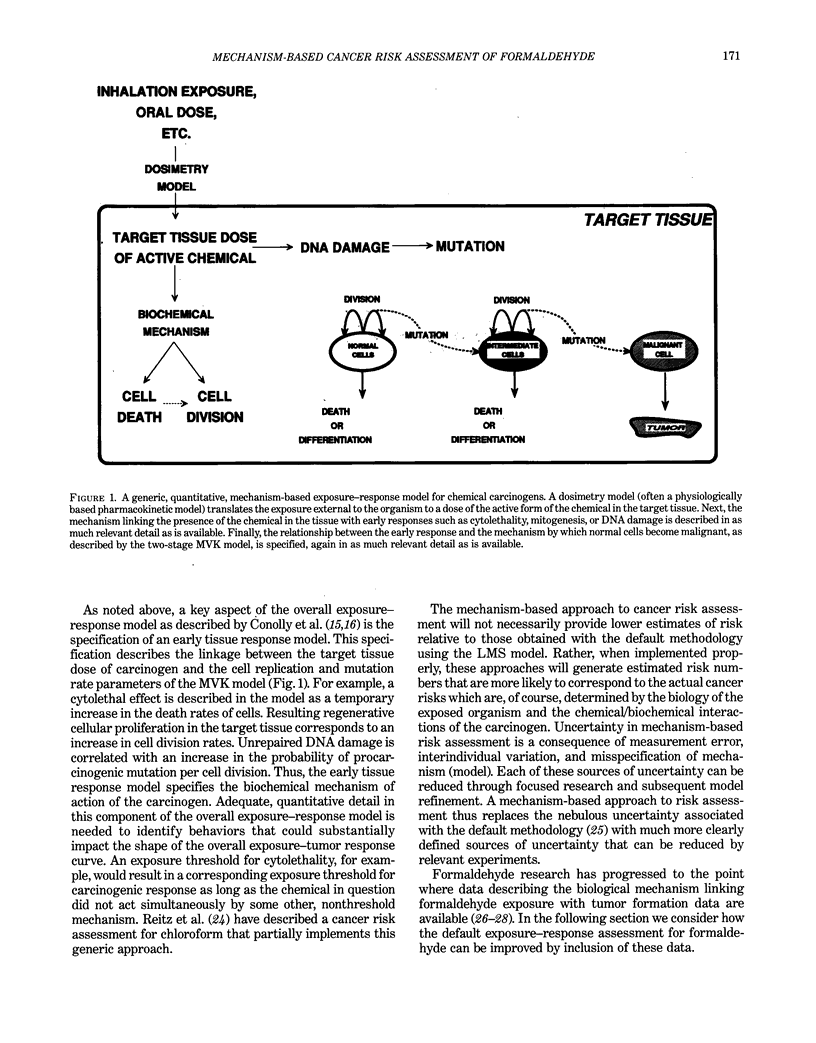
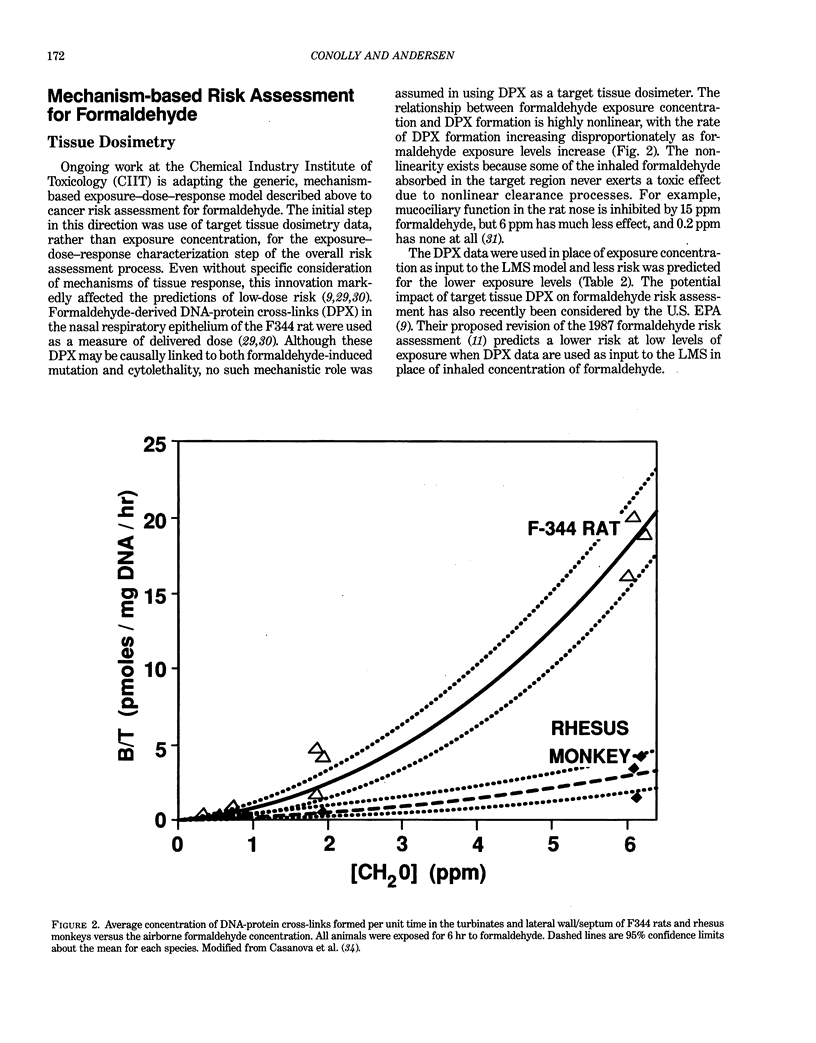
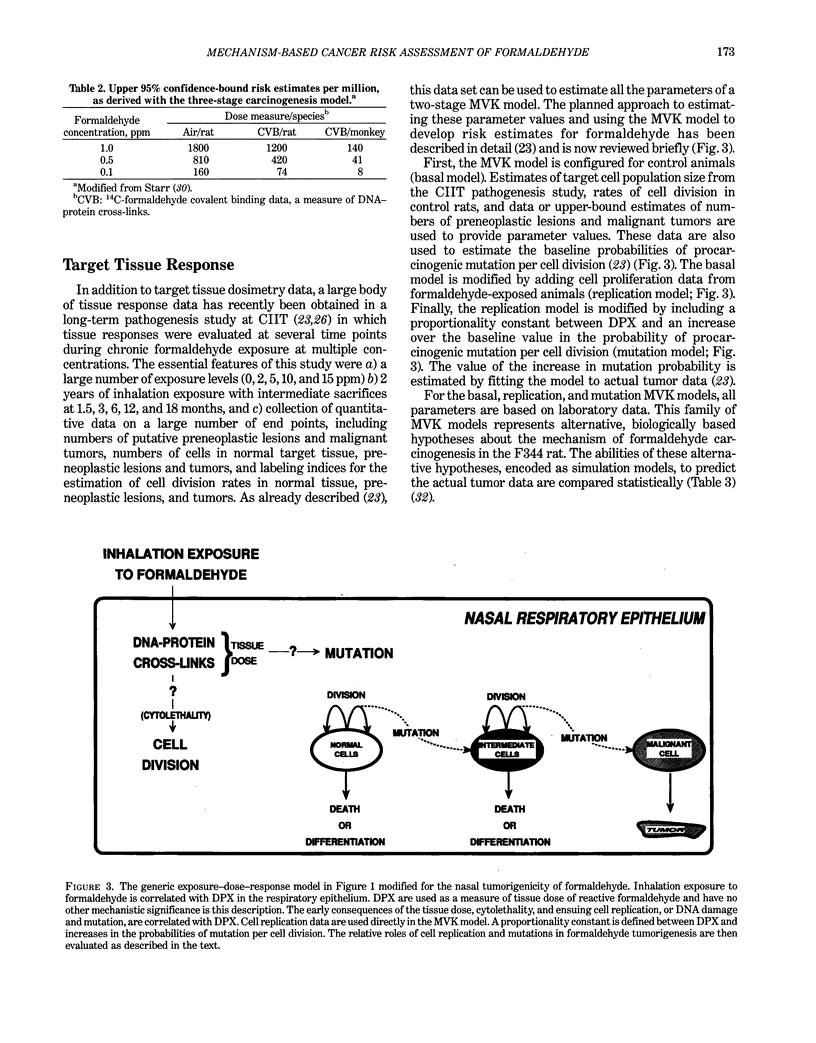
Abstract
The established carcinogenicity of formaldehyde in the rat and suggestive epidemiological evidence that formaldehyde may be a human carcinogen have led to its regulation by U.S. Federal agencies as a probable human carcinogen. These risk assessments have typically been based on tumor data in F344 rats exposed chronically to formaldehyde by inhalation and used the inhaled concentration as a measure of dose and the linearized multistage model (LMS) for dose-response characterization. Low-dose risks estimated with the LMS are thought to be conservative but are also generally acknowledged to be highly uncertain. In this manuscript, we first consider in generic terms how use of chemical-specific data on mechanisms of target tissue dosimetry and the series of tissue responses to the chemical that culminate in tumor formation can lead to more accurate dose-response characterization. A planned mechanism-based risk assessment for formaldehyde is then described. This risk assessment uses data on target tissue dosimetry, size of the target cell population in the rat nasal epithelium, number and size of putative preneoplastic lesions, and tumor incidence. These data establish parameter values for a biologically based, multistage cancer model that is then used to predict cancer risk at low exposure levels. Such work provides insights into the relative roles of formaldehyde-stimulated cell replication and procarcinogenic mutation in tumor formation. Finally, future directions are outlined for research on tissue dosimetry and scaling of the mechanism-based formaldehyde risk model from rats to people.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casanova M., Deyo D. F., Heck H. D. Covalent binding of inhaled formaldehyde to DNA in the nasal mucosa of Fischer 344 rats: analysis of formaldehyde and DNA by high-performance liquid chromatography and provisional pharmacokinetic interpretation. Fundam Appl Toxicol. 1989 Apr;12(3):397–417. doi: 10.1016/0272-0590(89)90015-8. [DOI] [PubMed] [Google Scholar]
- Casanova M., Morgan K. T., Steinhagen W. H., Everitt J. I., Popp J. A., Heck H. D. Covalent binding of inhaled formaldehyde to DNA in the respiratory tract of rhesus monkeys: pharmacokinetics, rat-to-monkey interspecies scaling, and extrapolation to man. Fundam Appl Toxicol. 1991 Aug;17(2):409–428. doi: 10.1016/0272-0590(91)90230-2. [DOI] [PubMed] [Google Scholar]
- Chang J. C., Steinhagen W. H., Barrow C. S. Effect of single or repeated formaldehyde exposure on minute volume of B6C3F1 mice and F-344 rats. Toxicol Appl Pharmacol. 1981 Dec;61(3):451–459. doi: 10.1016/0041-008x(81)90368-9. [DOI] [PubMed] [Google Scholar]
- Conolly R. B., Reitz R. H., Clewell H. J., 3rd, Andersen M. E. Pharmacokinetics, biochemical mechanism and mutation accumulation: a comprehensive model of chemical carcinogenesis. Toxicol Lett. 1988 Oct;43(1-3):189–200. doi: 10.1016/0378-4274(88)90028-8. [DOI] [PubMed] [Google Scholar]
- Crump K. S. An improved procedure for low-dose carcinogenic risk assessment from animal data. J Environ Pathol Toxicol Oncol. 1984 Jul;5(4-5):339–348. [PubMed] [Google Scholar]
- Crump K. S., Guess H. A., Deal K. L. Confidence intervals and test of hypotheses concerning dose response relations inferred from animal carcinogenicity data. Biometrics. 1977 Sep;33(3):437–451. [PubMed] [Google Scholar]
- Frederick C. B., Wilson A. G. Comments on incorporating mechanistic data into quantitative risk assessment. Risk Anal. 1991 Dec;11(4):581–582. doi: 10.1111/j.1539-6924.1991.tb00647.x. [DOI] [PubMed] [Google Scholar]
- Gerlowski L. E., Jain R. K. Physiologically based pharmacokinetic modeling: principles and applications. J Pharm Sci. 1983 Oct;72(10):1103–1127. doi: 10.1002/jps.2600721003. [DOI] [PubMed] [Google Scholar]
- Kerns W. D., Pavkov K. L., Donofrio D. J., Gralla E. J., Swenberg J. A. Carcinogenicity of formaldehyde in rats and mice after long-term inhalation exposure. Cancer Res. 1983 Sep;43(9):4382–4392. [PubMed] [Google Scholar]
- Leung H. W. Development and utilization of physiologically based pharmacokinetic models for toxicological applications. J Toxicol Environ Health. 1991 Mar;32(3):247–267. doi: 10.1080/15287399109531480. [DOI] [PubMed] [Google Scholar]
- Monticello T. M., Miller F. J., Morgan K. T. Regional increases in rat nasal epithelial cell proliferation following acute and subchronic inhalation of formaldehyde. Toxicol Appl Pharmacol. 1991 Dec;111(3):409–421. doi: 10.1016/0041-008x(91)90246-b. [DOI] [PubMed] [Google Scholar]
- Moolgavkar S. H., Dewanji A., Venzon D. J. A stochastic two-stage model for cancer risk assessment. I. The hazard function and the probability of tumor. Risk Anal. 1988 Sep;8(3):383–392. doi: 10.1111/j.1539-6924.1988.tb00502.x. [DOI] [PubMed] [Google Scholar]
- Moolgavkar S. H., Knudson A. G., Jr Mutation and cancer: a model for human carcinogenesis. J Natl Cancer Inst. 1981 Jun;66(6):1037–1052. doi: 10.1093/jnci/66.6.1037. [DOI] [PubMed] [Google Scholar]
- Morgan K. T., Kimbell J. S., Monticello T. M., Patra A. L., Fleishman A. Studies of inspiratory airflow patterns in the nasal passages of the F344 rat and rhesus monkey using nasal molds: relevance to formaldehyde toxicity. Toxicol Appl Pharmacol. 1991 Sep 1;110(2):223–240. doi: 10.1016/s0041-008x(05)80005-5. [DOI] [PubMed] [Google Scholar]
- Morgan K. T., Patterson D. L., Gross E. A. Responses of the nasal mucociliary apparatus of F-344 rats to formaldehyde gas. Toxicol Appl Pharmacol. 1986 Jan;82(1):1–13. doi: 10.1016/0041-008x(86)90431-x. [DOI] [PubMed] [Google Scholar]
- Ramsey J. C., Andersen M. E. A physiologically based description of the inhalation pharmacokinetics of styrene in rats and humans. Toxicol Appl Pharmacol. 1984 Mar 30;73(1):159–175. doi: 10.1016/0041-008x(84)90064-4. [DOI] [PubMed] [Google Scholar]
- Reitz R. H., Mendrala A. L., Corley R. A., Quast J. F., Gargas M. L., Andersen M. E., Staats D. A., Conolly R. B. Estimating the risk of liver cancer associated with human exposures to chloroform using physiologically based pharmacokinetic modeling. Toxicol Appl Pharmacol. 1990 Sep 15;105(3):443–459. doi: 10.1016/0041-008x(90)90148-n. [DOI] [PubMed] [Google Scholar]
- Starr T. B., Buck R. D. The importance of delivered dose in estimating low-dose cancer risk from inhalation exposure to formaldehyde. Fundam Appl Toxicol. 1984 Oct;4(5):740–753. doi: 10.1016/0272-0590(84)90095-2. [DOI] [PubMed] [Google Scholar]
- Starr T. B. Quantitative cancer risk estimation for formaldehyde. Risk Anal. 1990 Mar;10(1):85–91. doi: 10.1111/j.1539-6924.1990.tb01023.x. [DOI] [PubMed] [Google Scholar]


