Abstract
Cholera toxin (CT) is a strong mucosal adjuvant for codelivered antigens, whereas its nontoxic B subunit (CTB) is an efficient mucosal carrier molecule for the generation of immune responses to linked antigens. We investigated the effects of CT and CTB on the immunogenicity of in vitro-treated antigen-pulsed dendritic cells (DC) following intravenous injection into mice. Prior to infusion, DC were pulsed for 90 min with either free ovalbumin (OVA), OVA mixed with CT or CTB, or chemical conjugates of OVA with CT and CTB (OVA-CT and OVA-CTB). DC pulsed with OVA or with OVA and CTB gave rise to modest antibody and T-cell responses. Conjugation of OVA with CTB enhanced both the subsequent B-cell and T-cell responses to OVA and preferentially induced Th2 responses. CT was shown to be a strong adjuvant when it was coadministered to DC with OVA and was even stronger when it was coadministered with OVA-CTB and primed for a mixed Th1-Th2 response. The antibody and T-cell responses were further enhanced if OVA was coupled to CT, implying that CT can utilize a combined carrier and adjuvant function vis-a-vis linked antigens for DC vaccination. The immunopotentiating capacity of CT- and CTB-linked antigen was associated with both upregulated secretion of interleukin-1β by the pulsed DC and increased expression of CD80 and CD86 on the DC surface. These results imply that CT and CTB can be used to both markedly increase and partially direct the DC vaccine-induced immune response with respect to Th1 and Th2 responses, which has obvious implications for DC-based vaccine development.
Dendritic cells (DC) are professional antigen-presenting cells (APC) which act as sentinels throughout the body. Upon an encounter with an inflammatory signal, DC transport antigens to lymphoid organs, where they activate antigen-specific CD4+ and CD8+ T-cells and are thus one of the most important determinants of specific immune induction (2). The special ability of DC to activate naive T-cells is maturation dependent and is associated with the expression of high levels of cell surface major histocompatibility complex (MHC) molecules and costimulatory molecules, as well as the capacity to secrete chemokines that attract naive T cells. Due to the strong immunoactivating capacity of DC, administration of ex vivo antigen-pulsed DC has been shown both in animals and in humans to induce strong T-cell and B-cell responses (25, 28, 30, 37) and to induce protection against both tumors and infectious agents (14, 21, 25, 31, 34, 39).
Cholera toxin (CT) and the cholera toxin B subunit (CTB) have strong immunomodulatory properties both in vivo and in vitro. Both compounds bind to GM1 ganglioside receptors present on most cells in the body, including leucocytes. CT, the cholera-inducing enterotoxin produced by Vibrio cholerae O1 and O139, is one of the most potent mucosal immunogens and adjuvants known (9, 10, 22, 29). Full adjuvanticity requires an intact CT molecule consisting of the cell-binding pentameric B subunit noncovalently linked to the toxic ADP-ribosylating A subunit (23, 29). Although CT can also modulate both specific B-cell and T-cell activation by direct actions on these cells, its primary action as an adjuvant is probably mediated through a direct effect on APC. Thus, CT has been found to directly affect both the cytokine profile and the cell surface phenotype of APC (5, 12).
CTB, on the other hand, is nontoxic and a relatively inefficient adjuvant for admixed antigens, but it is a highly efficient mucosal carrier molecule for linked antigens. Chemical or genetic conjugation of some antigens to CTB can strongly enhance the induction of mucosal antibody responses to the linked antigen (8, 15, 20, 26), but with other antigens it can give rise to immune deviation, leading to so-called antigen-specific oral tolerance peripherally (1, 15, 40). Selected CTB-coupled antigens have thus been used in animal models to suppress T-cell-mediated autoimmune diseases (4, 42, 47), immunoglobulin E (IgE)-mediated allergic reactions (32, 46, 48), and infection-induced pathological inflammatory conditions (27, 41). The mechanism behind the efficacy of CTB as a carrier and immunodeviating molecule has not been fully defined, but it is believed to be associated with the strong binding of CTB to the GM1 receptor.
We have previously shown that conjugation of antigen to CTB decreases the dose of antigen required by APC for T-cell activation in vitro more than 10,000-fold (13). In this study we investigated the adjuvant effects of CTB and CT on the immunogenicity in vivo of DC treated in vitro. To do this, bone marrow-derived DC generated in vitro were pulsed with free or CT- or CTB-linked antigen in the presence or absence of CT and then injected into mice. Our studies showed that DC pulsed with CTB-conjugated antigen are superior to DC pulsed with free antigen for inducing both T-cell and B-cell responses in vivo and that CT has strong adjuvant properties when it is applied directly to the DC. Furthermore, the adjuvant and carrier functions of CT and CTB were combined when antigen was linked directly to CT. Conjugation of antigen to CTB preferentially induced Th2 responses, whereas CT also primed for Th1 responses. The strong immunopotentiating capacity of CT- and CTB-linked antigens was associated with upregulated production of interleukin-1β (IL-1β) and increased cell surface expression of CD80 and CD86 by the pulsed DC. These results have clear implications for the design of vaccines when DC are used as vaccine carriers.
MATERIALS AND METHODS
Chemical conjugation of OVA to CT or CTB.
Whole ovalbumin (OVA) (grade VI) was purchased from Sigma (St. Louis, Mo.). Recombinant CTB was produced and purified from V. cholerae strain 358 as described previously (19). CT was purchased from LIST Biological Laboratories, Inc. (Campbell, Calif.). OVA proteins were chemically coupled to recombinant CTB or CT by using N-succinimidyl (3-[2-pyridyl]-dithio)propionate (Pharmacia) as a bifunctional coupling reagent as described previously (32). Conjugated material was quantified and purified by fast protein liquid chromatography gel filtration (Superdex 200 16/60 column; Pharmacia Biotech, Uppsala, Sweden) by using the Biologic Workstation fast protein liquid chromatography system (Bio-Rad, Hercules, Calif.). The purified conjugates contained concentrations of OVA that were equal to those of CT and CTB. The conjugates were analyzed with a GM1 enzyme-linked immunosorbent assay (ELISA) by using biotinylated anti-CTB monoclonal antibodies (44) and were shown to have retained the GM1-binding activity.
Generation and in vitro pulsing of bone marrow-derived DC.
DC were generated from bone marrow precursors as described previously (34). Briefly, male BALB/c mice (B&K Universal AB, Stockholm, Sweden) were killed, and bone marrow was flushed from the femurs and tibias and depleted of erythrocytes with ammonium chloride. T cells, B cells, and MHC II-positive cells were removed by incubating the preparations with rat anti-mouse CD4, CD8, B220, and I-Ad antibodies (1 μg/ml each; Pharmingen, San Diego, Calif.) and anti-rat IgG-coated and anti-mouse IgG-coated beads (Dynal). The remaining cells were plated in 24-well plates (106 cells/well) in Iscoves medium supplemented with 10% fetal calf serum and 1,125 U of recombinant murine granulocyte-macrophage colony-stimulating factor (Pharmingen) per ml (DC medium). One-half of the DC medium was replaced every second day. On day 6, nonadherent cells were collected and further purified by metrizamide density centrifugation (18% metrizamide in phosphate-buffered saline [PBS]; Sigma) at 800 × g. More than 90% of the DC obtained were CD11c positive. In some experiments, DC were further purified by using anti-mouse CD11c microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions, which yielded >99% pure CD11c-positive DC.
DC were pulsed for 2 h at 37°C with OVA, OVA conjugated to CTB (OVA-CTB), or OVA conjugated to CT (OVA-CT) at equimolar concentrations of OVA (0.5 or 0.05 μM) in the presence or absence of CT (0.05 μM) or CTB (0.5 μM) and then extensively washed by three rounds of centrifugation prior to injection.
Fluorescence-acitivated cell sorter analysis.
Antigen-pulsed DC were incubated with murine anti-OVA serum (1/10 dilution), with serum from naive mice (control antibody; 1/10 dilution), or with a monoclonal antibody to CTB (LT39; supernatant at a 1/10 dilution [45]) for 30 min at 4°C, followed by incubation with fluorescein isothiocyanate-labeled goat anti-mouse IgG (Serotec), or they were directly labeled with phosphatidylethanolamine (PE)-conjugated anti-mouse CD80 clone 16-10A1 or PE-conjugated anti-mouse CD86 clone GL1 from Pharmingen or with PE-conjugated rat IgG2a(κ) and fluorescein isothiocyanate-conjugated Armenian hamster IgGgr2(κ) isotype controls.
Cytokine secretion by DC.
DC were incubated at a concentration of 105 cells/well in flat-bottom 96-well plates (Nunc) in the presence or absence of CT (0.05 μM) or CTB (0.5 μM) in 200 μl of complete medium. Culture supernatants were collected after 24 h and frozen at −70°C until they were assayed for cytokine content. Culture supernatants were analyzed for IL-1β by using specific Duoset ELISAs from R&D according to the manufacturer's instructions. IL-12 and IL-18 were similarly measured by using OptEIA mouse IL-12(p40) and IL-18 sets from Pharmingen.
Vaccinations and sample collection.
BALB/c mice were given two intravenous injections 2 weeks apart of 2 × 106 DC in 100 μl of PBS. Two weeks after the second DC vaccination, the mice were boosted with 3 μg of OVA in Freund's complete adjuvant (FCA) subcutaneously. Serum was taken prior to vaccination, at the time of boosting (day 14 after the second DC vaccination), and on day 9 after OVA-FCA administration and was frozen at −20°C until it was analyzed for OVA-specific antibody production. Mice were sacrificed on days 9 to 14 after the OVA booster injection. The spleens were collected, and single-cell suspensions were analyzed for OVA-specific gamma interferon (IFN-γ) production in vitro. These studies were approved by the Ethical Committee for Animal Experimentation, Göteborg, Sweden.
Antibody responses in serum.
High-binding microtiter plates (Greiner) were coated with OVA (20 μg/ml in PBS) overnight. Threefold serial dilutions of sera were incubated for 90 min in PBS containing 0.1% bovine serum albumin. After this, peroxidase-conjugated anti-mouse IgG (1/3,000), IgG1 (1/1,000), or IgG2a (1/1,000) (all obtained from Southern Biotechnology) was added for 90 min at room temperature. The reaction was developed with 100 μl of a 1-mg/ml solution of o-phenylenediamine dihydrochloride (Sigma) in 0.1 M citrate buffer (pH 4.5) containing 0.04% H2O2, and the results were read at 450 nm. The specific antibody titer was estimated by determining the interpolated sample dilution that gave an absorbance that was 0.4 U above the background level (the background level was defined as the absorbance obtained with 0.1% bovine serum albumin). No anti-OVA antibodies were detected in preimmunization sera. The assay was standardized by using a pool of sera from OVA-hyperimmunized mice.
Cytokine responses in spleen cell suspensions.
B-cell activation following DC vaccination was assessed by measuring anti-OVA serum immunoglobulin titers. T-cell analyses were hampered because the DC induced fetal calf serum-specific T-cell responses. To circumvent this problem, we used another source of serum (horse serum) in our in vitro analysis; in our hands, this method allowed only short assays (i.e., cytokine determinations but not proliferation assays).
IFN-γ production in spleen cells was measured by using a modified version of a cell ELISA method (3) with an IFN-γ ELISA kit (R&D) according to the manufacturer's instructions. Briefly, mononuclear spleen cell suspensions were seeded in duplicate at different cell densities into Iscoves medium containing 5% horse serum in the presence or absence of OVA (500 μg/ml) and incubated at 37°C for 24 h in anti-IFN-γ-coated flat-bottom 96-well plates. The cells were removed by extensive washing, and biotinylated anti-mouse IFN-γ was added overnight at 4°C; this was followed by 45 min of incubation with 2 μg of peroxidase-labeled avidin (Sigma) per ml at room temperature. Color was developed with 100 μl of peroxidase substrate containing 3,3′,5,5′-tetramethylbenzidine (0.1 mg/ml; Sigma) and 0.06% H2O2 in 0.05 M phosphate-citrate buffer at pH 5.0. The reaction was stopped by adding 25 μl of 1 M H2SO4, and the absorbance at 450 nm was determined. The concentration of IFN-γ was determined by extrapolation from a standard curve by using recombinant cytokine. The results are expressed below as the amount of IFN-γ secreted per 106 spleen cells.
In one experiment prior to analysis we depleted the spleen cell suspensions of CD4+ cells using rat anti-mouse CD4 antibodies or of CD8+ cells using rat anti-mouse CD8 antibodies (Pharmingen) and anti-rat IgG-coated beads (Dynal).
Statistical analyses.
Statistical analyses were performed by using a one-way analysis of variance with Dunnett's posttest.
RESULTS
CTB is an efficient carrier molecule for DC vaccination.
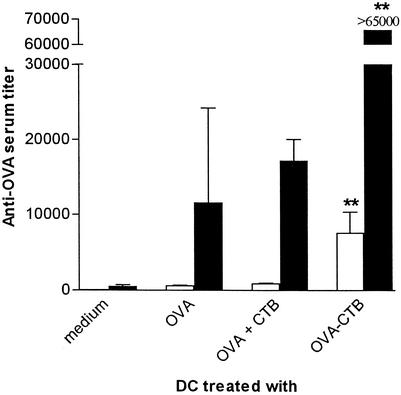
To investigate if conjugation of an antigen to CTB could potentiate DC vaccination, mice were injected twice with DC that had been prepulsed with either free or CTB-conjugated OVA, and the titers of OVA-specific serum IgG were measured 2 weeks later. We found that DC pulsed with OVA-CTB was superior to DC pulsed with OVA alone for evoking an anti-OVA antibody response (Fig. 1). Thus, low but consistent levels of anti-OVA IgG were found in mice that had been injected twice with OVA-pulsed DC, whereas no anti-OVA antibodies were detected in mice injected with mock-pulsed DC. The titers of anti-OVA IgG were 12 to 13 times higher (P < 0.01) in mice injected with OVA-CTB-pulsed DC than in mice that received DC pulsed with OVA alone or DC pulsed with a mixture of OVA and CTB (Fig. 1).
FIG. 1.
CTB is an efficient carrier molecule for DC vaccination. BALB/c mice were immunized twice with DC prepulsed with equimolar concentrations of either OVA, OVA plus CTB, or OVA-CTB (concentrations corresponding to 0.5 μM OVA and 0.5 μM CTB) and were boosted 2 weeks later with OVA in FCA. Serum was taken 14 days after the second DC vaccination (open bars) or 9 days after the booster dose (solid bars) and analyzed for OVA-specific IgG. The data are the arithmetic mean reciprocal titers and standard deviations for three or more mice. Two asterisks indicate that the P value was <0.01 for a comparison with mice that received OVA-pulsed DC or DC that were pulsed with OVA plus CTB.
When the DC-vaccinated mice were boosted with a low dose of OVA in FCA, the levels of anti-OVA IgG increased 5- to 10-fold in all primed mice but not in mice that had been vaccinated with mock-pulsed DC. Following the OVA booster, mice initially vaccinated with OVA-CTB-pulsed DC had significantly higher (more than sixfold higher) anti-OVA serum IgG titers than mice that had been primed with OVA-pulsed DC or with DC that were pulsed with OVA plus CTB (Fig. 1).
To ascertain that DC were responsible for the in vivo priming, we purified CD11c-positive cells from our bone marrow cultures and pulsed these cells, as well as nonpurified bone marrow-derived DC, with OVA-CTB prior to vaccination. Both populations of cells primed for B-cell and T-cell responses. There were no statistically significant differences in the anti-OVA serum titers or OVA-specific IFN-γ responses between the two groups of recipient mice (data not shown).
CT is a strong adjuvant for DC vaccination.
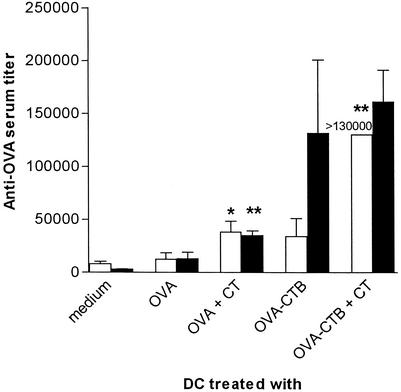
It has been postulated that CT exerts much of its adjuvant influence through a direct effect on APC (5, 7, 12). Therefore, we tested if CT adjuvant influenced the antibody responses following DC vaccination. Prior to injection, DC were pulsed with free or CTB-conjugated OVA in the presence or absence of CT, and the anti-OVA immune responses in recipient mice were then measured. CT treatment of DC significantly enhanced the anti-OVA titers (measured after two DC vaccinations) when both OVA-pulsed and OVA-CTB-pulsed DC were used (Fig. 2). The anti-OVA titers were especially impressive in mice immunized with OVA-CTB-pulsed CT-treated DC. Thus, the anti-OVA titers were higher after two DC vaccinations with OVA-CTB-pulsed and CT-treated DC than after two DC priming vaccinations with OVA-pulsed DC followed by one booster immunization with OVA in FCA (Fig. 1). After the OVA booster immunization, the groups primed with CT-treated DC continued to give higher anti-OVA titers than the corresponding groups primed with DC without any CT, but the differences were smaller than the differences after the primary immunizations (Fig. 2).
FIG. 2.
CT is a strong adjuvant for DC vaccination. BALB/c mice were immunized twice with DC prepulsed with either 0.5 μM OVA or 0.5 μM OVA-CTB in the presence or absence of 0.05 μM CT and were boosted 2 weeks later with OVA in FCA. Serum was taken 14 days after the second DC vaccination (open bars) or 9 days after the booster dose (solid bars) and analyzed for OVA-specific IgG. The data are the arithmetic mean reciprocal titers and standard deviations for three or more mice. Two asterisks indicate that the P value was <0.01 and one asterisk indicates that the P value was <0.05 for a comparison with mice that received antigen-pulsed DC in the absence of CT.
CT-treated DC promote both Th1 and Th2 responses.
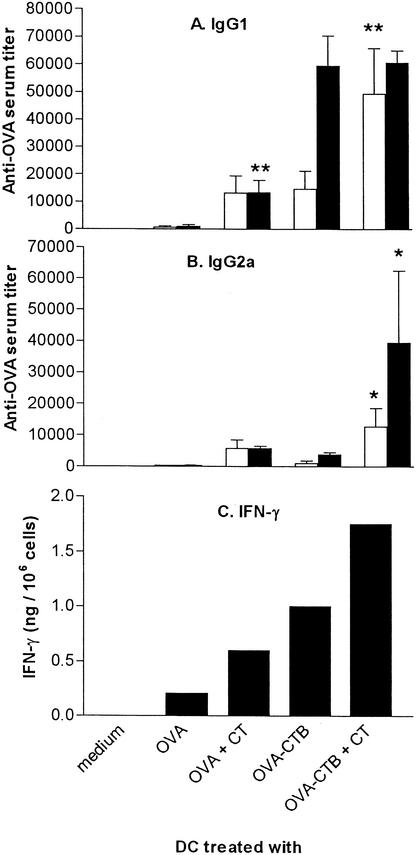
The adjuvant effect of CT is believed to be IL-12 independent and Th2 biased (5, 12). However, we found that CT promotes both Th1 and Th2 development. Thus, whereas vaccination with OVA-pulsed or OVA-CTB-pulsed DC preferentially induced anti-OVA IgG1 responses (Fig. 3A), vaccination with CT-treated DC also induced strong anti-OVA IgG2a responses (Fig. 3B). Furthermore, when spleen cell suspensions from these mice were activated in vitro with OVA and the IFN-γ production was measured, we found that vaccination with CT adjuvant-treated DC enhanced the OVA-specific IFN-γ responses when both OVA-pulsed and OVA-CTB-pulsed DC were used (Fig. 3C). We could not detect any IL-4 production by in vitro activated spleen cells, irrespective of the vaccination protocol (data not shown).
FIG. 3.
CT-treated DC promote Th1 responses. BALB/c mice were immunized twice with DC prepulsed with either 0.5 μM OVA or 0.5 μM OVA-CTB in the presence or absence of 0.05 μM CT and were boosted 2 weeks later with OVA in FCA. (A and B) Serum was taken 14 days after the second DC vaccination (open bars) or 9 days after the booster dose (solid bars) and analyzed for OVA-specific IgG1 (A) and OVA-specific IgG2a (B). The data are the arithmetic mean reciprocal titers and standard deviations for three or more mice. Two asterisks indicate that the P value was <0.01 and one asterisk indicates that the P value was <0.05 for a comparison with mice that received antigen-pulsed DC in the absence of CT. (C) Mice were sacrificed 9 days after the boost dose, and OVA-specific IFN-γ production was determined for pooled spleen cell suspensions from three mice. The data are the amounts of OVA-specific IFN-γ produced by 106 spleen cells.
CT is a highly efficient carrier for DC vaccination.
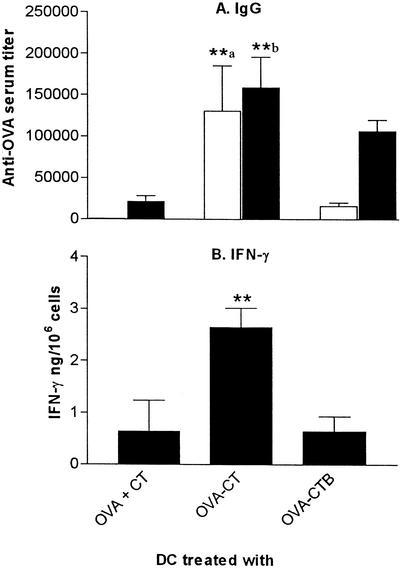
To combine the carrier and adjuvant functions of CT and CTB, we coupled OVA to CT and pulsed DC with this construct. Due to the toxic side effects of CT, we used a 10-fold-lower dose of antigen than we used in the experiments described above (corresponding to 0.05 μM OVA and 0.05 μM CT or 0.05 μM CTB). Treatment with OVA coupled to CT was superior to both treatment with OVA-CTB and treatment with OVA plus CT for inducing both B-cell and T-cell responses. Significantly stronger anti-OVA serum antibody responses were obtained after two vaccinations with OVA-CT-pulsed DC, a time when the antibody responses to DC treated with OVA plus CT and to DC treated with OVA-CTB were still modest (Fig. 4A). Similarly, the OVA-specific IFN-γ production was more than fourfold higher (P < 0.01) following vaccination with OVA-CT-pulsed DC than following vaccination with DC pulsed with OVA plus CT or DC pulsed with OVA-CTB (Fig. 4B). Depletion of either CD4+ or CD8+ T-cell subsets prior to the cell ELISA analysis revealed that the OVA-specific IFN-γ responses obtained were mediated by CD4+ T cells (data not shown).
FIG. 4.
CT is a highly efficient carrier for DC vaccination. BALB/c mice were immunized twice with DC prepulsed with either 0.05 μM OVA-CTB or 0.05 μM OVA-CT in the presence or absence of 0.05 μM CT and were boosted 2 weeks later with OVA in FCA. (A) Serum was taken 14 days after the second DC vaccination (open bars) or 9 days after the booster dose (solid bars) and analyzed for OVA-specific IgG. The data are the arithmetic mean reciprocal titers and standard deviations for three or more mice. Two asterisks followed by the letter a indicate that the P value was <0.01 for a comparison with mice that received DC pulsed with OVA-CTB or OVA plus CT, and two asterisks followed by the letter b indicate that the P value was <0.01 for a comparison with mice that received DC pulsed with OVA plus CT. (B) Mice were sacrificed 9 days after the booster dose, and OVA-specific IFN-γ production was determined in spleen cell suspensions. The bars indicate the mean amounts of OVA-specific IFN-γ produced by 106 spleen cells for three mice; the error bars indicate standard deviations. Two asterisks indicate that the P value was <0.01 for a comparison with mice that received DC pulsed with OVA-CTB or with OVA plus CT.
Cell surface expression of OVA and of costimulatory molecules on pulsed DC.
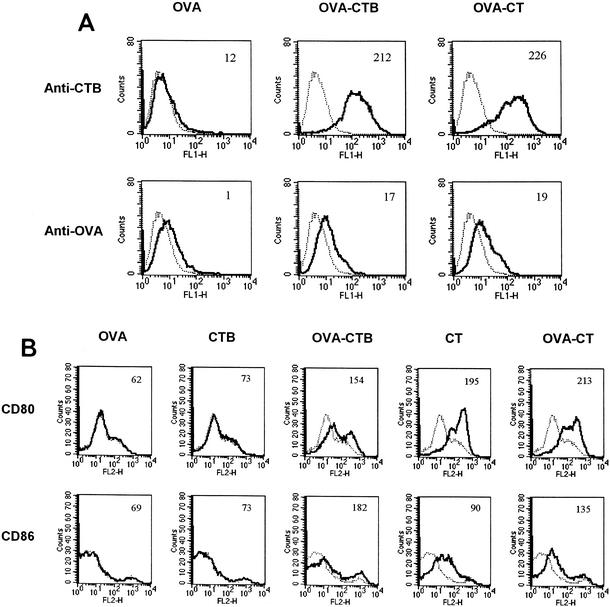
To investigate if the levels of cell surface-bound OVA were correlated with immunogenicity, we compared the densities of OVA and CT or CTB on DC following 90 min of incubation with OVA, OVA-CTB, or OVA-CT. No CTB was detected on the surface of OVA-pulsed cells, whereas high densities of CTB were found on OVA-CT- and OVA-CTB-pulsed cells (Fig. 5A). Low levels of cell surface exposed OVA were detected in OVA-pulsed DC. The levels were similarly low in OVA-CT- and OVA-CTB-pulsed DC (Fig. 5A).
FIG. 5.
Cell surface expression of OVA, CTB, and costimulatory molecules on antigen-pulsed DC. (A) OVA-, OVA-CTB-, or OVA-CT-pulsed DC were analyzed for cell surface expression of OVA or CTB immediately after antigen pulsing. The solid lines indicate the results obtained for anti-OVA and anti-CTB antibodies, whereas the dotted lines indicate the results obtained for control antibody. The mean fluorescence is indicated in the top right corner of each graph. (B) OVA-, CTB-, CT-, OVA-CTB-, or OVA-CT-pulsed DC were analyzed for cell surface expression of CD80 or CD86 24 h after antigen pulsing. The solid lines indicate the results obtained for anti-CD80 and anti-CD86 antibodies, whereas the dotted lines indicate the results obtained for unstained cells. The mean fluorescence is indicated in the top right corner of each graph.
CT has previously been shown to enhance the cell surface levels of the costimulatory molecules CD80 and CD86 on DC. We found that OVA-CTB, OVA-CT, and CT, but not free OVA or free CTB, enhanced the expression of CD80 and CD86 on the pulsed DC (Fig. 5B). Neither CT nor CTB affected the background staining when isotype-matched control antibodies were used (data not shown).
Cytokine production by pulsed DC.
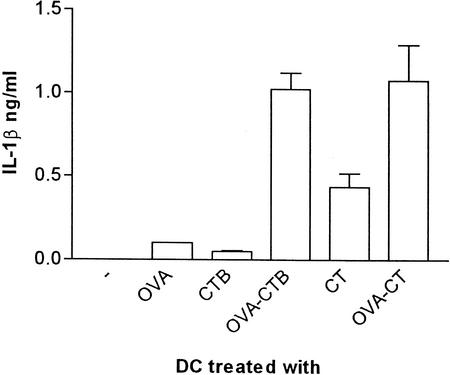
The levels of IL-1β, IL-12(p40), and IL-18 were measured in 24-h culture supernatants from DC pulsed with either free OVA, CTB, or CT or with the OVA-CTB and OVA-CT constructs. We did not detect IL-18 in any of the samples (data not shown). Similarly, overall, the levels of secreted IL-12 were low (data not shown). Instead, native CT, OVA-CT, and OVA-CTB had a more pronounced effect on the production of IL-1β. Thus, pulsing of DC with CT, but not pulsing of DC with OVA or CTB, induced an IL-1β response. This IL-1β response was even stronger when DC were pulsed with either OVA-CTB or OVA-CT (Fig. 6).
FIG. 6.
Secretion of IL-1β by antigen-pulsed DC. Twenty-four-hour cultures of OVA-, CTB-, CT-, OVA-CTB-, or OVA-CT-pulsed DC were analyzed for levels of IL-1β by ELISA. The data are the arithmetic means and standard deviations for at least two individual experiments.
DISCUSSION
We investigated the immunomodulating effects of CT and CTB on DC vaccination. Prior to injection into syngeneic mice, bone marrow-derived DC were pulsed with either free OVA, OVA linked to CT or CTB, or mixtures of OVA and CT or CTB. The subsequent immune responses were measured after two DC vaccinations and then again after a subsequent OVA challenge. We found that CTB is an efficient carrier molecule for ex vivo antigen pulsing of DC, promoting a Th2-like response. Treatment of DC with CT adjuvant markedly enhanced the immunostimulatory capacity of the cells and predisposed them for a combined Th1-Th2 response. The apparently different carrier and adjuvant functions of CT and CTB could be combined for maximal potency if the antigen was linked directly to CT prior to DC pulsing, and this predisposed the cells for an even stronger Th1 response. The immunoenhancing capacity of CT, OVA-CT, and OVA-CTB could be linked to upregulated expression of CD80 and CD86 on the DC in combination with enhanced production of IL-1β.
Our finding that CTB was an efficient carrier protein for DC vaccination in vivo corroborates our previous in vitro findings. In the previous studies coupling of antigen to CTB increased the efficacy of antigen presentation by DC and other APC and dramatically lowered the dose of antigen required for efficient presentation, effects that could be linked to increased uptake of the coupled antigen via binding to the GM1 receptor and upregulated expression of CD40 and CD86 on the APC (13). The responses obtained in this study following vaccination with OVA-CTB-pulsed DC were Th2 biased (high titers of OVA-specific IgG1 but no IgG2a), which most likely reflects the fact that CTB treatment of DC did not induce the production of Th1-promoting cytokines, such as IL-12 or IL-18. Instead, CD40, which is upregulated on OVA-CTB-treated DC following interaction with T cells (13), has recently been shown to induce Th2 development (24). Furthermore, these results are in line with in vivo data showing that orally administered CTB-coupled antigen can be used as a treatment to change the immune response from Th1-mediated pathology (42, 43, 47).
We also found that CT is a strong adjuvant for DC vaccination, enhancing both T-cell and B-cell responses. CT has previously been shown to enhance the cell surface expression of both MHC, costimulatory molecules, and chemokine receptors on DC and to affect the secretion of cytokines, such as IL-12 and tumor necrosis factor alpha (12). We also found that there was strong upregulation of costimulatory molecules, particularly CD80. However, contrary to previous reports (12), we found that CT by itself induced the secretion of IL-1β from DC. Similar observations have been made with related cell types, including macrophages (6, 7, 11). IL-1 not only induces the maturation of DC (33) but also is an efficient mucosal adjuvant when it is coadministered with protein antigens (38), and it might be responsible for a substantial part of CT's adjuvant activity (6, 38).
Coupling of antigen directly to CT was superior to pulsing DC with OVA-CTB in the presence of CT, which could reflect the joint targeting of antigen and adjuvanticity to the same DC or possibly to the same subcellular compartment. The alternative possibility, that there is competition between CT and OVA-CTB for GM1 binding which leads to lower uptake of OVA in DC pulsed with OVA-CTB plus CT than in DC pulsed with OVA-CT, is less likely inasmuch as other in vitro studies have shown that such competition would require >100-fold-higher concentrations of CT than those used in the present study (J. Holmgren, unpublished observations).
The adjuvant effect of CT on DC vaccination was also associated with the induction of a Th1-type response. This occurred despite a lack of IL-12 or IL-18 priming and could not be attributed to IL-1β production (as OVA-CTB and OVA-CT induced comparable levels of IL-1β). One possibility is that the increased levels of IgG2a and IFN-γ obtained following vaccination with CT-treated DC simply reflect the overall stronger immune response obtained with CT-treated DC. However, other factors could influence the Th1-Th2 balance. CT-pulsed DC carried considerable quantities of CT adjuvant on their cell surfaces. We do not know to what extent such cell surface-bound CT retains adjuvant properties towards adjacent cells and whether cell surface-bound CT potentiates Th1 development. However, our preliminary in vitro data show that the toxic effects of CT on T cells are lost if CT is attached to DC, suggesting that DC cell surface-bound CT does not enter adjacent cells. It is also debated whether free CT can induce a Th1 response and/or a Th2 response. Several studies have shown that CT by itself primes for an exclusive Th2 response by inhibiting IL-12 production (5, 12). However, when CT is administered mucosally as an adjuvant, it induces a combined Th1-Th2 response (16, 17) like our CT-pulsed DC, and, in contrast to CTB, it aggravates Th1-mediated delayed-type hypersensitivity and autoimmune tissue reactions (42). Another possibility is that the levels of costimulatory molecules on the injected CT-pulsed DC affect Th1 and Th2 commitment. CT-treated or OVA-CT-pulsed DC upregulated their expression of CD80 to a much greater degree than OVA-CTB-pulsed DC. Even though no consensus has been reached regarding the roles of CD80 and CD86 in Th1 and Th2 development, there have been several reports which have shown that high levels of CD80 do drive Th1 commitment, either through a Th1-specific pathway or simply by offering more costimulation (18, 35, 36). Furthermore, we cannot rule out the possibility that CT and CTB affect intracellular processing and loading of OVA onto MHC molecules in different ways, nor can we rule out the possibility that chemokine receptor expression, chemokine secretion patterns, and intracellular survival of OVA-CTB- and OVA-CT-pulsed DC might differ.
Irrespective of the underlying mechanism(s), the use of CT as a DC adjuvant should be especially interesting for DC vaccination against selected virus infections or tumors, where a Th1 response is desired. The normal toxicity of CT, which precludes its use as an adjuvant for in vivo administered vaccines, appears to be irrelevant for DC vaccination, in which the CT treatment takes place in vitro and there is extensive washing of the DC before they are infused in vivo.
It was interesting that chemical conjugates of OVA-CTB and OVA-CT had a greater impact on cytokine production than any of the native proteins alone, including CT. Thus, the highest levels of IL-12 and IL-1β produced by the DC were induced by the chemical conjugates. CT, OVA-CT, and OVA-CTB, but not free OVA or free CTB, also induced enhanced expression of CD80 and CD86. We do not know why chemical conjugates have such strong immunomodulatory effects on the production of cytokines and on the levels of costimulatory molecules, but we believe that these effects are related to chemical modifications induced by the coupling procedure. Irrespective of the underlying mechanism, these strong effects on DC phenotype and on IL-1β production could explain part of the immunopotentiating capacity of CT and CTB conjugates.
In summary, both CT and CTB can be utilized to enhance the responsiveness to DC vaccination. Conjugating antigen to CT or CTB prior to DC pulsing could enhance the subsequent immune response through several mechanisms, including (i) enhanced uptake and presentation of the conjugated protein, (ii) IL-1β costimulation, and (iii) enhanced expression of costimulatory molecules. Furthermore, through selective use of CTB and CT as carrier antigens it is possible to direct the immune response towards a Th2 response and towards a combined Th1-Th2 response, respectively, which has obvious potential implications for the induction of a desired type of immune response through the DC vaccination approach.
Acknowledgments
We thank Marianne Lindblad for help with CTB preparation, conjugation, purification, and characterization.
This study was supported by the Swedish Science Council (Medicine), by the SIDA/SAREC Special Program for AIDS and Related Diseases, by the EU cluster program on mucosal immunization (MUCIMM), by Stiftelsen Lars Hiertas Minne, and through support to GUVAX from the Knut and Alice Wallenberg Foundation.
Editor: V. J. DiRita
REFERENCES
- 1.Arakawa, T., J. Yu, D. K. Chong, J. Hough, P. C. Engen, and W. H. Langridge. 1998. A plant-based cholera toxin B subunit-insulin fusion protein protects against the development of autoimmune diabetes. Nat. Biotechnol. 16:934-938. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 3.Beech, J. T., T. Bainbridge, and S. J. Thompson. 1997. Incorporation of cells into an ELISA system enhances antigen-driven lymphokine detection. J. Immunol. Methods 205:163-168. [DOI] [PubMed] [Google Scholar]
- 4.Bergerot, I., C. Ploix, J. Petersen, V. Moulin, C. Rask, N. Fabien, M. Lindblad, A. Mayer, C. Czerkinsky, J. Holmgren, and C. Thivolet. 1997. A cholera toxoid-insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes. Proc. Natl. Acad. Sci. USA 94:4610-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun, M. C., J. He, C.-Y. Wu, and B. L. Kelsall. 1999. Cholera toxin suppresses interleukin (IL)-12 production and IL-12 receptor β1 and β2 chain expression. J. Exp. Med. 189:541-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bromander, A. K., J. Holmgren, and N. Lycke. 1991. Cholera toxin stimulates IL-1 production and enhances antigen presentation by macrophages in vitro J. Immunol. 146:2908-2914. [PubMed] [Google Scholar]
- 7.Cong, Y., C. T. Weaver, and C. O. Elson. 1997. The mucosal adjuvanicity of cholera toxin involves enhancement of costimulatory activity by selective up-regulation of B7.2 expression. J. Immunol. 159:5301-5308. [PubMed] [Google Scholar]
- 8.Czerkinsky, C., M. W. Russell, N. Lycke, M. Lindblad, and J. Holmgren. 1989. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect. Immun. 57:1072-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elson, C. O., and W. Ealding. 1984. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J. Immunol. 133:2892-2897. [PubMed] [Google Scholar]
- 10.Elson, C. O., and W. Ealding. 1984. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J. Immunol. 132:2736-2741. [PubMed] [Google Scholar]
- 11.Foss, D. L., M. J. Zilliox, and M. P. Murtaugh. 1999. Differential regulation of macrophage interleukin-1 (IL-1), IL-12, and CD80-CD86 by two bacterial toxins. Infect. Immunol. 67:5275-5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagliardi, M. C., F. Sallusto, M. Marinaro, A. Langenkamp, A. Lanzavecchia, and M. T. De Magistris. 2000. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming Eur. J. Immunol. 30:2394-2403. [DOI] [PubMed] [Google Scholar]
- 13.George-Chandy, A., K. Eriksson, M. Lebens, I. Nordström, E. Schön, and J. Holmgren. 2001. Cholera toxin B subunit as a carrier protein promotes antigen presentation and increases CD40 and CD86 expression on antigen-presenting cells. Infect. Immun. 69:5716-5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George-Chandy, A., N. Mielcarek, I. Nordström, J. Holmgren, and K. Eriksson. 2001. Vaccination with autologous or heterologous dendritic cells protects mice against Bordetella pertussis lung infection. Infect. Immunol. 69:4120-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmgren, J., C. Czerkinsky, J.-B. Sun, and A.-M. Svennerholm. 1996. Oral vaccination, mucosal immunity and oral tolerance with special reference to cholera toxin, p. 437-458. In S. H. E. Kaufmann (ed.), Concepts in vaccine development. Walter de Gruyter, Berlin, Germany.
- 16.Hörnquist, E., and N. Lycke. 1993. Cholera toxin adjuvant greatly promotes antigen priming of T cells. Eur. J. Immunol. 23:2136-2143. [DOI] [PubMed] [Google Scholar]
- 17.Kjerrulf, M., D. Grdic, L. Ekman, K. Schön, M. Vajdy, and N. Lycke. 1997. Interferon-γ receptor-deficient mice exhibit impaired gut mucosal immune responses but intact oral tolerance. Immunology 92:60-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuchroo, V. K., M. P. Das, J. A. Brown, A. M. Ranger, S. S. Zamvil, R. A. Sobel, H. L. Weiner, N. Nabavi, and L. H. Glimcher. 1995. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell 80:707-718. [DOI] [PubMed] [Google Scholar]
- 19.Lebens, M., S. Johansson, J. Osek, M. Lindblad, and J. Holmgren. 1993. Large-scale production of Vibrio cholerae toxin B subunit for use in oral vaccines. Bio/Technology 11:1574-1578. [DOI] [PubMed] [Google Scholar]
- 20.Lipscombe, M., I. G. Charles, M. Roberts, G. Dougan, J. Tite, and N. F. Fairweather. 1991. Intranasal immunization using the B subunit of the Escherichia coli heat-labile toxin fused to an epitope of the Bordetella pertussis P.69 antigen. Mol. Microbiol. 5:1385-1392. [DOI] [PubMed] [Google Scholar]
- 21.Ludewig, B., S. Ehl, U. Karrer, B. Odermatt, H. Hengartner, and R. M. Zinkernagel. 1998. Dendritic cells efficiently induce protective antiviral immunity. J. Virol. 72:3812-3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lycke, N., and J. Holmgren. 1986. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology 59:301-308. [PMC free article] [PubMed] [Google Scholar]
- 23.Lycke, N., T. Tsuij, and J. Holmgren. 1992. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur. J. Immunol. 22:2277-2281. [DOI] [PubMed] [Google Scholar]
- 24.MacDonald, A. S., A. D. Straw, N. M. Dalton, and E. J. Pearce. 2002. Th2 response induction by dendritic cells: a role for CD40. J. Immunol. 168:537-540. [DOI] [PubMed] [Google Scholar]
- 25.Mayordomo, J. I., T. Zorina, W. J. Storkus, L. Zitvogel, C. Celluzzi, L. D. Falo, C. J. Melief, S. T. Ildstad, W. Martin Kast, A. B. Deleo, and M. T. Lotze. 1995. Bone marrow-derived dendritic cells pulsed with synthetic peptides elicit protective and therapeutic antitumour immunity. Nat. Med. 1:1297-1302. [DOI] [PubMed] [Google Scholar]
- 26.McKenzie, S. J., and J. F. Halsey. 1984. Cholera toxin B subunit as a carrier protein to stimulate a mucosal immune response. J. Immunol. 133:1818-1824. [PubMed] [Google Scholar]
- 27.McSorley, S. J., C. Rask, R. Pichot, V. Julia, C. Czerkinsky, and N. Glaichenhaus. 1998. Selective tolerization of Th1-like cells after nasal administration of a cholera toxoid-LACK conjugate. Eur. J. Immunol. 28:424-432. [DOI] [PubMed] [Google Scholar]
- 28.Nestle, F. O., S. Alijagic, M. Dilliet, Y. Sun, S. Grabbe, R. Dummer, G. Burg, and D. Schadendorf. 1998. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat. Med. 4:328-332. [DOI] [PubMed] [Google Scholar]
- 29.Pierce, N. F., and J. L. Gowans. 1978. The role of antigen form and function in the primary and secondary intestinal immune responses to cholera toxin and toxoid in rats. J. Exp. Med. 148:195-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porgador, A., and E. Gilboa. 1995. Bone marrow-derived dendritic cells pulsed with a class I-restricted peptide are potent inducers of cytotoxic T lymphocytes. J. Exp. Med. 182:255-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porgador, A., D. Snyder, and E. Gilboa. 1996. Induction of antitumor immunity using bone marrow-generated dendritic cells. J. Immunol. 156:2918-2926. [PubMed] [Google Scholar]
- 32.Rask, C., J. Holmgren, M. Fredriksson, M. Lindblad, I. Nordström, J.-B. Sun, and C. Czerkinsky. 2000. Prolonged oral treatment with low doses of allergen conjugated to cholera toxin B subunit suppresses immunoglobulin E antibody responses in sensitized mice. Clin. Exp. Allergy 30:1024-1032. [DOI] [PubMed] [Google Scholar]
- 33.Sallusto, F., M. Cella, C. Danieli, and A. Lanzavecchia. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schön, E., A. M. Harandi, I. Nordström, J. Holmgren, and K. Eriksson. 2001. Dendritic cell vaccination protects mice against lethality caused by genital herpes virus type 2 infection. J. Reprod. Immunol. 50:87-106. [DOI] [PubMed] [Google Scholar]
- 35.Schweitzer, A. N., F. Borriello, R. C. Wong, A. K. Abbas, and A. H. Sharpe. 1997. Role of costimulators in T cell differentiation: studies using antigen-presenting cells lacking expression of CD80 or CD86. J. Immunol. 158:2713-2722. [PubMed] [Google Scholar]
- 36.Soos, J. M., T. A. Ashley, J. Morrow, J. C. Patarroyo, B. E. Szente, and S. S. Zamvil. 1999. Differential expression of B7 co-stimulatory molecules by astrocytes correlates with T cell activation and cytokine production. Int. Immunol. 11:1169-1179. [DOI] [PubMed] [Google Scholar]
- 37.Sornasse, T., V. Flamand, G. De Becker, H. Bazin, F. Thielemans, K. Thielemans, J. Urbain, O. Leo, and M. Moser. 1992. Antigen-pulsed dendritic cells efficiently induce an antibody response in vivo. J. Exp. Med. 175:15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staats, H. F., and F. A. Ennis, Jr. 1999. IL-1 is an effective adjuvant for mucosal and systemic immune responses when coadministered with protein immunogens. J. Immunol. 162:6141-6147. [PubMed] [Google Scholar]
- 39.Steinman, R. M., and M. Dhodapkar. 2001. Active immunization against cancer with dendritic cells: the near future. Int. J. Cancer 94:459-473. [DOI] [PubMed] [Google Scholar]
- 40.Sun, J. B., J. Holmgren, and C. Czerkinsky. 1994. Cholera toxin B subunit: an efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc. Natl. Acad. Sci. USA 91:10795-10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun, J. B., N. Mielcarek, M. Lakew, J. M. Grzych, A. Capron, J. Holmgren, and C. Czerkinsky. 1999. Intranasal administration of a Schistosoma mansoni glutathione S-transferase-cholera toxoid conjugate vaccine evokes antiparasitic and antipathological immunity in mice. J. Immunol. 163:1045-1052. [PubMed] [Google Scholar]
- 42.Sun, J. B., C. Rask, T. Olsson, J. Holmgren, and C. Czerkinsky. 1996. Treatment of experimantal autoimmune encephalomyelitis by feeding myelin basic protein conjugated to cholera toxin B subunit. Proc. Natl. Acad. Sci. USA 93:7196-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun, J. B., B. G. Xiao, M. Lindblad, B. L. Li, H. Link, C. Czerkinsky, and J. Holmgren. 2000. Oral administration of cholera toxin B subunit conjugated to myelin basic protein protects against experimental autoimmune encephalomyelitis by inducing transforming growth factor-beta-secreting cells and suppressing chemokine expression. Int. Immunol. 19:1449-1457. [DOI] [PubMed] [Google Scholar]
- 44.Svennerholm, A.-M., and J. Holmgren. 1978. Identification of Escherichia coli heat-labile enterotoxin by means of a ganglioside immunosorbant assay (GM1-ELISA) procedure. Curr. Microbiol. 1:19-23. [Google Scholar]
- 45.Svennerholm, A.-M., M. Wikström, M. Lindblad, and J. Holmgren. 1986. Monoclonal antibodies to Escherichia coli heat-labile enterotoxins: neutralizing activity and differentiation of human and porcine LTs and cholera toxin. Med. Biol. 64:23-30. [PubMed] [Google Scholar]
- 46.Tamura, S., E. Hatori, T. Tsuruhara, C. Aizawa, and T. Kurata. 1997. Suppression of delayed-type hypersensitivity and IgE antibody responses to ovalbumin by intranasal administration of Escherichia coli heat-labile enterotoxin B subunit-conjugated ovalbumin. Vaccine 15:225-229. [DOI] [PubMed] [Google Scholar]
- 47.Tarkowski, A., J.-B. Sun, R. Holmdahl, J. Holmgren, and C. Czerkinsky. 1999. Treatment of experimental autoimmune arthritis by nasal administration of a type II collagen-cholera toxoid conjugate vaccine. Arthritis Rheum. 42:1628-1634. [DOI] [PubMed] [Google Scholar]
- 48.Wiedermann, U., B. Jahn-Schmid, M. Lindblad, C. Rask, J. Holmgren, D. Kraft, and C. Ebner. 1999. Suppressive versus stimulatory effects of allergen/cholera toxoid (CTB) conjugates depending on the nature of the antigen in a murine model of type I allergy. Int. Immunol. 11:1717-1724. [DOI] [PubMed] [Google Scholar]