Abstract
High levels of d-serine occur in mammalian brain, where it appears to be an endogenous ligand of the glycine site of N-methyl-d-aspartate receptors. In glial cultures of rat cerebral cortex, d-serine is enriched in type II astrocytes and is released upon stimulation with agonists of non-N-methyl-d-aspartate glutamate receptors. The high levels of d-serine in discrete areas of rat brain imply the existence of a biosynthetic pathway. We have purified from rat brain a soluble enzyme that catalyzes the direct racemization of l-serine to d-serine. Purified serine racemase has a molecular mass of 37 kDa and requires pyridoxal 5′-phosphate for its activity. The enzyme is highly selective toward l-serine, failing to racemize any other amino acid tested. Properties such as pH optimum, Km values, and the requirement for pyridoxal phosphate resemble those of bacterial racemases, suggesting that the biosynthetic pathway for d-amino acids is conserved from bacteria to mammalian brain.
d-Amino acids are prominent in bacteria whereas in animal tissues l-amino acids occur exclusively, though there have been occasional reports of d-amino acids, usually in invertebrates (1, 2). Recently, d-serine (3–6) and d-aspartate (7, 8) were reported in mammalian tissues, especially in the nervous system. Using highly selective antibodies, we localized d-aspartate to neuroendocrine tissues (9), whereas the immunohistochemical localizations of d-serine closely resemble N-methyl-d-aspartate (NMDA) receptors for the neurotransmitter glutamate, as the distribution of d-serine measured chemically (10, 11). Glutamate cannot activate the NMDA receptor in the absence of glycine, indicating a “glycine site” for the receptor (12, 13). d-Serine is up to three times more potent than glycine at this site (14), suggesting that d-serine is the endogenous ligand for this site. d-Serine is localized exclusively to type II astrocytes, a form of glia concentrated in gray matter in the same areas of the brain as NMDA receptors (10). Stimulation of the kainate subtype of glutamate receptors releases d-serine from type II astrocytes, implying that synaptic release of glutamate triggers release of d-serine from the astrocytes to activate NMDA receptors physiologically (10). Although in most parts of the brain the distribution of d-serine resembles NMDA receptors far better than glycine, in some areas glycine and NMDA receptors are colocalized, suggesting that d-serine is the predominant ligand for the receptor in most brain areas but that glycine serves this purpose in some sites (11).
Understanding the neurobiology of d-serine requires delineation of its biosynthesis. d-Serine might be formed by direct racemization from l-serine. Dunlop and Neidle (15) reported the transformation of radiolabeled l-serine to d-serine in intact rats, but this might have involved multiple steps rather than any direct enzymatic racemization. In the present study we describe an enzyme activity that directly racemizes l-serine to d-serine and that is localized to the brain. We report purification to homogeneity of this enzyme and its requirement for pyridoxal phosphate.
MATERIALS AND METHODS
Materials.
Amino acids, aminooxyacetic acid (AOAA), catalase, oxidized glutathione, hydroxylamine, leupeptin, luminol (sodium salt), pepstatin, phenylmethylsulfonyl fluoride, pyridoxal 5′-phosphate (PLP), o-phthaldialdehyde (OPA), l-homocysteic acid, and Tris were obtained from Sigma. d-Amino acid oxidase from pig kidney (EC 1.4.3.3), DTT, and horseradish peroxidase were obtained from Boehringer Mannheim. Ammonium sulfate, KH2PO4, and KH2PO4 were purchased from J. T. Baker. Butyl Sepharose 4 fast flow, Q-Sepharose, and Mono Q HR 5/5 were obtained from Pharmacia. Macro-prep ceramic hydroxyapatite type I (20 μm) was purchased from Bio-Rad. N-tert-butyloxycarbonyl-l-cysteine (l-Boc-cys) was obtained from Nova Biochem. Other reagents were of analytical grade.
Assay of Serine Racemase.
d-serine formation was monitored by a chemiluminescent assay that specifically detects d-serine. Racemase activity was performed in the presence of 50 mM Tris⋅HCl, pH 8.0/18 μl enzyme extract/1 mM EDTA/2 mM DTT/15 μM PLP/20 mM l-serine. After 0.5–8 h of incubation at 37°C, the reaction was terminated by the addition of trichloroacetic acid (TCA) to a final concentration of 5%. Blanks used boiled enzyme extract. The precipitated protein was removed by centrifugation, and the supernatant was extracted two times with 1 ml of water-saturated diethyl ether to remove TCA. d-Serine was determined by incubation of the samples with d-amino acid oxidase, which specifically degrades d-amino acids, generating an α-keto acid, NH3, and hydrogen peroxide (16). The generation of hydrogen peroxide was quantitated by the use of peroxidase and luminol, which emits light. A 10-μl sample aliquot was added to 100 μl of medium containing 100 mM Tris⋅HCl, pH 8.8, 10 units/ml peroxidase, and 8 μM luminol. After a 10- to 20-min delay, required to decrease the nonspecific luminol luminescence, 10 μl of d-amino acid oxidase (75 units/ml) was added and the tubes were mixed gently with a pipette tip. Maximum luminescence was recorded after 10–15 min at room temperature by using a Monolight 2010 luminometer (Analytical Luminescence Laboratory). The amount of d-serine in each sample was calculated by comparing with standard curves. The measurements were reliable in the range of 50–2,000 pmol d-serine per sample. Addition of mM concentrations of l-serine did not alter the values measured for d-serine. Alternatively, amino acid enantiomers were separated by HPLC by using a carbon 18 reverse-phase column (RP18 Spheri-5, 22 cm × 4.6 mm; Perkin–Elmer) with fluorimetric detection after derivatization with l-Boc-cys and OPA, as described (17). The results obtained with the chemiluminescent assay were identical to those obtained using HPLC. The presence of trace amounts of d-serine in the commercial l-serine reagent generates high blank values. Thus, the stock solution of l-serine (100 mM) was pretreated routinely for 3 days with 30 units of d-amino acid oxidase and 500 units of catalase to remove any d-serine contaminant. The enzymes were precipitated by the addition of 5% TCA. After removal of TCA by extraction with diethyl ether, l-serine solution was neutralized with NaOH and could be used without further purification, being virtually free of any d-serine contaminant. The same purification procedure was applied for l-alanine and l-threonine.
Purification of Serine Racemase.
Sixty brains from 10- to 14-day-old Sprague–Dawley rats were homogenized by using a Polytron in 5 vol of ice-cold buffer A (10 mM KPi, pH 7.2/50 mM KCl/1 mM EDTA/2 mM DTT/15 μM PLP/0.2 mM freshly prepared phenylmethylsulfonyl fluoride/1 μg/ml leupeptin/1 μg/ml pepstatin). All subsequent steps were performed at 4°C. The homogenate was centrifuged at 40,000 × g for 20 min, and the supernatant was brought to 45% ammonium sulfate saturation under continuous stirring. After 40-min precipitation, the solution was centrifuged at 20,000 × g for 20 min. The pellet was resuspended in 20% ammonium sulfate in buffer A. The suspension was left on ice for 1 h and then centrifuged at 20,000 × g for 20 min to remove insoluble aggregates. The supernatant was loaded at 3 ml/min onto a 70-ml butyl-Sepharose column preequilibrated with 20% ammonium sulfate in buffer A. The column was washed with 210 ml of 10% ammonium sulfate, and the active fraction was eluted with 5% ammonium sulfate in buffer A. The eluted material was concentrated by precipitation with 50% ammonium sulfate and centrifugation at 20,000 × g for 20 min. The pellet was resuspended in 6–8 ml buffer A and dialyzed overnight against 4 liters of buffer B (10 mM KPi, pH 7.2/50 mM KCl/1 mM EDTA/2 mM DTT/15 μM PLP). After dialysis, the suspension was centrifuged at 20,000 × g for 20 min to remove insoluble aggregates, and the supernatant was loaded at 0.5 ml/min onto a 3-ml Q-Sepharose column. After washing with 10 ml loading buffer, the protein was eluted with 250 mM NaCl in buffer B. The eluted material was concentrated with centriprep 30 (Amicon) and diluted in buffer B without KCl to decrease the salt concentration to 50 mM. Then, the suspension was loaded at 0.5 ml/min onto a Mono Q column. The column was washed with buffer B containing 150 mM KCl, and the protein was eluted with a linear gradient of KCl in the range of 158–188 mM KCl. The active fractions were pooled, concentrated with centriplus 30 (Amicon), and diluted in buffer B without EDTA and KPi. The procedure was repeated once to decrease the EDTA and KPi concentrations to 20 μM and 0.75 mM, respectively. To this suspension, CaCl2 was added (300 μM final concentration) to improve the protein binding to hydroxyapatite. The protein was applied at 0.1 ml/min to a 1-ml hydroxyapatite column and eluted with a linear gradient of 0.75–400 mM KPi in buffer containing 50 mM KCl, 2 mM DTT, and 15 μM PLP. The purified protein typically was eluted in the range of 0.75–30 mM KPi. For most applications the purified protein was concentrated further using centriplus 30. Protein concentration was determined with Coomassie blue plus protein assay reagent (Pierce).
Spectrophotometric Assays.
Enzyme-bound PLP was determined at room temperature by recording the absorbance of the purified protein in the range of 500–280 nm using a Lambda Bio spectrophotometer (Perkin–Elmer).
RESULTS
Uo et al. (18) recently described serine racemase activity in silkworms and purified the activity approximately 5-fold. They failed to detect serine racemase activity in crude homogenates, but observed activity in a fraction partially purified by ammonium sulfate fractionation. Similarly, in rat brain we cannot detect the conversion of l-serine to d-serine, monitored by HPLC, in homogenates, but can demonstrate this activity in a fraction precipitated with 45% ammonium sulfate (Table 1). With the partially purified enzyme, a simple routine assay monitors the conversion by added d-amino acid oxidase of d-serine to an α keto acid and H2O2. H2O2 then is transformed into a luminescent compound in the presence of peroxidase and luminol.
Table 1.
Purification of serine racemase
| Fraction | Protein, mg | Specific activity, μmol l-Ser/mg per h | Fold purification | Total activity | Yield, % |
|---|---|---|---|---|---|
| Homogenate | 4,744 | ND | — | — | — |
| (NH4)2SO4 fractionation | 624 | 0.0004 | 1 | 0.249 | 100 |
| Butyl-sepharose | 34 | 0.003 | 7.5 | 0.102 | 41 |
| Q-sepharose | 6.6 | 0.029 | 73 | 0.191 | 77 |
| Mono Q | 0.22 | 0.833 | 2,082 | 0.183 | 73 |
| Hydroxyapatite | 0.015 | 5.0 | 12,500 | 0.075 | 30 |
Enzyme was purified and fractions were assayed as described. Data represent a typical purification, which was repeated six times with similar results. ND, not detected.
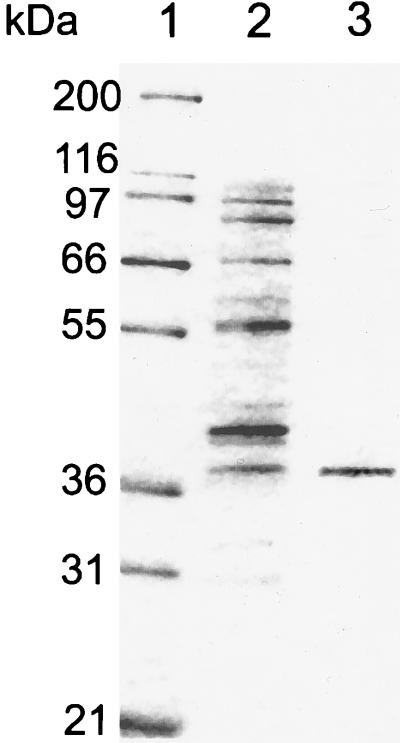
We have purified the enzyme to homogeneity by using sequentially ammonium sulfate fractionation, butyl-Sepharose, Q-Sepharose, mono-Q, and hydroxyapatite chromatography steps (Table 1). The overall purification from the ammonium sulfate fraction was 12,500. Enzyme activity was obtained in 30% yield. Interestingly, there was an apparent increase in yield after the Q-Sepharose step, suggesting the removal of an inhibitor. SDS gel electrophoresis revealed a single band of about 37 kDa for the purified protein (Fig. 1).
Figure 1.
SDS/PAGE analysis of purified serine racemase. A 12% polyacrylamide gel was stained with Coomassie blue. Lane 1, molecular mass markers: myosin (200 kDa), β-galactosidase (116.7 kDa), phosphorylase b (97.4 kDa), BSA (66.3 kDa), glutamic dehydrogenase (55.4 kDa), lactate dehydrogenase (36.5 kDa), carbonic anhydrase (31 kDa), and trypsin inhibitor (21.5 kDa). Lane 2, Mono Q column eluate containing 1 μg protein. Lane 3, hydroxyapatite column eluate containing 0.5 μg purified protein. Silver staining of the purified preparation showed no additional bands.
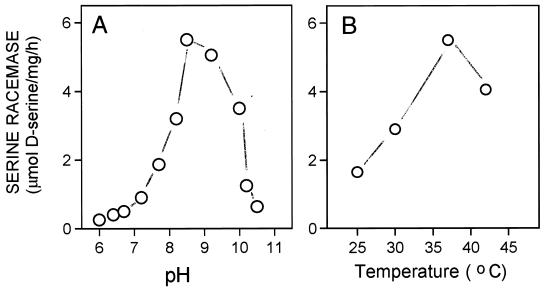
The enzyme was stable with no loss of activity when stored for 4 days at 4°C. Only modest loss of activity occurred after two cycles of freezing and thawing. The enzyme appeared to be soluble with no activity detected in membrane preparations. Activity displayed a sharp pH optimum in the alkaline range with optimal activity at pH 8–9 being about 10 times higher than at pH 7 (Fig. 2). Enzyme activity was maximal at 37°C and was abolished by boiling.
Figure 2.
pH and temperature dependence of racemase activity. (A) Racemase activity was assayed at 37°C in media containing 50 mM Mes-Tris (pH 6.0–6.5), 50 mM Tris⋅HCl (pH 6.8–8.8) of 50 mM CAPS-NaOH (pH 9–10.5), 20 mM l-serine, 100 μg/ml purified enzyme, 1 mM EDTA, 2 mM DTT, and 15 μM PLP. (B) Racemase activity was performed at different temperatures in a medium containing 50 mM Tris⋅HCl, pH 8.0/20 mM l-serine, 100 μg/ml purified enzyme, 1 mM EDTA, 2 mM DTT, and 15 μM PLP. The reaction was stopped after 4 h and analyzed both by chemiluminescence and HPLC assay. The experiment was replicated three times by using different preparations with similar results.
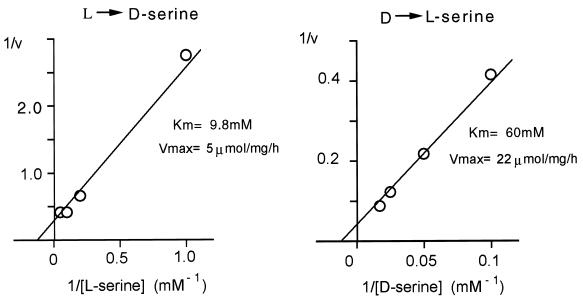
Enzyme activity obeys Michaelis–Menten kinetics (Fig. 3). Monitoring the conversion of l- to d-serine, the Km was about 10 mM with a Vmax of 5 μmol/mg per h. The enzyme also can convert d- to l-serine but with lesser affinity, as the Km in this direction was 60 mM, though the Vmax was higher, at 22 μmol/mg per h.
Figure 3.
Kinetic parameters of racemization reaction. Initial rate of racemase activity was measured at 37°C in medium containing 50 mM Tris⋅HCl, pH 8.0, 35 μg/ml purified enzyme, 1 mM EDTA, 2 mM DTT, and 15 μM PLP and different concentrations of either l- or d- serine. The reaction was stopped after 2 h when less than 10% substrate was consumed. Values for Km and Vmax were calculated by using the Michaelis–Menten equation. The values are representative of three experiments with different enzyme preparations.
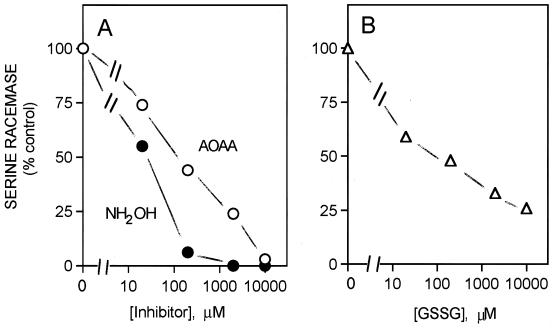
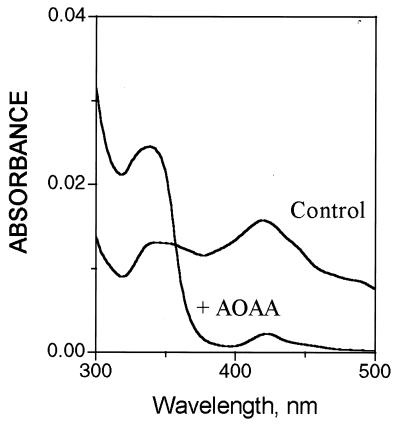
Serine racemase requires PLP. Dialysis for 16 h against 1,000 volumes of the purification buffer without PLP abolishes enzyme activity, which can be restored by the addition of PLP (data not shown). AOAA and hydroxylamine, which inactivate PLP, inhibit enzyme activity (Fig. 4). Examination of the absorption spectrum of the enzyme confirmed the importance of PLP. Thus, the normal enzyme preparation displayed absorption peaks at 420 and 340 nm, characteristic of PLP-dependent enzymes. The peak at 420 nm, corresponding to a Schiff’s base complex of PLP with an active site lysine, was abolished by treatment with AOAA (Fig. 5).
Figure 4.
Inhibition of serine racemase by PLP inhibitors and sulfhydryl oxidation. (A) Enzyme activity was monitored at 37°C in a medium containing 50 mM Tris⋅HCl, pH 8.0/20 mM l-serine, 100 μg/ml purified enzyme, 1 mM EDTA, 2 mM DTT, and 10 μM PLP and different concentrations of either AOAA (○) or hydroxylamine (•). (B) Reaction medium and conditions were as described in A, except that DTT was omitted from the last step of the enzyme preparation. The enzyme was preincubated for 10 min in the presence of different concentrations of oxidized glutathione (GSSG).
Figure 5.
Absorption spectra of purified serine racemase. Purified enzyme (70 μg/ml) was preincubated for 10 min in medium containing 10 mM KPi (pH 7.2), 2 mM DTT, 1 mM EDTA, and 10 μM PLP, either in the absence (Control) or in the presence of 1 mM AOAA. The distinct peaks of absorbance at 420 and 340 nm were not observed in the presence of buffer alone or when BSA was used instead of serine racemase.
Sulfhydryl groups seem to be important for enzyme activity, as oxidized glutathione markedly reduced enzyme activity (Fig. 4).
We wondered whether conversion of l- to d-serine might be a by-product of a different enzyme activity with nonenzymatic racemization, giving rise to d-serine. Accordingly, we monitored by HPLC the levels of l- and d-serine at different incubation times (Table 2). Increases in formation of d-serine are paralleled by stoichiometric decreases in levels of l-serine, making it unlikely that l-serine is converted to any other compound by the enzyme. Additionally, we examined the purified enzyme for the presence of other enzyme activities that might contribute to d-serine formation indirectly. We found no evidence for serine:pyruvate aminotransferase activity, as l-serine levels are not altered in the presence of pyruvate. We did not observe serine hydroxymethyltransferase activity inasmuch as we failed to detect the formation of glycine after extensive incubation of the enzyme with l-serine. There is no evidence for serine dehydratase activity that would be associated with a marked decrease in l-serine levels.
Table 2.
Racemization of l-Serine
| Time, h | [l-Serine] | [d-Serine] |
|---|---|---|
| mM | ||
| 0 | 4.00 | 0.0007 |
| 0.5 | 3.96 | 0.042 |
| 1 | 3.93 | 0.101 |
| 4 | 3.65 | 0.342 |
Racemase activity was assayed at 37°C in a medium containing 50 mM Tris⋅HCl, pH 8.0, 4 mM l-serine, 40 μg/ml purified enzyme, 1 mM EDTA, 2 mM DTT, and 15 μM PLP. Samples were analyzed for amino acid emantiomers by HPLC as described.
Serine racemase is highly selective for l-serine (Table 3). The enzyme displays about 1.5% as much activity toward l-alanine as for l-serine while no activity is demonstrated with l-threonine or l-aspartate.
Table 3.
Substrate specificity of serine racemase
| Amino acid | Specific activity, μmol/mg per h | % control |
|---|---|---|
| l-Serine | 4.8 | 100 |
| l-Alanine | 0.012 | 1.5 |
| l-Threonine | 0 | 0 |
| l-Aspartate | 0 | 0 |
Racemase activity was assayed at 37°C in a medium containing 50 mM Tris⋅HCl, pH 8.0, 20 mM l-amino acids, 100 μg/ml purified enzyme, 1 mM EDTA, 2 mM DTT, and 15 μM PLP. After 8 h, the reaction was terminated by the addition of 5% TCA, and samples were analyzed by HPLC as described. The data represent a typical experiment that was replicated three times by using different preparations with similar results.
DISCUSSION
Our isolation of serine racemase represents the purification of an enzyme that directly converts l-serine to d-serine. Bacteria contain substantial levels of d-serine and many other d-amino acids. Though a number of amino acid racemases have been purified from bacteria, no serine-specific enzyme has been identified previously (19–21). Serine racemase appears to be a very conserved enzyme. The pH optimum in the rat brain enzyme we have purified as well as its requirement for PLP and behavior in chromatographic systems resemble the activity characterized in crude preparations of silkworm (18). Bacterial amino acid racemases display properties resembling serine racemase including Km values, alkaline pH optimum, and the requirement for PLP. The alkaline pH optimum might reflect the mechanism of racemization, as PLP nonenzymatically racemizes amino acids at alkaline pH (22).
Serine racemase displays a Km value in the direction of l- to d-serine resembling brain levels of l-serine and favoring the physiologic synthesis of d-serine. Because of the much higher Km value in the direction of d- to l-serine, under physiological conditions the enzyme should predominantly make d-serine.
Serine racemase is a relatively small soluble protein of 37 kDa. Its absorption spectrum indicates that no minor, undetected protein could account for enzyme activity. Thus, the magnitude and ratios of absorption at 280, 340, and 420 nm closely resemble values for known PLP enzymes (20, 23, 24). This could be possible only if essentially all the protein is the PLP requiring racemase.
d-Amino acid oxidase has been well known for many years, but its function was obscure until the demonstration of d-serine in mammalian brain. d-Amino acid oxidase is highly selective for d-serine, which thus is likely its principal, if not its sole, physiologic substrate. Our demonstration of serine racemase now establishes that both biosynthetic and degrading enzymes for d-serine exist in mammalian brain.
Accumulating evidence establishes that d-serine is the predominant endogenous ligand for the “glycine site” of the NMDA receptor. Its localization in most brain areas resemble NMDA receptor distribution more closely than glycine (10, 11). It is released from type II astrocytes by glutamatergic stimulation (10), and it is at least as active as glycine at the “glycine site” (14). Moreover, recently, we have shown that endogenous d-serine is required for physiologic NMDA neurotransmission. Thus, treatment of brain slices or cultures with d-amino acid oxidase, under conditions in which d-serine is completely degraded, greatly reduces NMDA transmission measured electrophysiologically or biochemically in terms of stimulation of nitric oxide synthase activity and levels of cyclic GMP (J. P.M., A. Parent, D. Linden, H.W., C.D.F., R.O.B., M. A. Rogawski, and S.H.S., unpublished data).
We have obtained amino acid sequence for mammalian serine racemase, reflecting a protein with no major similarity to any other known protein (H.W., S. Blackshaw, and S.H.S., unpublished data). Targeted deletion of this enzyme protein may enhance substantially our understanding of NMDA neurotransmission. Drugs inhibiting serine racemase may provide a useful therapeutic approach in disease entities for which NMDA receptor antagonists have displayed efficacy, such as in the treatment of stroke and other forms of overexcitation in the brain.
Acknowledgments
This work was supported by U.S. Public Health Service Grant MH-18501, the Theodore and Vada Stanley Foundation, and Research Scientist Award DA00074 (to S.H.S.), a summer fellowship from Pfizer (to K.S.), and a Howard Hughes fellowship for physicians (to C.D.F.). H.W. is a Pew fellow.
ABBREVIATIONS
- AOAA
aminooxyacetic acid
- NMDA
N-methyl-d-aspartate
- PLP
pyridoxal 5′-phosphate
- TCA
trichloroacetic acid
References
- 1.Corrigan J J. Science. 1969;164:142–149. doi: 10.1126/science.164.3876.142. [DOI] [PubMed] [Google Scholar]
- 2.Corrigan J J, Srinivasan N G. Biochemistry. 1966;5:1185–1190. doi: 10.1021/bi00868a010. [DOI] [PubMed] [Google Scholar]
- 3.Nagata Y, Konno R, Yasumura Y, Akino T. Biochem J. 1989;257:291–292. doi: 10.1042/bj2570291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashimoto A, Nishikawa T, Hayashi T, Fujii N, Harada K, Oka T, Takahashi K. FEBS Lett. 1992;296:33–36. doi: 10.1016/0014-5793(92)80397-y. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto A, Kumashiro S, Nishikawa T, Oka T, Takahashi K, Mito T, Takashima S, Doi N, Mizutani Y, Yamazaki T, Kaneko T, Ootomo E. J Neurochem. 1993;61:783–786. doi: 10.1111/j.1471-4159.1993.tb03575.x. [DOI] [PubMed] [Google Scholar]
- 6.Nagata Y, Horiike K, Maeda T. Brain Res. 1994;634:291–295. doi: 10.1016/0006-8993(94)91932-1. [DOI] [PubMed] [Google Scholar]
- 7.Dunlop D S, Neidle A, McHale D, Dunlop D M, Lajtha A. Biochem Biophys Res Commun. 1986;141:27–32. doi: 10.1016/s0006-291x(86)80329-1. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto A, Oka T, Nishikawa T. Eur J Neurosci. 1995;7:1657–1663. doi: 10.1111/j.1460-9568.1995.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 9.Schell M J, Cooper O B, Snyder S H. Proc Natl Acad Sci USA. 1995;94:2013–2018. doi: 10.1073/pnas.94.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schell M J, Molliver M E, Snyder S H. Proc Natl Acad Sci USA. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schell M J, Brady R O, Jr, Molliver M E, Snyder S H. J Neurosci. 1997;17:1604–1615. doi: 10.1523/JNEUROSCI.17-05-01604.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson J W, Ascher P. Nature (London) 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 13.Kleckner N W, Dingledine R. Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- 14.Matsui T, Sekiguchi M, Hashimoto A, Tomita U, Nishikawa T, Wada K. J Neurochem. 1995;65:454–458. doi: 10.1046/j.1471-4159.1995.65010454.x. [DOI] [PubMed] [Google Scholar]
- 15.Dunlop D S, Neidle A. Biochem Biophys Res Commun. 1997;235:26–30. doi: 10.1006/bbrc.1997.6724. [DOI] [PubMed] [Google Scholar]
- 16.Scannone H, Wellner D, Novogrodsky A. Biochemistry. 1964;11:1742–1745. doi: 10.1021/bi00899a027. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto A, Nishikawa T, Oka T, Takahashi K, Hayashi T. J Chromatogr. 1992;582:41–48. doi: 10.1016/0378-4347(92)80300-f. [DOI] [PubMed] [Google Scholar]
- 18.Uo T, Yoshimura T, Shimizu S, Esaki N. Biochem Biophys Res Commun. 1998;246:31–34. doi: 10.1006/bbrc.1998.8561. [DOI] [PubMed] [Google Scholar]
- 19.Wood W A, Gunsalus I C. J Biol Chem. 1951;190:403–416. [PubMed] [Google Scholar]
- 20.Yorifuji T, Misono H, Soda K. J Biol Chem. 1971;246:5093–5101. [PubMed] [Google Scholar]
- 21.Svensson M L, Gatenbeck S. Arch Microbiol. 1981;129:213–215. [Google Scholar]
- 22.Olivard J, Metzler D E, Snell E E. J Biol Chem. 1952;191:669–674. [PubMed] [Google Scholar]
- 23.Manohar R, Apu Rao A G, Appaji Rao N. Biochemistry. 1984;23:4116–4122. doi: 10.1021/bi00313a016. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa K, Kaneko E, Ichiyama A. J Biochem. 1996;119:970–978. doi: 10.1093/oxfordjournals.jbchem.a021337. [DOI] [PubMed] [Google Scholar]