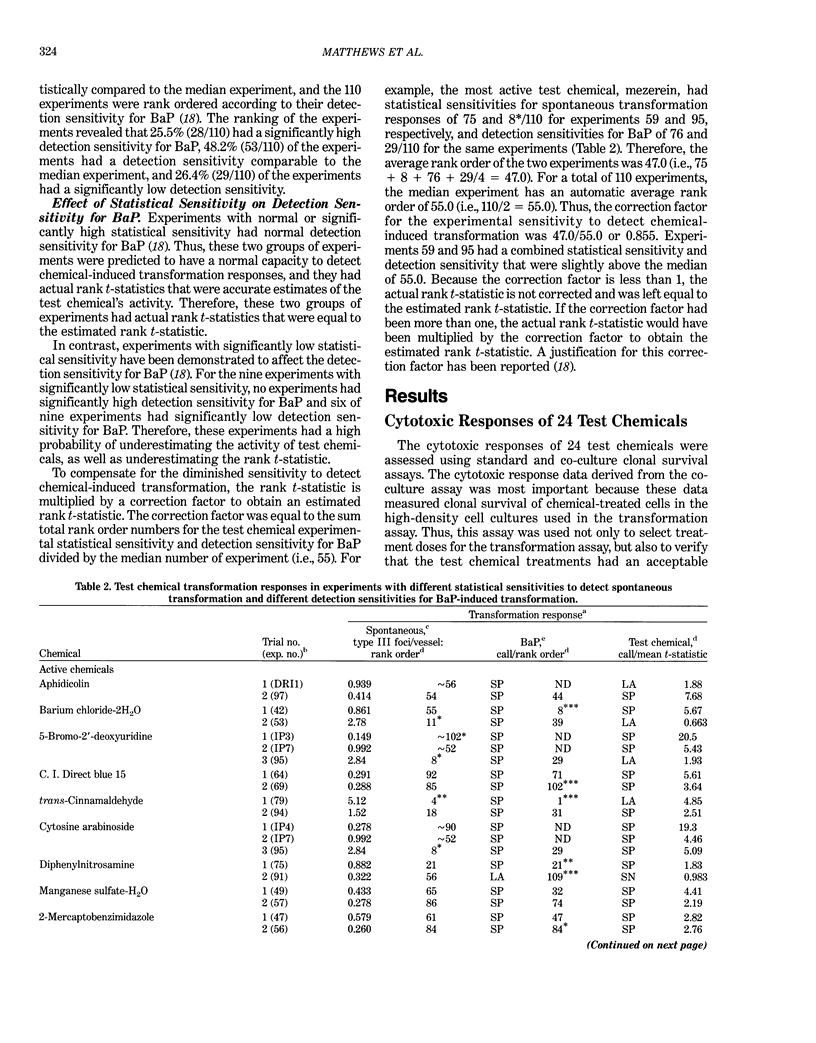
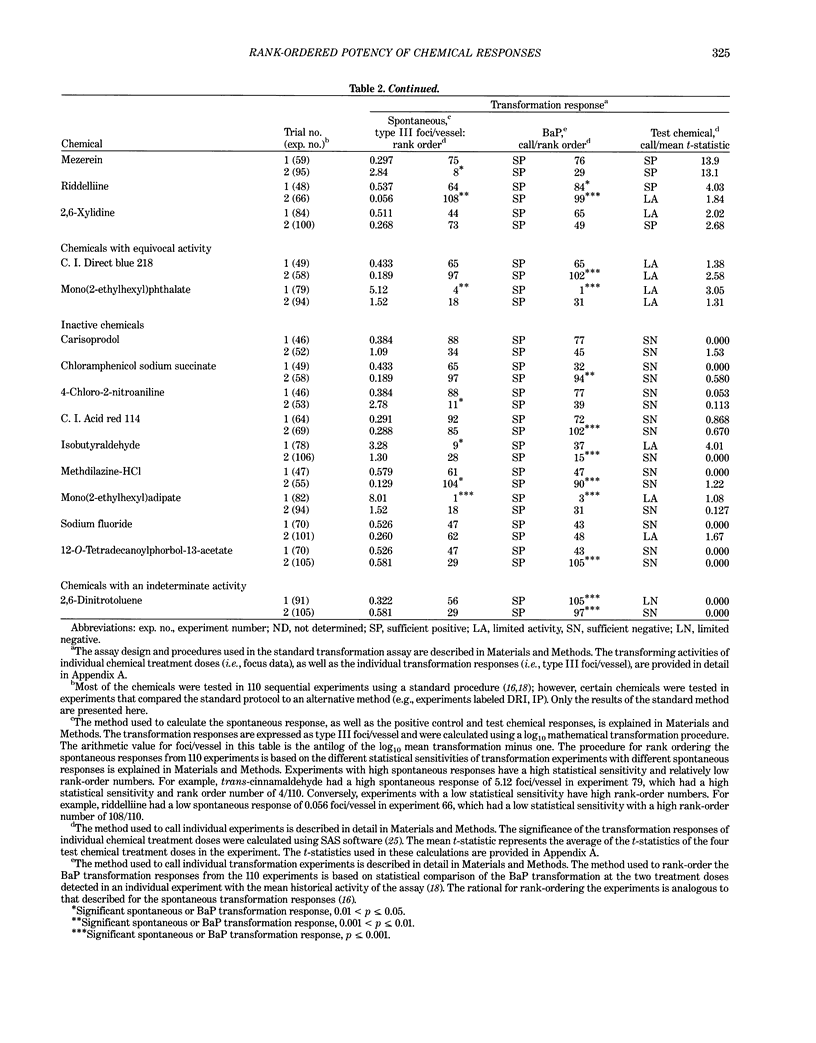
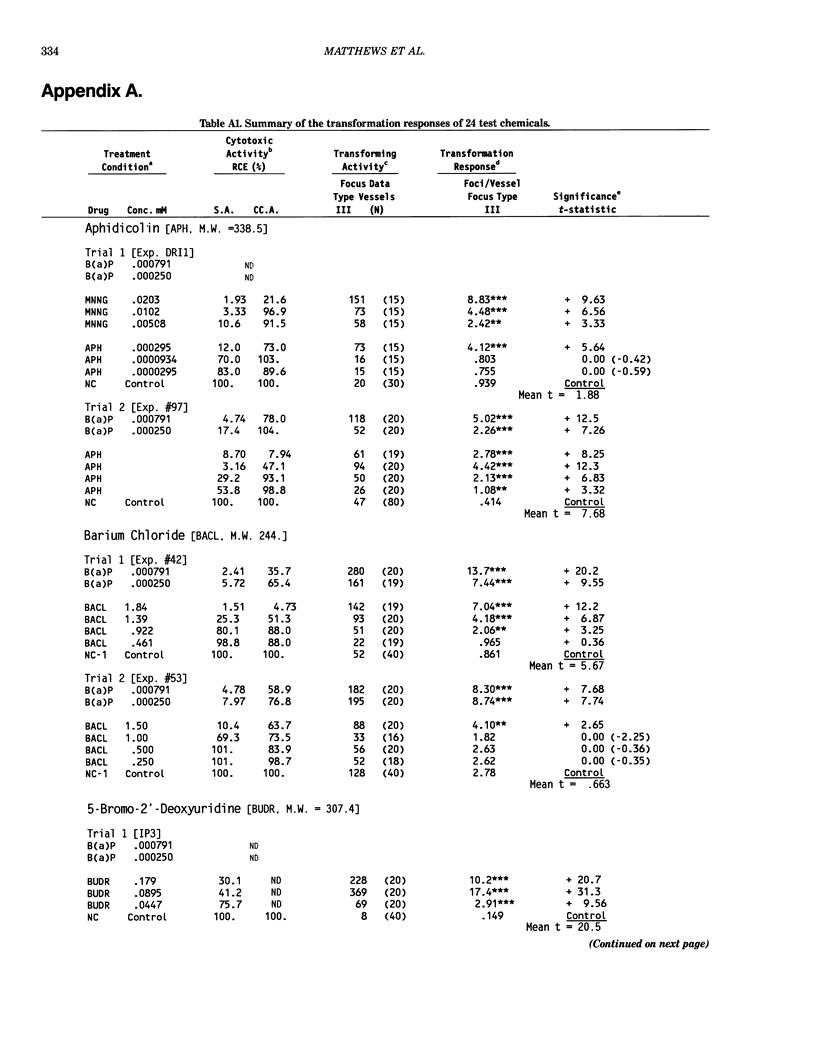
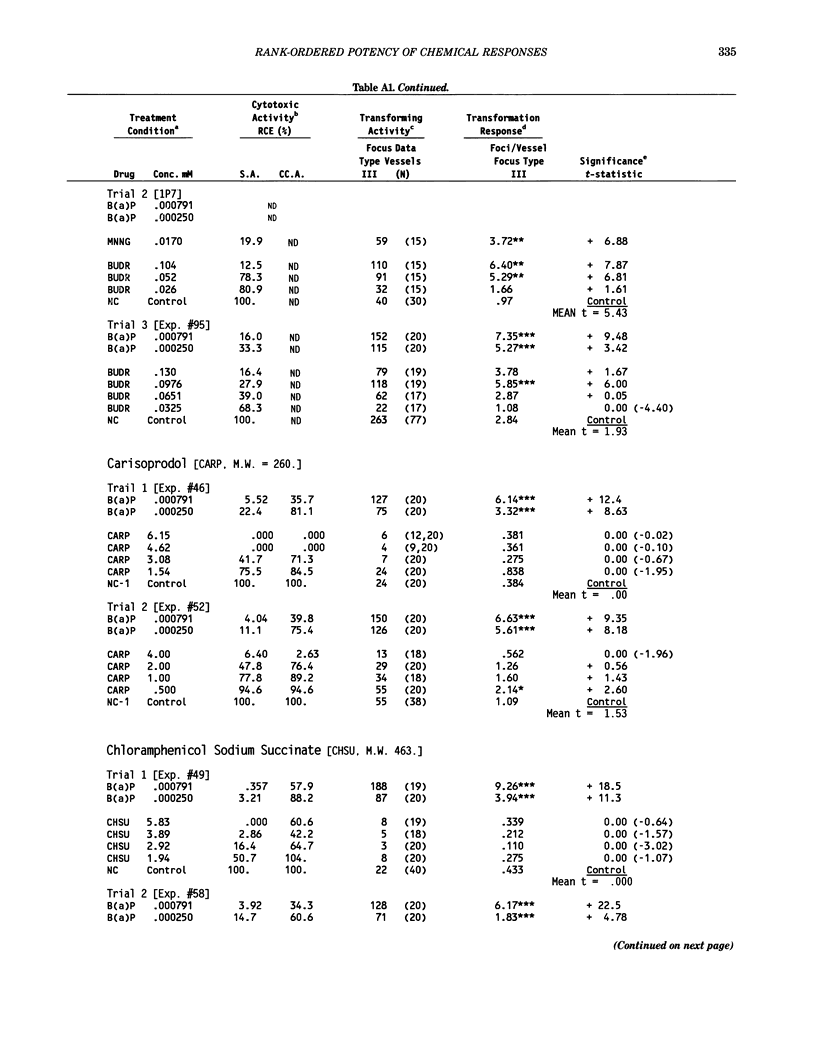
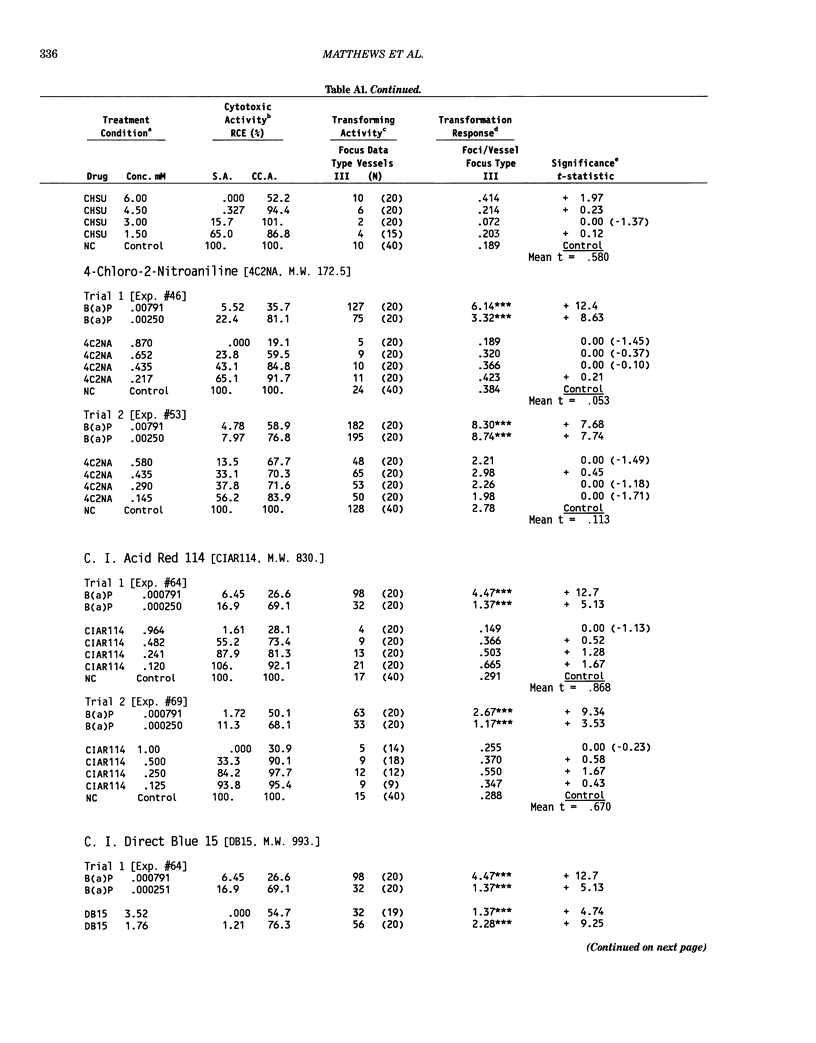
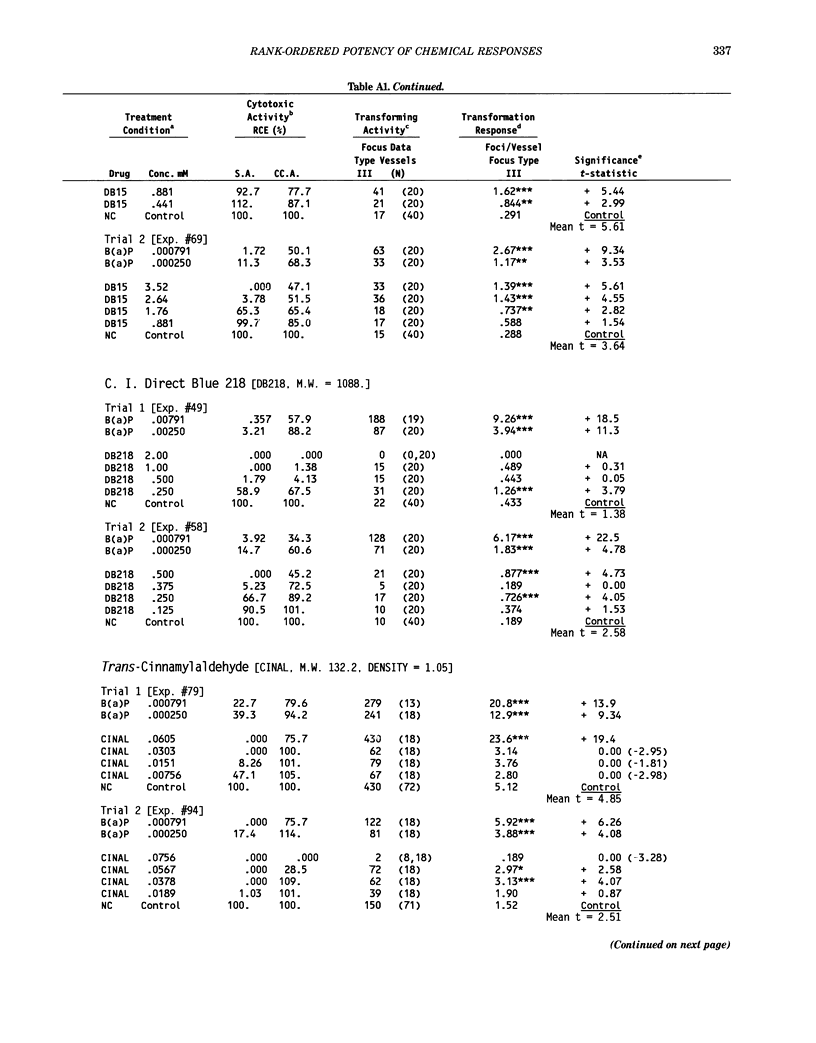
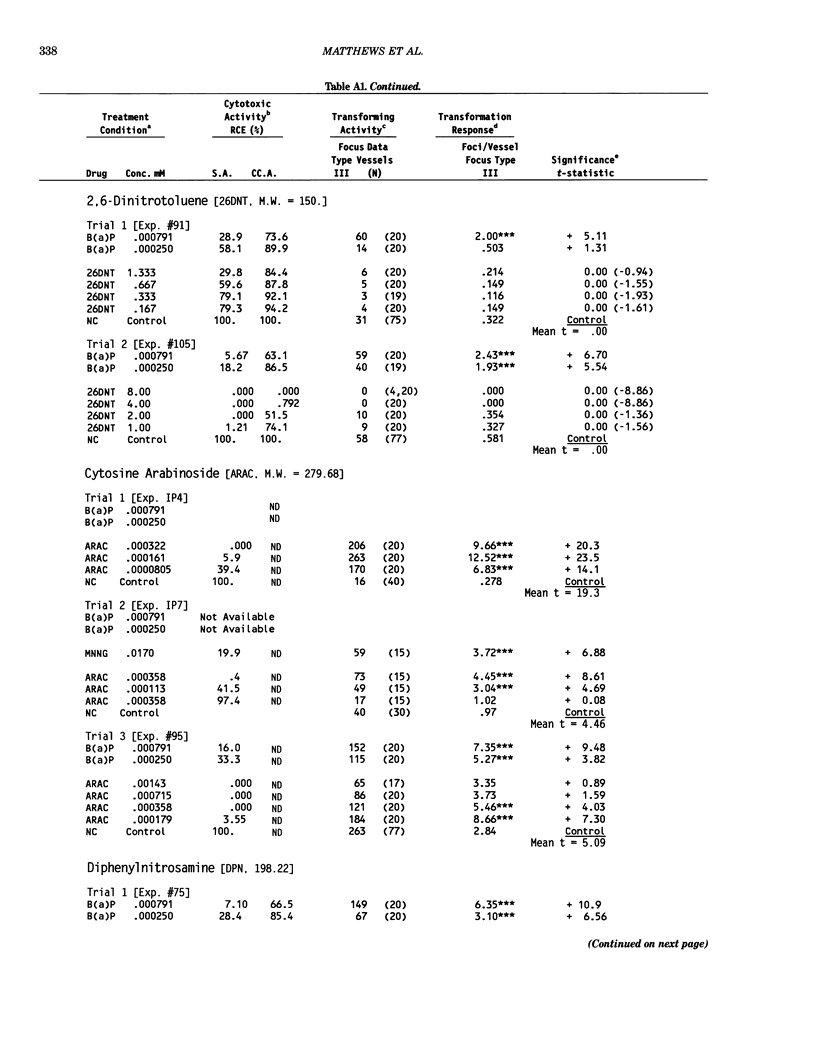
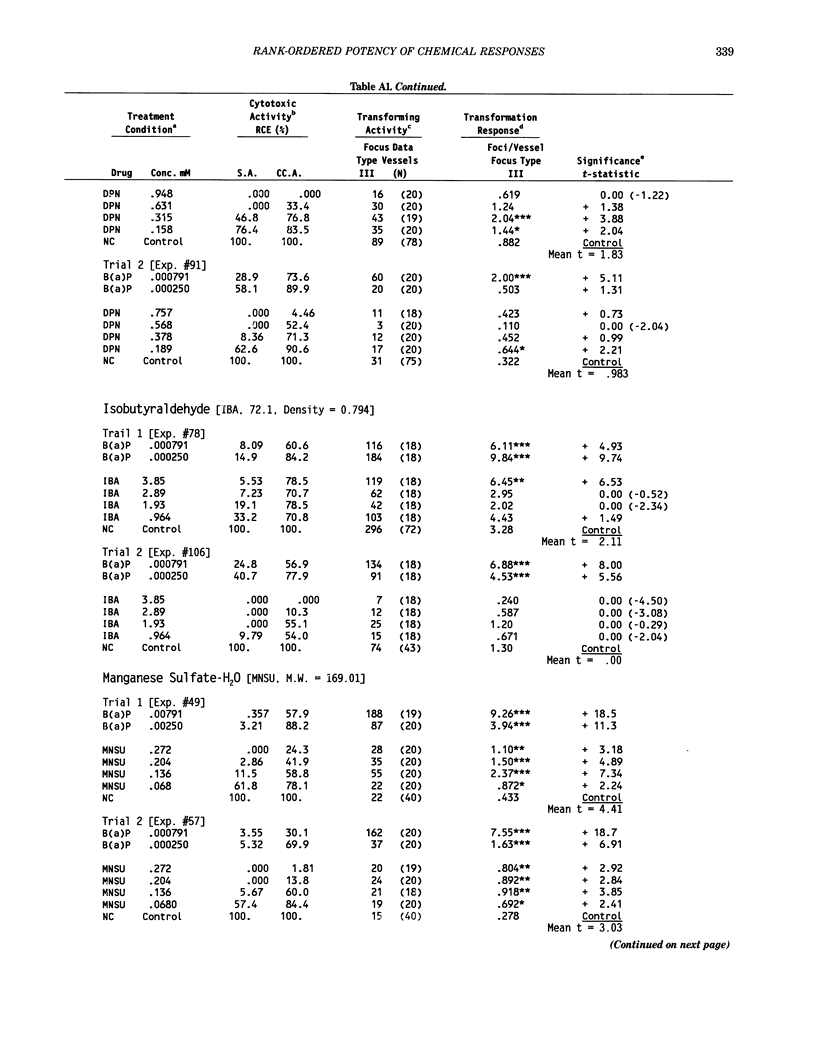
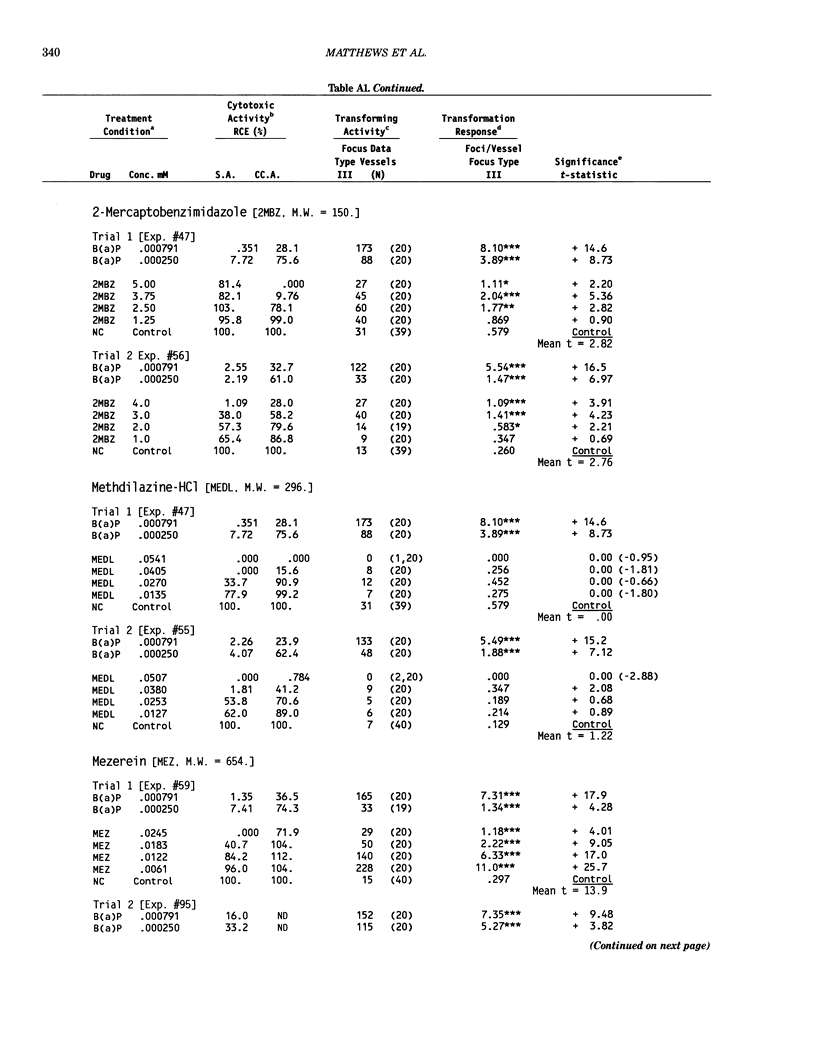
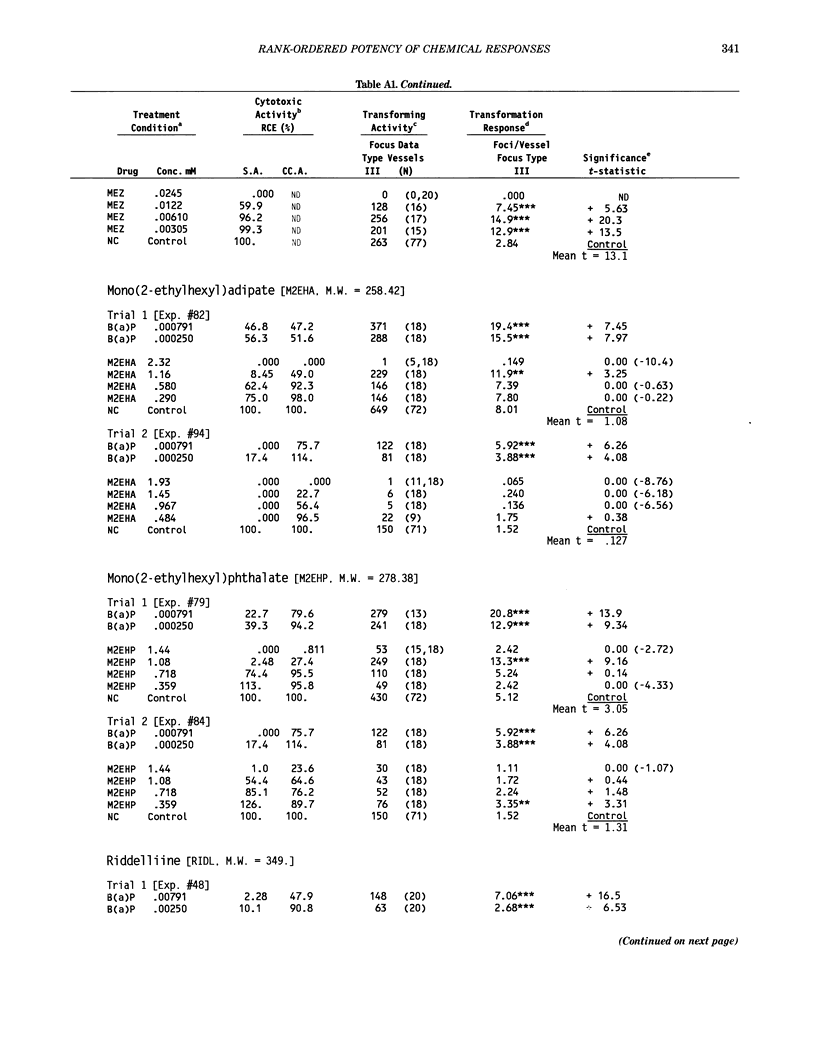
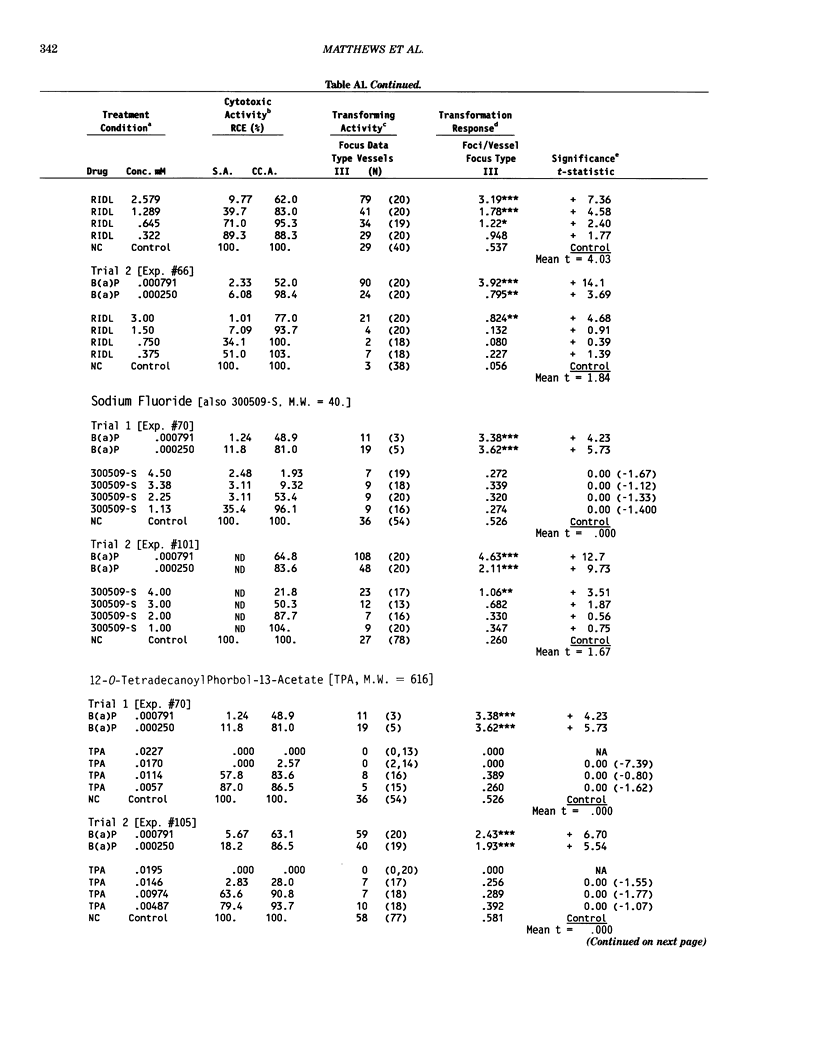
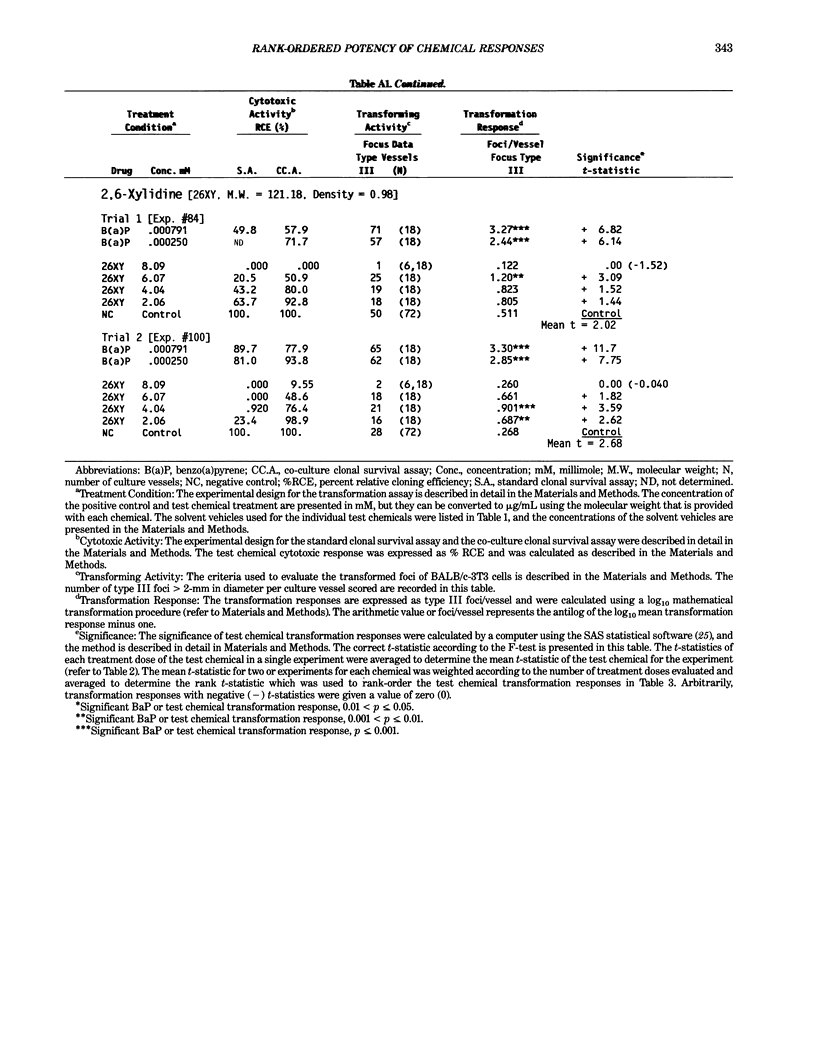
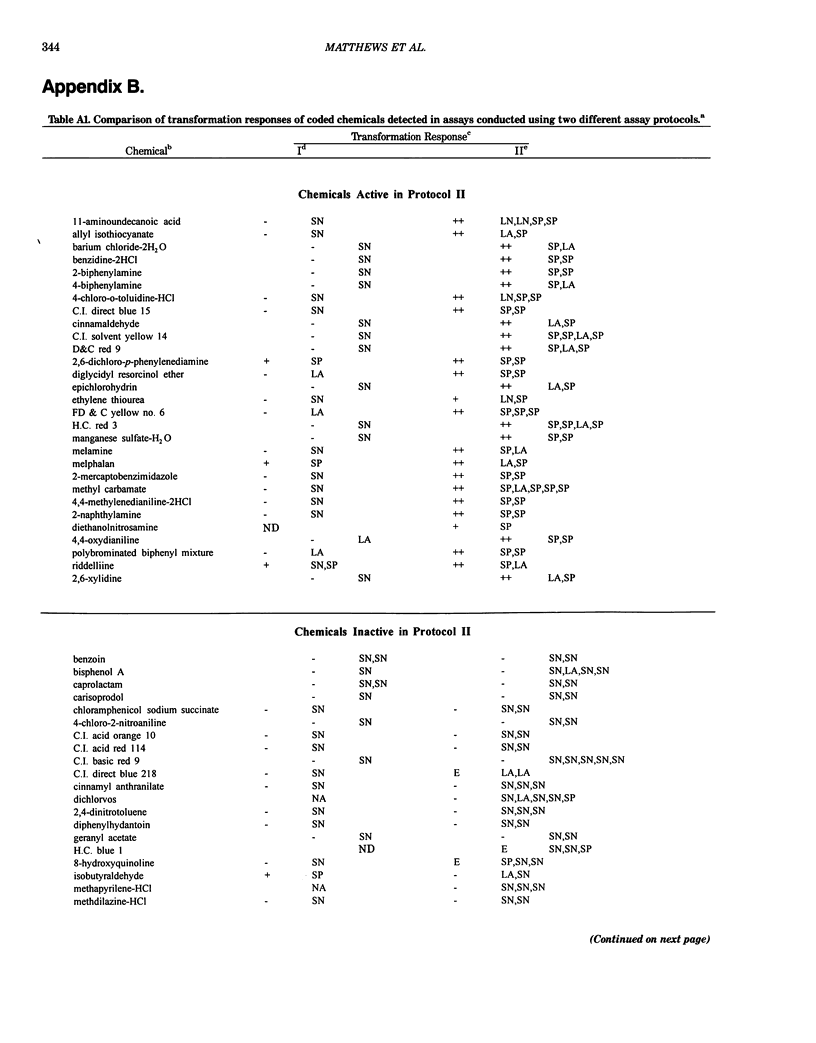
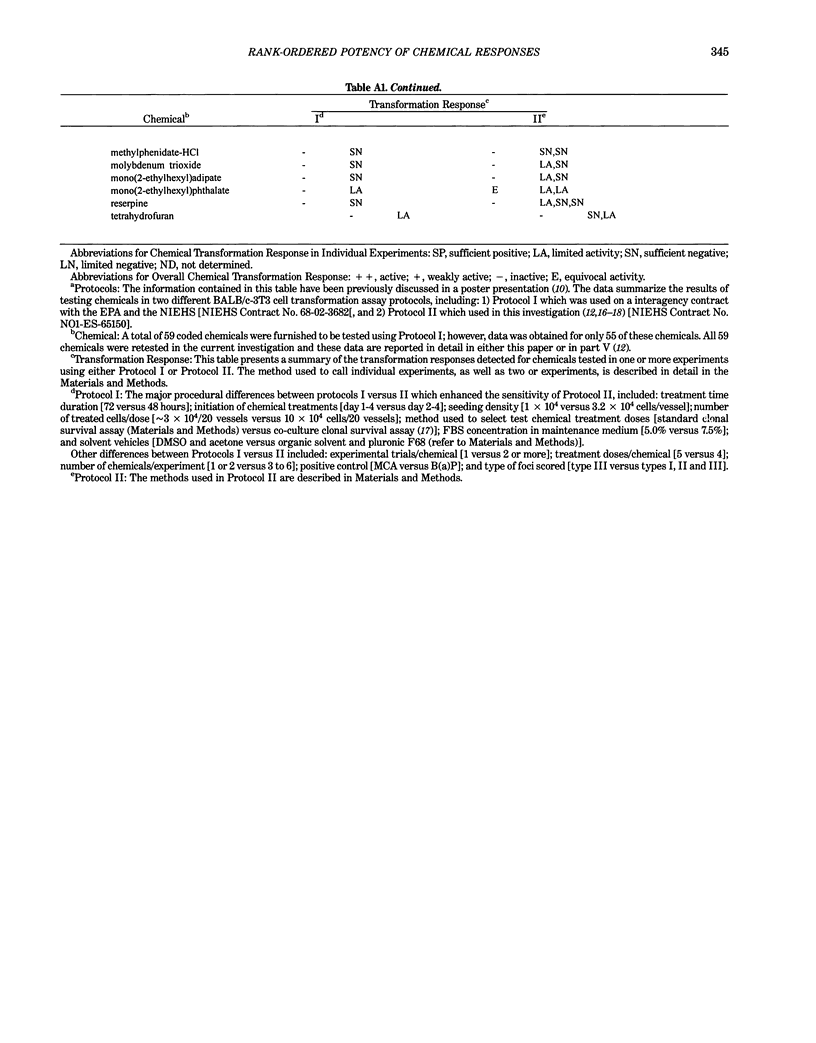
This report introduces an improved method of detecting chemical-induced morphological transformation of A-31-1-13 BALB/c-3T3 cells. The new procedure uses an increased target cell population to assess chemical-induced damage by increasing the initial seeding density and by delaying the initiation time of chemical treatment. Furthermore, a newly developed co-culture clonal survival assay was used to select chemical doses for the transformation assay. This assay measured the relative cloning efficiency (RCE) of chemical treatments in high-density cell cultures. In addition, transformation assay sensitivity was enhanced through the use of improved methods to solubilize many chemicals. From a group of 24 chemicals tested in at least two trials, clear evidence of chemical-induced transformation was detected for 12 chemicals (aphidicolin, barium chloride-2H2O, 5-bromo-2'-deoxyuridine, C.I. direct blue 15, trans-cinnamaldehyde, cytosine arabinoside, diphenylnitrosamine, manganese sulfate-H2O, 2-mercaptobenzimidazole, mezerein, riddelliine, and 2,6-xylidine); 2 chemicals had equivocal activity [C.I. direct blue 218 and mono(2-ethylhexyl)phthalate], 9 chemicals were inactive [carisoprodol, chloramphenicol sodium succinate, 4-chloro-2-nitroaniline, C.I. acid red 114, isobutyraldehyde, mono(2-ethylhexyl)adipate, sodium fluoride, and 12-O-tetradecanoylphorbol-13-acetate), and 1 chemical had an indeterminate response (2,6-dinitrotoluene). All positive responses were detected in the absence of an exogenous activation system and exhibited significant activity at two or more consecutive doses. This report also presents a mathematical method that uses t-statistics for rank-ordering the potency of chemical-induced transformation responses. This model detects sensitivity differences in experiments used to evaluate chemical-induced transformation. Furthermore, it provides a method to estimate a chemical's transformation response in terms of the historical behavior of the assay, as well as its future activity. The most active of the 24 chemicals was mezerein, and the least active chemical was diphenylnitrosamine.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby J., Tennant R. W. Chemical structure, Salmonella mutagenicity and extent of carcinogenicity as indicators of genotoxic carcinogenesis among 222 chemicals tested in rodents by the U.S. NCI/NTP. Mutat Res. 1988 Jan;204(1):17–115. doi: 10.1016/0165-1218(88)90114-0. [DOI] [PubMed] [Google Scholar]
- Ashby J., Tennant R. W., Zeiger E., Stasiewicz S. Classification according to chemical structure, mutagenicity to Salmonella and level of carcinogenicity of a further 42 chemicals tested for carcinogenicity by the U.S. National Toxicology Program. Mutat Res. 1989 Jun;223(2):73–103. doi: 10.1016/0165-1218(89)90037-2. [DOI] [PubMed] [Google Scholar]
- Dunkel V. C., Pienta R. J., Sivak A., Traul K. A. Comparative neoplastic transformation responses of Balb/3T3 cells, Syrian hamster embryo cells, and Rauscher murine leukemia virus-infected Fischer 344 rat embryo cells to chemical compounds. J Natl Cancer Inst. 1981 Dec;67(6):1303–1312. [PubMed] [Google Scholar]
- Heidelberger C., Freeman A. E., Pienta R. J., Sivak A., Bertram J. S., Casto B. C., Dunkel V. C., Francis M. W., Kakunaga T., Little J. B. Cell transformation by chemical agents--a review and analysis of the literature. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat Res. 1983 Apr;114(3):283–385. doi: 10.1016/0165-1110(83)90036-2. [DOI] [PubMed] [Google Scholar]
- Kakunaga T. A quantitative system for assay of malignant transformation by chemical carcinogens using a clone derived from BALB-3T3. Int J Cancer. 1973 Sep 15;12(2):463–473. doi: 10.1002/ijc.2910120217. [DOI] [PubMed] [Google Scholar]
- Kakunaga T., Crow J. D. Cell variants showing differential susceptibility to ultraviolet light--induced transformation. Science. 1980 Jul 25;209(4455):505–507. doi: 10.1126/science.7394516. [DOI] [PubMed] [Google Scholar]
- Kuroki T., Sasaki K. Relationship between in-vitro cell transformation and in-vivo carcinogenesis based on available data on the effects of chemicals. IARC Sci Publ. 1985;67:93–118. [PubMed] [Google Scholar]
- Lo K. Y., Kakunaga T. Similarities in the formation and removal of covalent DNA adducts in benzo(a)pyrene-treated BALB/3T3 variant cells with different induced transformation frequencies. Cancer Res. 1982 Jul;42(7):2644–2650. [PubMed] [Google Scholar]
- Malcolm A. R., Mills L. J., Trosko J. E. Effects of ethanol, phenol, formaldehyde, and selected metabolites on metabolic cooperation between Chinese hamster V79 lung fibroblasts. Carcinog Compr Surv. 1985;8:305–318. [PubMed] [Google Scholar]
- Matthews E. J., Spalding J. W., Tennant R. W. Transformation of BALB/c-3T3 cells: V. Transformation responses of 168 chemicals compared with mutagenicity in Salmonella and carcinogenicity in rodent bioassays. Environ Health Perspect. 1993 Jul;101 (Suppl 2):347–482. doi: 10.1289/ehp.93101s2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. J. Transformation of BALB/c-3T3 cells: I. Investigation of experimental parameters that influence detection of spontaneous transformation. Environ Health Perspect. 1993 Jul;101 (Suppl 2):277–291. doi: 10.1289/ehp.93101s2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. J. Transformation of BALB/c-3T3 cells: II. Investigation of experimental parameters that influence detection of benzo[a]pyrene-induced transformation. Environ Health Perspect. 1993 Jul;101 (Suppl 2):293–310. doi: 10.1289/ehp.93101s2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. J. Transformation of BALB/c-3T3 cells: III. Development of a co-culture clonal survival assay for quantification of chemical cytotoxicity in high-density cell cultures. Environ Health Perspect. 1993 Jul;101 (Suppl 2):311–318. doi: 10.1289/ehp.93101s2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesnow S., Argus M., Bergman H., Chu K., Frith C., Helmes T., McGaughy R., Ray V., Slaga T. J., Tennant R. Chemical carcinogens. A review and analysis of the literature of selected chemicals and the establishment of the Gene-Tox Carcinogen Data Base. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat Res. 1987 Jan-Mar;185(1-2):1–195. doi: 10.1016/0165-1110(87)90017-0. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Tennant R. W., Margolin B. H., Shelby M. D., Zeiger E., Haseman J. K., Spalding J., Caspary W., Resnick M., Stasiewicz S., Anderson B. Prediction of chemical carcinogenicity in rodents from in vitro genetic toxicity assays. Science. 1987 May 22;236(4804):933–941. doi: 10.1126/science.3554512. [DOI] [PubMed] [Google Scholar]
- Whorton E. B., Jr, Ward J. B., Jr, Morris D. L. Experimental design and statistical analysis considerations for in vitro mammalian cell transformation assays with BALB/3T3 cells. Environ Mutagen. 1982;4(5):595–603. doi: 10.1002/em.2860040511. [DOI] [PubMed] [Google Scholar]


