Abstract
The search to identify Mycobacterium tuberculosis antigens capable of conferring protective immunity against tuberculosis has received a boost owing to the resurgence of tuberculosis over the past two decades. It has long been recognized that lymphoid cells are required for protection against M. tuberculosis. While traditionally the CD4+ populations of T cells were believed to predominantly serve this protective function, a pivotal role for CD8+ T cells in this task has been increasingly appreciated. We show that the 50- to 55-kDa Apa protein, specified by the Rv1860 gene of M. tuberculosis, can elicit both lymphoproliferative response and gamma interferon (IFN-γ) production from peripheral blood mononuclear cells (PBMC) of purified protein derivative (PPD)-positive individuals, with significant differences recorded in the levels of responsiveness between PPD-positive healthy controls and pulmonary tuberculosis patients. Flow cytometric analysis of whole blood stimulated with the recombinant Apa protein revealed a sizeable proportion of CD8+ T cells in addition to CD4+ T cells contributing to IFN-γ secretion. PBMC responding to the Apa protein produced no interleukin-4, revealing a Th1 phenotype. A DNA vaccine and a poxvirus recombinant expressing the Apa protein were constructed and tested for their ability to protect immunized guinea pigs against a challenge dose of virulent M. tuberculosis. Although the DNA vaccine afforded little protection, the poxvirus recombinant boost after DNA vaccine priming conferred a significant level of protective immunity, bringing about a considerable reduction in mycobacterial counts from the challenge bacilli in spleens of immunized guinea pigs, a result comparable to that achieved by BCG vaccination.
The dominant contribution of cell-mediated immune responses in protection against tuberculosis (TB) has been extensively documented. The early observations of Mackaness and Blanden (22) revealed the controlling role for lymphocytes in the antimycobacterial function of macrophages. That lymphocytes dominate the protective response through soluble mediators (5, 8), subsequently called the cytokines, was also discovered soon thereafter. The ability of T cells to adoptively transfer resistance in mice against a challenge from virulent Mycobacterium tuberculosis was demonstrated by Orme (28), who also reported the preeminent role of live bacilli in the acquisition and recall of protective immunity to TB (27). Working with secreted culture filtrate proteins of M. tuberculosis, Andersen et al. (4) showed that T cells, predominantly of the L3T4+ subtype from mice infected with live, but not dead bacilli, recognized the secreted proteins. The two subsets of T cells were also implicated in protection against TB by other workers (18, 20, 25, 27).
Subsequent studies have enhanced our understanding of the role for TH1 subtype of T cells in protective immunity against TB. The increased susceptibility to TB of mice with disruptions in the gene for gamma interferon (IFN-γ) (12), the cardinal cytokine that marks the Th1 response and activates macrophages to a heightened state of cytocidal functions with potential to eliminate intracellular bacilli, highlighted the crucial role for this cytokine in antitubercular immunity. The vital protective role played by IFN-γ in human immunity to TB was also unequivocally demonstrated by the extreme susceptibility to TB and even BCG of individuals with mutations in genes for IFN-γ-receptor ligand-binding chain and IFN-γ-receptor signaling chain, as well as interleukin-12 (IL-12) p40 subunit and IL-12 receptor β1 chain (1), whose defective functioning results in failure to induce IFN-γ production.
The essential function of the CD8+ subset of T cells in protection against TB in mice has also been known for some time (27, 43), with mice carrying a disruption in the β2-microglobulin gene showing enhanced susceptibility to M. tuberculosis infection (13). Although the contribution of M. tuberculosis-specific cytolytic CD8+ T cells in protecting humans remains to be established, their existence has been amply demonstrated (6, 36, 40). The potential of this subset of T cells to destroy M. tuberculosis-infected cells and thereby the intracellular bacilli, thus providing long-lasting immunity to TB, resulted in efforts that led to the discovery of a battery of M. tuberculosis proteins capable of eliciting human CD8+ T cells in purified protein derivative (PPD)-positive individuals, such as the 6-kDa ESAT6 protein (19), the 19-kDa lipoprotein (24), antigens 85A and 85B (37), CFP10.4 (35), and the 39-kDa secreted protein (21). In addition, the discovery of human CD1b-restricted CD8+ T cells capable of killing intracellular M. tuberculosis through the combined action of perforin and granulysin (39) suggests that CD8+ T cells play a prime role in controlling human TB infection.
We identified the Apa protein specified by the Rv1860 gene of M. tuberculosis in an extensive screen of several recombinant proteins from a lambda ZAP II::M. tuberculosis expression library (2) for their ability to elicit lymphoproliferative responses in PPD-positive individuals. The Apa protein homologue of Mycobacterium bovis BCG was originally described for its unusual potency in eliciting both antibodies and T cells from guinea pigs immunized with live but not dead organisms (31, 33). We show that both human CD4+ and CD8+ T cells respond to the Apa protein of M. tuberculosis by synthesizing the protective type I cytokine IFN-γ, with significantly higher levels observed in PPD-positive healthy controls compared to pulmonary TB patients. That Apa protein-specific immune responses can protect against TB was revealed upon challenge of guinea pigs immunized with a combination of a DNA vaccine and poxvirus expressing the Apa protein with virulent M. tuberculosis.
MATERIALS AND METHODS
Study population.
Newly diagnosed TB patients (14 males [mean age, 29 ± 8 years; mean weight, 54 ± 6 kg] and 17 females [mean age, 26 ± 6 years; mean weight, 45 ± 5 kg]) who were admitted to the Shantabai Devarao Shivaram Tuberculosis and Chest Diseases Hospital, Bangalore, India, formed part of the study cohort. Diagnosis of TB was based on presence of acid-fast bacilli in sputum. PPD-positive healthy controls (12 males [mean age, 27 ± 6 years; mean weight, 52 ± 8 kg] and 10 females [mean age, 24 ± 5 years; mean weight, 51 ± 9 kg]) who had never suffered from clinical symptoms of TB were drawn from the staff of the above mentioned hospital, Indian Institute of Science or National Tuberculosis Institute, Bangalore, India. Active pulmonary TB was excluded from the healthy controls by chest roentgenogram and sputum smears for acid-fast bacilli. All individuals tested with recombinant proteins were PPD positive with minimum induration readings of 15 mm in the Mantoux test and had received M. bovis BCG vaccinations as children. The clinical conditions of human immunodeficiency virus infection/AIDS and hepatitis were ruled out in the study population by the hospital. The study was approved by the institutional ethics committee, and signed informed consents were obtained from all individuals prior to enrollment in the study once the purpose and consequences of the study had been fully explained.
Isolation of PBMC and T-cell proliferation assays.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood samples by the Ficoll-Hypaque density gradient method. The cells were cultured at 1.5 × 105 cells per well in RPMI 1640 supplemented with 10% heat-inactivated human AB serum, 2 mM l-glutamine, and antibiotics (Gibco-BRL, Grand Island, N.Y.) in flat-bottom 96-well microtiter plates (Corning Costar, Cambridge, Mass.) with 50 μg of recombinant antigen/ml in a humidified atmosphere of 5% CO2 at 37°C for 5 days. Incubations with M. tuberculosis sonicate (5 μg/ml) and the mitogen phytohemagglutinin A (PHA; Sigma, St. Louis, Mo.) at 10 μg/ml were carried out for a period of 3 days. Lymphocyte proliferation was measured by the incorporation of [3H]thymidine (NEN Dupont, Boston, Mass.) that was added at 0.5 μCi per well for the final 18 to 20 h of culture. The cells were harvested by using a PHD cell harvester (Cambridge Technology, Inc., Watertown, Mass.), and the amount of incorporated radiolabel was determined in a LKB Rack Beta liquid scintillation counter (model 1209; LKB-Wallac, Turku, Finland). All tests were performed in triplicate wells.
The proliferative response was expressed as the stimulation index (SI), which was calculated as follows: SI = mean counts per minute (cpm) of test antigen-stimulated cultures/mean cpm of unstimulated cultures in triplicate wells. A positive response to the Apa protein was scored based on the criterion that the SI was ≥2.0, with the average cpm obtained on stimulation with recombinant antigen being ≥500 and the presence of a significant increase in cpm induced by the test antigen, as ascertained by the Student t test. Similarly, the cutoff value for a positive M. tuberculosis sonicate-directed response was an SI of >3.0. No significant differences were observed in the recovery of PBMC from blood (1 × 106 to 2 × 106 PBMC/ml) between TB patients and PPD-positive healthy donors.
Cloning procedures and recombinant expression of the M. tuberculosis Apa protein.
The recombinant M. tuberculosis clone MTB65 in the pBS-SK vector derived by excision from the corresponding clone in the lambda ZAPII::M. tuberculosis expression library (2) contained the gene Rv1860 coding for the Apa protein missing the first 93 nucleotides (nt) from the start codon ATG, thus giving rise to a truncated Apa protein lacking the N-terminal 31 amino acids, as a fusion to the vector sequences of pBS-SK. The Apa open reading frame from M. tuberculosis clone MTB65 was excised as a 890-nt fragment extending from the vector cloning site EcoRI to the Klenow-filled BamHI site immediately downstream of the termination codon of the open reading frame and cloned into pRSETC between the EcoRI and Klenow-filled HindIII sites. This construct pRSETC-65 expressed the N-terminally truncated Apa protein as a fusion to six histidine residues at its N terminus upon induction with isopropyl-β-d-thiogalactopyranoside (IPTG) in the Escherichia coli strain BL21(DE3).
For expression of the Apa protein in DNA vaccine and poxvirus vectors, the apa gene from M. tuberculosisclone MTB65 was first cloned into the E. coli expression vector pRSETB as follows: the insert from M. tuberculosis clone MTB65 was excised as a 1.1-kb SacI-to-EcoRV fragment that was blunt ended by sequential treatment with Klenow enzyme and T4 DNA polymerase. This fragment was ligated to pRSETB digested with SacI and PvuII. The vector segment containing the multiple cloning sites upstream of the M. tuberculosis apa gene was then deleted from this construct by digestion with NheI and NotI, with Klenow filling to blunt the ends, followed by reclosing the molecule by ligation. The insert of apa gene obtained from this construct along with the start codon ATG as a 1.1-kb fragment by digestion with NdeI and NcoI was blunted with Klenow polymerase and ligated to the DNA vaccine vector pUMVC3 (Aldevron, Fargo, N.D.) cut with EcoRV or the BglII-cut and Klenow-blunted MVA transfer vector pMCO3 (7). Recombination into the MVA genome, followed by purification and expansion of recombinant APAMVA poxvirus, was carried out according to standard procedures (7). Expression of Apa protein from the above constructs was verified by Western immunoblotting of pNGVLApa (APADNA)-transfected CV-1 and APAMVA-infected BHK21 cell lysates, respectively, with mouse anti-Apa serum.
Protein preparations.
pRSETC-65 was transformed into E. coli BL21(DE3) cells, and the expression of protein was induced with 1 mM IPTG at an optical density of 0.5 A600 units for 4 h. Cells were harvested by centrifugation at 12,000 × g for 5 min, and the pellet was resuspended in lysis buffer (50 mM Tris, pH 8.0; 150 mM NaCl) and sonicated for 15 min by using a Branson sonifier model 450. The lysate was adjusted to 0.5 M NaCl and 10 mM imidazole and was bound to a 5-ml bed volume column of Ni2+-nitrilotriacetic acid (NTA) agarose beads at 4°C in a Pharmacia fast-performance liquid chromatograph (FPLC) model GP250. After the column was washed with 10 column volumes of wash buffer (50 mM Tris, pH 8.0; 1.0 M NaCl; 10 mM imidazole), bound protein was eluted by using a 0 to 1.0 M gradient of imidazole in 10 mM Tris (pH 7.0) at 4°C. Fractions containing the Apa protein were pooled, concentrated by precipitation with ammonium sulfate, and dialyzed against phosphate-buffered saline at 4°C. Protein purity was confirmed by N-terminal sequencing by using Edman chemistry, and fractions were stored in aliquots at −75°C. The absence of contaminating bacterial endotoxins in the purified protein preparations was ascertained by using the Pyrogent plus Gel-clot LAL test kit (BioWhittaker, Inc., Walkersville, Md.). Generation of mouse antiserum to recombinant protein and preparation of mycobacterial sonicates were carried out as previously described (3).
Quantitation of cytokines by ELISA.
IFN-γ enzyme-linked immunosorbent assays (ELISAs) were carried out in duplicate wells to measure the cytokine levels in cell culture supernatants 96 h after antigen addition by using commercially available antibody pairs (Endogen, Woburn, Mass.). Measurement of IFN-γ in M. tuberculosis sonicate- and PHA-stimulated PBMC cultures were carried out by using supernatants harvested after 72 h of stimulation. Quantitative IL-4 and IL-10 measurements were performed similarly with 48 h supernatants from PBMC cultures. The lower limit of detection for all of the cytokines as determined by using the kits described above was 15 pg/ml.
Intracellular cytokine detection.
Antigenic stimulation of 0.5 ml of whole blood was carried out as described elsewhere (26) with 50 μg each of M. tuberculosis sonicate or recombinant Apa protein. Intracellular IFN-γ was detected by using an antibody cocktail made up of anti-CD3-fluorescein isothiocyanate (clone UCHT1; BD Pharmingen, San Diego, Calif.), anti-CD4/CD8-biotin (clones 3B5 and S3.5, respectively; Caltag Laboratories, Burlingame, Calif.), and anti-IFN-γ-phycoerythrin (clone B27; Caltag Laboratories), followed by Streptavidin-Cy-Chrome (Pharmingen). Data was acquired on a FACScan flow cytometer (Becton Dickinson, San Jose, Calif.). Small lymphocytes were collected after exclusion of the dead cells and debris by gating on the forward versus side scatter with fluorescence triggering in the FL1 channel to gate on the CD3+ T lymphocytes. For each analysis, a total of 50,000 CD4+ or CD8+ T-cell subsets were acquired, and data were analyzed by using Winlist software (Verity Software House, Inc., Topsham, Maine). Positive staining was affirmed by using isotype-matched controls and by comparing the dot plots of unstimulated and antigen-stimulated cultures.
Immunization and challenge of guinea pigs.
The experiments used 300 to 350 g outbred male guinea pigs from the colony of the National Tuberculosis Institute. Animals were immunized intradermally in groups of four with one of the following: (i) control DNA, 200 μg, given three times, 4 weeks apart (at weeks 0, 4, and 8), followed by MVA wild type, 107 PFU, given 4 weeks after the third DNA (at week 12); (ii) APADNA, 200 μg, given three times, 4 weeks apart (at weeks 0, 4, and 8), followed by MVA wild type, 107 PFU, given 4 weeks after the third DNA (at week 12); (iii) APADNA, 200 μg, given three times, 4 weeks apart (at weeks 0, 4, and 8), followed by APAMVA, 107 PFU, given 4 weeks after the third DNA (at 12 weeks); and (iv) BCG Guindy, 106 CFU, given one time only (at week 0). Animals were challenged intramuscularly in the thigh muscle at 4 weeks after the last immunization with 2 × 105 viable M. tuberculosis strain NTI64719. This challenge dose was sufficient to cause measurable clinical illness in all control animals within a relatively short time frame of 6 weeks. Animals were allowed free access to standard laboratory food and water, observed for illness, and weighed weekly for 6 weeks, after which the spleens were removed aseptically, inspected immediately for pathology, and cultured for CFU of M. tuberculosis as described previously (29).
Statistical analysis.
The results of lymphoproliferative assays are represented as mean SI values, each of which was performed in triplicate. The results of ELISAs are expressed as picograms per milliliter (means of duplicate wells). Age, weight, and sex trends for SI and IFN-γ responses were analyzed by using logistical regression and were found not to significantly influence the outcome. Comparisons between the PPD-positive healthy contact and patient groups were made by using the Student t test, followed by the nonparametric Mann-Whitney U test, as well as the Fisher exact test wherever applicable. Comparisons between groups of guinea pigs were performed by using Student t tests on log-transformed data. All statistical comparisons were carried out by using the GraphPad Prism version 3.00 for Windows (GraphPad Software, San Diego, Calif.). P values of <0.05 were considered statistically significant.
RESULTS
Expression and purification of M. tuberculosis Apa protein.
We screened proteins expressed by several recombinants from a lambda ZAP II::M. tuberculosis library (2) for their ability to stimulate T-cell lymphoproliferative responses in PPD-positive individuals. Sequence analysis of one such positive clone, MTB65, revealed the gene Rv1860 coding for the 50- to 55-kDa Apa protein of M. tuberculosis (10, 11). The insert from MTB65 was subcloned into the pRSETC vector, in which the protein was expressed as a N-terminal fusion to six histidine residues and recombinant Apa protein, high-pressure liquid chromatography-purified by affinity binding to and elution from Ni2+-NTA agarose beads (Fig. 1, lane 2) was used in T-lymphoproliferation assays.
FIG. 1.
Purification of recombinant Apa protein. The insert from ARRMTB65 was cloned into pRSETC as described in Materials and Methods. Lanes: 1, IPTG-induced lysate from E. coli BL21(DE3) carrying the pRSETC-65 recombinant after induction with IPTG; 2, Apa protein from lane 1 FPLC-purified by using Ni2+-NTA agarose.
T-cell lymphoproliferative response to the 50- to 55-kDa antigen of M. tuberculosis in PPD-positive healthy contacts and TB patients.
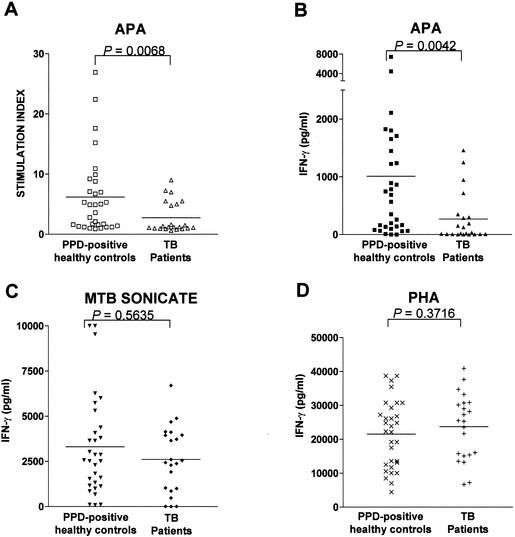
Although the Apa protein has already been established to be a strong stimulator of antibody production in TB patients, information on its ability to stimulate human T-cell responses is lacking. Our studies revealed an SI value of >2 in the PBMC of 19 of the 31 healthy PPD-positive volunteers upon stimulation with Apa. Seven of the twenty-two pulmonary TB patients also had positive SI responses. Although the proportion of responding individuals between these two groups was not significantly different (P = 0.0514 [Fisher exact test]), the difference in mean SI values showed a high level of significance (mean levels of 6.17 ± 1.18 among PPD-positive healthy contacts and 2.75 ± 0.56 among patients, Fig. 2A [P = 0.0068, Mann-Whitney test]). Since all healthy controls included in the study were PPD positive, they all displayed positive lymphoproliferative responses to total M. tuberculosis sonicate antigen, as expected (mean SI ± standard error of the mean = 33.45 ± 5.10 [range, 4.5 to 55.4]). Lower M. tuberculosis sonicate-specific SI values were observed in patients (mean SI ± standard error of the mean = 12.38 ± 2.26 [range, 3.2 to 37.7]), in keeping with reduced activation status of T cells previously reported in TB patients (36).
FIG. 2.
Human PBMC respond to recombinant Apa protein by lymphoproliferation and IFN-γ production. (A) The SI values plotted for 30 PPD-positive healthy controls and 22 pulmonary TB patients were derived as a ratio of the counts incorporated by PBMC in the presence of Apa antigen to that observed with unstimulated cells in triplicate cultures. (B, C, and D) IFN-γ production by PBMC from the same group of volunteers shown in panel A in response to the Apa protein, total M. tuberculosis sonicate, and the mitogen PHA, respectively. The horizontal lines specify the mean SI or IFN-γ values observed in each group. P values for significance are shown for each data set. P values of <0.05 are significant. Evidence of age-sex interaction with SI or IFN-γ response to the stimulants within the study groups, treated separately or in concert, did not reach statistical significance (P > 0.05).
Cytokine profile of Apa-stimulated human PBMC.
We evaluated the production of the Th1 and Th2 cytokines IFN-γ and IL-4, respectively, by the responding lymphocyte population by using a capture ELISA. The mean levels of IFN-γ induced by Apa in the PPD-positive healthy control and TB patient groups were 1,009 ± 273 (range, 15 to 7,454) pg/ml and 268 ± 91 (range, <15 to 1,458) pg/ml, respectively, and the difference between the two groups was significant (Fig. 2B [P = 0.0042, Mann-Whitney test]). The proportion of PPD-positive healthy volunteers whose PBMC secreted >100 pg of IFN-γ/ml when stimulated with the Apa protein, namely, 77% (24 of 31, Fig. 2B) was significantly higher compared to the 46% among TB patients (10 of 22, P = 0.0223 [Fisher exact test]). On the other hand, the difference between the mean levels of IFN-γ produced by the members of the two groups in response to total M. tuberculosis sonicate was not significant (P = 0.5635 [Mann-Whitney test]), although the mean value among TB patients was lower than that produced by healthy PPD-positive volunteers (3,305 ± 497 and 2,608 ± 390 pg/ml for the PPD-positive healthy and patient groups, respectively; Fig. 2C). These observations are in keeping with previously reported reduced PPD-specific IFN-γ production in TB patients in comparison with normal controls (16). Production of IFN-γ in response to the mitogen PHA was, however, found to be comparable between the two groups (21,520 ± 1,779 and 23,720 ± 2,056 pg/ml, respectively, for the healthy PPD-positive and TB patient groups; Fig. 2D) and fell in the range normally observed in healthy immunocompetent individuals (17). IL-4 production by the PBMC samples stimulated with M. tuberculosis sonicate were all below the limit of detection of 15 pg/ml. A similar absence of IL-4 production in response to total mycobacterial proteins has also been reported earlier (16). Low and comparable levels of IL-10 production (30 to 55 pg/ml) were observed in the two groups in response to M. tuberculosis sonicate. The Apa protein also did not induce detectable levels of the Th2 cytokines IL-4 and IL-10 (<15 pg of both cytokines/ml) in either group, thus highlighting the potential of this protein in eliciting a predominantly Th1 type of T-cell response.
Phenotype analysis of responding T cells.
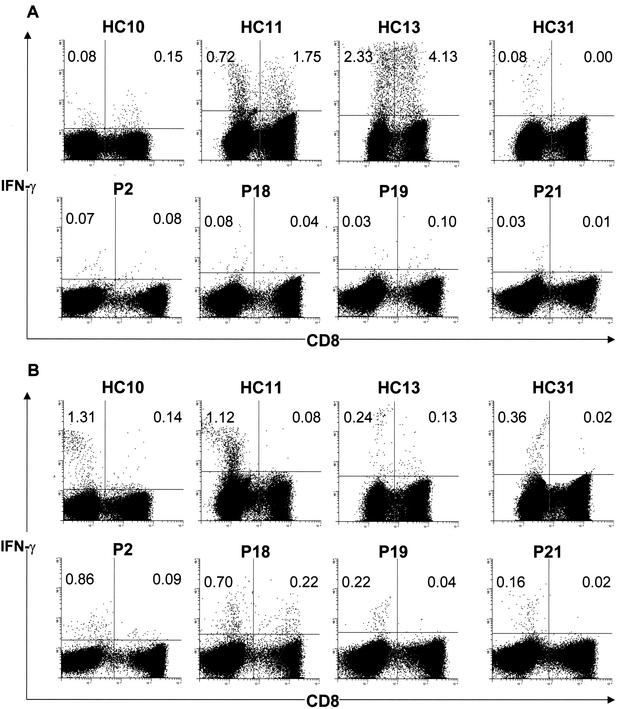
In order to determine the phenotype of lymphocytes responding to the Apa protein, we resorted to flow cytometry with antigen-stimulated whole-blood samples stained simultaneously for surface CD3 and CD4/CD8, along with intracellular IFN-γ. The Apa protein induced production of IFN-γ from CD4+ T cells, as well as CD8+ T cells, whose frequencies in nine PPD-positive healthy controls and eight TB patients for whom fluorescence-activated cell sorting (FACS) analysis could be performed are summarized in Table 1. Apa-specific CD4+ T cells contributed to IFN-γ production in all PPD-positive healthy controls tested whose PBMC had secreted significant levels of IFN-γ by ELISA, with as many as seven also displaying measurable frequencies of CD8+ T cells. Volunteers HC11, HC12, and HC13, whose PBMC had secreted some of the highest levels of IFN-γ, also displayed the largest percentages of Apa-specific CD4+ T cells (0.72, 0.79, and 2.33%, respectively; Fig. 3A and Table 1). HC13 also possessed the highest frequencies (4.33%) of IFN-γ-producing CD8+ T cells. In HC31 alone, the T-cell response to Apa comprised uniquely CD4+ T cells (0.08%, Fig. 3A). The frequencies of responding T cells measured by FACS analysis of 6-h-stimulated whole-blood samples were in reasonable accordance with the levels of secreted IFN-γ measured in PBMC cultures after a 4-day incubation with antigen. In contrast to the above scenario observed among the healthy PPD-positive donors, TB patients displayed a striking paucity of Apa-induced IFN-γ production from both subsets of T cells, as would be predicted from the ELISA values for this cytokine. Among the patients who were tested by flow cytometry, only P2, P7, and P9, who were incidentally also among the best responders in secreted IFN-γ measurements, had moderate proportions of responding T cells, with 0.07, 0.06, and 0.17% of CD4+ T cells and 0.08, 0.12, and 0.08% of CD8+ T cells (Table 1). In keeping with the low levels detected in PBMC cultures, the Apa protein stimulated synthesis of IFN-γ in low proportions of either subset of T cells in all of the remaining patients tested (Table 1). Again, both CD4+ T cells and CD8+ T cells produced IFN-γ in response to Apa in most patients, excluding P21, whose Apa-specific T cells were predominantly of the CD4+ subtype (Fig. 3A). Stimulation of whole blood from both PPD-positive healthy controls and TB patients with M. tuberculosis sonicates, however, resulted in the predominant staining of CD4+ T cells for IFN-γ by flow cytometry (Fig. 3B and Table 1) in both groups. Thus, the Apa protein joins the select band of M. tuberculosis proteins known to stimulate human T cells, especially the CD8+ T-cell subset.
TABLE 1.
T-cell responses to total mycobacterial sonicate and the Apa protein of M. tuberculosis among PPD-positive human volunteersa
| PPD-positive healthy controls | TB patients
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serial no. | Apa protein
|
MTB sonicate
|
Serial no. | APA protein
|
MTB sonicate
|
|||||||||
| SI (IFN-γ) | % T-cell subset
|
SI (IFN-γ) | % T-cell subset
|
|
SI (IFN-γ) | % T-cell subset
|
SI (IFN-γ) | % T-cell subset
|
||||||
| CD4+ | CD8+ | CD4+ | CD8+ | CD4+ | CD8+ | CD4+ | CD8+ | |||||||
| HC1 | 7.1 (1,825) | 0.40 | 0.30 | 11.3 (5,309) | 1.34 | 0.14 | P2 | 7.0 (947) | 0.07 | 0.08 | 35.0 (4,889) | 0.86 | 0.09 | |
| HC2 | 3.6 (162) | 0.02 | 0.00 | 15.6 (1,127) | 0.08 | 0.01 | P7 | 5.5 (717) | 0.06 | 0.12 | 27.0 (3,995) | 0.89 | 0.06 | |
| HC10 | 6.7 (746) | 0.08 | 0.15 | 30.7 (6,267) | 1.31 | 0.14 | P8 | 1.0 (6) | 0.0 | 0.0 | 37.7 (2,374) | 0.38 | 0.02 | |
| HC11 | 8.8 (4,472) | 0.72 | 1.75 | 10.6 (4,374) | 1.12 | 0.08 | P9 | 5.5 (1,250) | 0.17 | 0.08 | 19.2 (6,700) | 1.35 | 0.09 | |
| HC12 | 15.2 (1,708) | 0.79 | 1.50 | 10.7 (1,541) | 0.20 | 0.18 | P17 | 4.8 (192) | 0.01 | 0.03 | 17.2 (3,724) | 0.62 | 0.06 | |
| HC13 | 26.9 (7,454) | 2.33 | 4.13 | 17.7 (2,510) | 0.24 | 0.13 | P18 | 3.6 (350) | 0.08 | 0.04 | 19.0 (2,606) | 0.70 | 0.22 | |
| HC21 | 10.9 (1,802) | 0.32 | 0.28 | 20.8 (3,028) | 0.62 | 0.06 | P19 | 5.0 (287) | 0.03 | 0.10 | 12.6 (1,920) | 0.22 | 0.04 | |
| HC22 | 5.3 (1,448) | 0.16 | 0.33 | 17.4 (3,650) | 0.37 | 0.02 | P21 | 1.0 (127) | 0.03 | 0.01 | 9.4 (1,002) | 0.16 | 0.02 | |
| HC31 | 7.0 (1,237) | 0.08 | 0.00 | 36.7 (4,074) | 0.36 | 0.02 | ||||||||
The SI values shown were obtained by dividing the mean cpm of triplicate cultures stimulated with antigen by that of unstimulated control wells. The IFN-γ levels shown in parentheses are in picograms/milliliter and were obtained by subtracting IFN-γ levels in culture supernatants of unstimulated cells from those of cells stimulated with antigen. % T-cell subset values are shown as percentages of each T-cell subset (either CD4+ or CD8+) after deducting the background frequencies for each subset obtained in the absence of antigen. The percentages of the CD4+ cells shown were obtained by independent staining with antibodies to human CD4.
FIG. 3.
Flow cytometric detection of intracellular IFN-γ in Apa-stimulated (A) and M. tuberculosis sonicate-stimulated (B) T cells. Whole blood was stimulated with recombinant Apa protein of M. tuberculosis or M. tuberculosis sonicate for 6 h, fixed, and stained for CD3 (fluorescein isothiocyanate), CD8 (phycoerythrin), and IFN-γ (Cychrome). Cells were gated sequentially on lymphocytes, followed by CD3, and analyzed for expression of CD8 and IFN-γ. The frequencies in the upper quadrants are IFN-γ-producing cells as percentages of the total CD4+ (left quadrants) or CD8+ (right quadrants) T cells.
Apa protects guinea pigs against M. tuberculosis challenge.
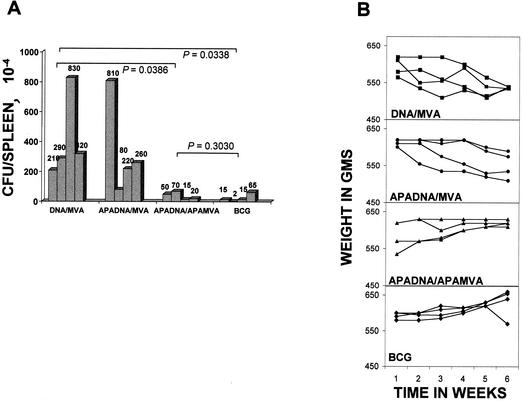
In order to assess the role of Apa protein-specific immune responses in protection against TB, we constructed a DNA vaccine expressing the Apa protein under the control of the cytomegalovirus promoter in the vector pUMVC3 (APADNA). Challenge of guinea pigs immunized with this construct with virulent M. tuberculosis resulted in a burden of viable challenge bacilli in the spleens similar to control animals immunized with the plasmid vector alone, with mean values of 330 × 104 and 342 × 104 CFU/spleen, respectively. In contrast, animals given a boost with recombinant APAMVA after the above APADNA vaccine showed an impressive level of protection compared to control group i (mean CFU values of 38.75 versus 412.5 × 104 per spleen in immunized and control groups, respectively; P = 0.0386) that was comparable to that afforded by BCG vaccination (24.25 × 104 CFU per spleen; P = 0.3030; Fig. 4A). APADNA vaccinated animals boosted with wild-type poxvirus MVA (group ii) did not show any degree of protection (Fig. 4A). The protected animals (group iii) were also spared the weight loss that went hand in hand with the lack of protection in the control group and that immunized with APADNA, followed by wild-type MVA, in a manner similar to BCG-vaccinated animals (Fig. 4B). Thus, although a possible beneficial effect of the APADNA priming event on the effectiveness of the subsequent poxvirus boost cannot be ruled out in the absence of data from animals administered control DNA, followed by APAMVA, the Apa protein of M. tuberculosis delivered as a live immunizing preparation did appear to be as effective as the whole M. bovis BCG in conferring immunity to M. tuberculosis challenge in the highly susceptible guinea pig model.
FIG. 4.
Animals immunized with Apa-expressing DNA, followed by a poxvirus boost, are protected against challenge with M. tuberculosis. (A) Animals immunized with APADNA/APAMVA have significantly fewer M. tuberculosis bacilli in the lungs than control animals. Challenged animals were sacrificed at the end of the 6-week observation period, and the CFU of M. tuberculosis in the spleen were enumerated. The data are given as the CFU per spleen for each animal in the groups. P values for significance between the mean CFU values for each group are shown. P values of <0.05 are significant. The lower limit of detection was 50 bacilli per organ (one CFU on a plate seeded with 100 μl of an undiluted 1% sample [5 ml] of a spleen). (B) Weekly weights of animals for 6 weeks after challenge with M. tuberculosis by the intramuscular route in the immunization groups described. Weights are plotted against time in weeks for each animal. Symbols: ▪, DNA/MVA; •, DNAAPA/MVA; ▴, DNAAPA/MVAAPA; ⧫, BCG.
DISCUSSION
A characteristic feature of M. tuberculosis is the presence of multiple families of closely related proteins, such as ESAT-6, Ag85A, and CFP10, many of which have in fact been shown to stimulate human T cells (19, 35, 37). A recent report has suggested that these proteins perhaps perform vital functions, and the presence of multiple copies of their genes carrying alterations in those segments that encode strong T-cell epitopes may effectively aid the organism in evading the host immune system (21). We report here that the 50- to 55-kDa Apa protein (11), one of the rare glycosylated M. tuberculosis proteins which also appears to represent a member of one such family, is capable of stimulating human T cells with the ability to secrete IFN-γ. The M. bovis BCG homologue of the Apa protein, a member of the 45- to 47-kDa antigen complex, was originally identified in culture filtrate as an immunodominant target for antibody responses after immunization of guinea pigs with live but not dead BCG (33). Subsequently, humoral responses to the M. tuberculosis homologue of the Apa protein were found preferentially in TB patients but not in PPD-positive healthy individuals (10). The potential of the Apa protein to function as a target of T cells has thus far been investigated only in guinea pigs where immunization with live BCG revealed a predominantly CD4+ T-cell response to the Apa protein (32), which was in fact totally lost upon deglycosylation. Our studies carried out with bacterially expressed nonglycosylated recombinant M. tuberculosis Apa protein has pointed to the existence of human T-cell epitopes on the Apa polypeptide backbone.
The ability to produce IFN-γ, the hallmark of a TH1 type of T-cell response, is widely accepted as a strong correlate of protective immunity to TB (9), and M. tuberculosis has been shown to downregulate the production of this cytokine in TB patients (16, 41). Accordingly, although Apa-specific T cells were also primed in TB patients, the proliferation and IFN-γ production by Apa-specific T cells was depressed in TB patients compared to healthy PPD-positive individuals. However, we detected no more than 30 to 55 pg of IL-10/ml in both TB patients and PPD-positive healthy controls in response to stimulation by Apa. Although there are conflicting reports about the ability of IL-10 to enhance the antibacterial activity of macrophages (14), IL-10 has been consistently reported to cause depression of Th1 T-cell responses (15) and has been thought to mediate the T-cell anergy observed in full-blown TB patients. The lack of IL-10 production by PBMC in response to Apa, in contrast to the IL-10-producing tendency of the 30-kDa protein of M. tuberculosis reported earlier (42), bodes well for the success of this protein as a candidate TB vaccine.
Many of the T-cell-stimulatory antigens of M. tuberculosis have turned out to be culture filtrate proteins. A striking feature of protective immunity to TB is the unique efficacy of live but not killed bacilli to prime the immune responses that mediate protection. This requirement for live organisms has been consistently attributed to the presence of substantial quantities of secreted proteins elaborated by live bacteria but missing in killed preparations (4, 34). It is significant that the Apa protein, identified in our study in a completely random search also represents one of the secreted proteins of M. tuberculosis. Of special note is the vastly improved efficacy of the live recombinant APAMVA poxvirus boost after a priming dose of APADNA over the APADNA vaccine alone in protecting guinea pigs from a challenge dose of M. tuberculosis, suggesting recruitment of common antigen presentation pathways by both live mycobacteria and live poxvirus, that are perhaps out of bounds for proteins generated in situ by using a naked DNA vector. The inherent advantage of a live vector expressing the Apa protein in conferring protection can, however, be gauged only by challenging guinea pigs immunized with APAMVA alone.
The presence in PPD-positive healthy individuals of CD8+ T cells preferentially recognizing live M. tuberculosis-derived antigens has been demonstrated (6). Many of the T-cell-stimulatory proteins of M. tuberculosis have in fact also been shown to stimulate human CD8+ T cells (19, 21, 37). The possible mechanism(s) by which an intracellular pathogen such as M. tuberculosis might prime T cells of the CD8+ phenotype has been an exciting area of TB immunology. The continued presence of the tubercle bacilli within the endocytic vacuole after entry into the macrophage would be expected to prevent access to the major histocompatibility complex (MHC) class I presentation pathway required to prime CD8+ T cells. The elegant experiments showing that soluble exogenous antigens accompanying live mycobacteria but not live E. coli can be presented on MHC class I molecules (23) and stimulate CD8+ T cells revealed an innate ability of live mycobacteria to gain access to the host cytoplasm. One might reasonably conjecture from these observations that secreted proteins of M. tuberculosis which are available in free form within the vacuolar compartment would be the most likely candidates to leak out of the vacuole and access the class I MHC presentation pathway. Secreted proteins may also be deployed by the organism to modulate host macrophage functions, inadvertently rendering them vulnerable to recognition by CD8+ T cells. The secreted nature of the Apa protein may therefore explain its observed strong potential for priming CD8+ T cells in M. tuberculosis-infected individuals. The production of IFN-γ by CD8+ T cells has special import in M. tuberculosis infection, in which downregulation of the MHC class II-mediated antigen presentation (23) and sequestration of intracellular bacilli from the CD4+ T cells (30) has been demonstrated.
Preferential T-cell responses among healthy PPD-positive individuals but not TB patients to antigens of M. tuberculosis have been previously reported. Of note are the reported higher levels of CD8+ T cells recognizing Ag85A and Ag85B in healthy PPD-positive humans (36) and the depressed production of IL-12 p40 subunit, as well as IFN-γ, in response to the 30- or 32-kDa antigens of M. tuberculosis in TB patients (38). In both of the above studies, these findings seemed to implicate these immune parameters in protective immunity. Our results also showed a similar preferential Th1 type of T-cell response to the Apa protein in PPD-positive healthy controls compared to patients. The subsequent demonstration of the ability of the Apa protein to protect against M. tuberculosis challenge in the highly susceptible guinea pig animal model suggests that T-cell activity to the Apa protein may be an important component of the protective immune response to mycobacterial infection. This approach of initial screening for T-cell response in healthy PPD-positive humans is bound to lead to the identification of many more such T-cell antigens of M. tuberculosis that would comprise rational components of an effective TB vaccine.
Acknowledgments
This work was funded by a grant BT/Med/Tuberculosis/02/99 from the Department of Biotechnology of the Government of India.
We thank the FACS facility of the Indian Institute of Science for help with acquisition of flow cytometric data. We thank all of the volunteers who donated blood for the study, as well as the staff of the National Tuberculosis Institute and the SDS tuberculosis hospital, who provided technical assistance for the study. R.R.A. and P.K. were recipients of the Senior Research Fellowship of the Council for Scientific and Industrial Research.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Altare, F., E. Jouanguy, S. Lamhamedi, R. Doffinger, A. Fischer, and J. L. Casanova. 1998. Mendelian susceptibility to mycobacterial infection in man. Curr. Opin. Immunol. 10:413-417. [DOI] [PubMed] [Google Scholar]
- 2.Amara, R. R., and V. Satchidanandam. 1996. Analysis of a genomic DNA expression library of Mycobacterium tuberculosis using tuberculosis patient sera: evidence for modulation of host immune response. Infect. Immun. 64:3765-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amara, R. R., S. Shanti, and V. Satchidanandam. 1998. Characterization of novel immunodominant antigens of Mycobacterium tuberculosis. Microbiol. 144(Pt. 5):1197-1203. [DOI] [PubMed] [Google Scholar]
- 4.Andersen, P., D. Askgaard, L. Ljungqvist, M. W. Bentzon, and I. Heron.1991. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect. Immun. 59:1558-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloom, B. R., and B. Bennett. 1966. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science 153:80-82. [DOI] [PubMed] [Google Scholar]
- 6.Canaday, D. H., C. Ziebold, E. H. Noss, K. A. Chervenak, C. V. Harding, and W. H. Boom. 1999. Activation of human CD8+ alpha beta TCR+ cells by Mycobacterium tuberculosis via an alternate class I MHC antigen-processing pathway. J. Immunol. 162:372-379. [PubMed] [Google Scholar]
- 7.Carroll, M. W., and B. Moss. 1995. Escherichia coli β-glucuronidase (GUS) as a marker for recombinant vaccinia viruses. BioTechniques 19:352-356. [PubMed] [Google Scholar]
- 8.David, J. R. 1966. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc. Natl. Acad. Sci. USA 56:72-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellner, J. J., C. S. Hirsch, and C. C. Whalen. 2000. Correlates of protective immunity to Mycobacterium tuberculosis in humans. Clin. Infect. Dis. 30(Suppl. 3):S279-S282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espitia, C., R. Espinosa, R. Saavedra, R. Mancilla, F. Romain, A. Laqueyrerie, and C. Moreno. 1995. Antigenic and structural similarities between Mycobacterium tuberculosis 50- to 55-kilodalton and Mycobacterium bovis BCG 45- to 47-kilodalton antigens. Infect. Immun. 63:580-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espitia, C., and R. Mancilla. 1989. Identification, isolation and partial characterization of Mycobacterium tuberculosis glycoprotein antigens. Clin. Exp. Immunol. 77:378-383. [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn, J. L., M. M. Goldstein, K. J. Triebold, and B. R. Bloom. 1993. Major histocompatibility complex class I-restricted T cells are necessary for protection against Mycobacterium tuberculosis in mice. Infect. Agents Dis. 2:259-262. [PubMed] [Google Scholar]
- 14.Fortsch, D., M. Rollinghoff, and S. Stenger. 2000. IL-10 converts human dendritic cells into macrophage-like cells with increased antibacterial activity against virulent Mycobacterium tuberculosis. J. Immunol. 165:978-987. [DOI] [PubMed] [Google Scholar]
- 15.Gong, J. H., M. Zhang, R. L. Modlin, P. S. Linsley, D. Iyer, Y. Lin, and P. F. Barnes. 1996. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infect. Immun. 64:913-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch, C. S., Z. Toossi, C. Othieno, J. L. Johnson, S. K. Schwander, S. Robertson, R. S. Wallis, K. Edmonds, A. Okwera, R. Mugerwa, P. Peters, and J. J. Ellner. 1999. Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J. Infect. Dis. 180:2069-2073. [DOI] [PubMed] [Google Scholar]
- 17.Katial, R. K., D. Sachanandani, C. Pinney, and M. M. Lieberman. 1998. Cytokine production in cell culture by peripheral blood mononuclear cells from immunocompetent hosts. Clin. Diagn. Lab. Immunol. 5:78-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufmann, S. H., S. Chiplunkar, I. Flesch, and G. De Libero. 1986. Possible role of helper and cytolytic T cells in mycobacterial infections. Lepr. Rev. 57(Suppl. 2):101-111. [DOI] [PubMed] [Google Scholar]
- 19.Lalvani, A., R. Brookes, R. J. Wilkinson, A. S. Malin, A. A. Pathan, P. Andersen, H. Dockrell, G. Pasvol, and A. V. Hill. 1998. Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 95:270-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leveton, C., S. Barnass, B. Champion, S. Lucas, B. De Souza, M. Nicol, D. Banerjee, and G. Rook. 1989. T-cell-mediated protection of mice against virulent Mycobacterium tuberculosis. Infect. Immun. 57:390-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewinsohn, D. A., R. A. Lines, and D. M. Lewinsohn. 2002. Human dendritic cells presenting adenovirally expressed antigen elicit Mycobacterium tuberculosis-specific CD8+ T cells. Am. J. Respir. Crit. Care Med. 166:843-848. [DOI] [PubMed] [Google Scholar]
- 22.Mackaness, G. B., and R. V. Blanden. 1967. Cellular immunity. Prog. Allergy 11:89-140. [PubMed] [Google Scholar]
- 23.Mazzaccaro, R. J., M. Gedde, E. R. Jensen, H. M. van Santen, H. L. Ploegh, K. L. Rock, and B. R. Bloom. 1996. Major histocompatibility class I presentation of soluble antigen facilitated by Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA 93:11786-11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohagheghpour, N., D. Gammon, L. M. Kawamura, A. van-Vollenhoven, C. J. Benike, and E. G. Engleman. 1998. CTL response to Mycobacterium tuberculosis: identification of an immunogenic epitope in the 19-kDa lipoprotein. J. Immunol. 161:2400-2406. [PubMed] [Google Scholar]
- 25.Muller, I., S. P. Cobbold, H. Waldmann, and S. H. Kaufmann. 1987. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect. Immun. 55:2037-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nomura, L. E., J. M. Walker, and H. T. Maecker. 2000. Optimization of whole blood antigen-specific cytokine assays for CD4+ T cells. Cytometry 40:60-68. [DOI] [PubMed] [Google Scholar]
- 27.Orme, I. M. 1988. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J. Immunol. 140:3589-3593. [PubMed] [Google Scholar]
- 28.Orme, I. M. 1987. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J. Immunol. 138:293-298. [PubMed] [Google Scholar]
- 29.Pal, P. G., and M. A. Horwitz. 1992. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect. Immun. 60:4781-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pancholi, P., A. Mirza, N. Bhardwaj, and R. M. Steinman. 1993. Sequestration from immune CD4+ T cells of mycobacteria growing in human macrophages. Science 260:984-986. [DOI] [PubMed] [Google Scholar]
- 31.Romain, F., J. Augier, P. Pescher, and G. Marchal. 1993. Isolation of a proline-rich mycobacterial protein eliciting delayed-type hypersensitivity reactions only in guinea pigs immunized with living mycobacteria. Proc. Natl. Acad. Sci. USA 90:5322-5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romain, F., C. Horn, P. Pescher, A. Namane, M. Riviere, G. Puzo, O. Barzu, and G. Marchal. 1999. Deglycosylation of the 45/47-kilodalton antigen complex of Mycobacterium tuberculosis decreases its capacity to elicit in vivo or in vitro cellular immune responses. Infect. Immun. 67:5567-5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romain, F. E., A. Laqueyrerie, P. Militzer, P. Pescher, P. Chavarot, M. Lagranderie, G. Auregan, M. Gheorghiu, and G. Marchal. 1993. Identification of a Mycobacterium bovis BCG 45/47-kilodalton antigen complex, an immunodominant target for antibody response after immunization with living bacteria. Infect. Immun. 61:742-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rook, G. A., J. Steele, S. Barnass, J. Mace, and J. L. Stanford. 1986. Responsiveness to live Mycobacterium tuberculosis, and common antigens, of sonicate-stimulated T-cell lines from normal donors. Clin. Exp. Immunol. 63:105-110. [PMC free article] [PubMed] [Google Scholar]
- 35.Skjot, R. L. V., I. Brock, S. M. Arend, M. E. Munk, M. Theisen, T. H. M. Ottenhoff, and P. Andersen. 2002. Epitope mapping of the immunodominant antigen TB10.4 and the two homologous proteins TB10.3 and TB12.9, which constitute a subfamily of the ESAT-6 gene family. Infect. Immun. 70:5446-5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, S. M., R. Brookes, M. R. Klein, A. S. Malin, P. T. Lukey, A. S. King, G. S. Ogg, A. V. Hill, and H. M. Dockrell. 2000. Human CD8+ CTL specific for the mycobacterial major secreted antigen 85A. J. Immunol. 165:7088-7095. [DOI] [PubMed] [Google Scholar]
- 37.Smith, S. M., A. S. Malin, T. Pauline, Lukey, S. E. Atkinson, J. Content, K. Huygen, and H. M. Dockrell. 1999. Characterization of human Mycobacterium bovis bacille Calmette-Guerin-reactive CD8+ T cells. Infect. Immun. 67:5223-5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song, C. H., H. J. Kim, J. K. Park, J. H. Lim, U. O. Kim, J. S. Kim, T. H. Paik, K. J. Kim, J. W. Suhr, and E. K. Jo. 2000. Depressed interleukin-12 (IL-12), but not IL-18, production in response to a 30- or 32-kilodalton mycobacterial antigen in patients with active pulmonary tuberculosis. Infect. Immun. 68:4477-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stenger, S., D. A. Hanson, R. Teitelbaum, P. Dewan, K. R. Niazi, C. J. Froelich, T. Ganz, S. Thoma-Uszynski, A. Melian, C. Bogdan, S. A. Porcelli, B. R. Bloom, A. M. Krensky, and R. L. Modlin. 1998. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282:121-125. [DOI] [PubMed] [Google Scholar]
- 40.Stenger, S., R. J. Mazzaccaro, K. Uyemura, S. Cho, P. F. Barnes, J. P. Rosat, A. Sette, M. B. Brenner, S. A. Porcelli, B. R. Bloom, and R. L. Modlin. 1997. Differential effects of cytolytic T-cell subsets on intracellular infection. Science 276:1684-1687. [DOI] [PubMed] [Google Scholar]
- 41.Ting, L. M., A. C. Kim, A. Cattamanchi, and J. D. Ernst. 1999. Mycobacterium tuberculosis inhibits IFN-gamma transcriptional responses without inhibiting activation of STAT1. J. Immunol. 163:3898-3906. [PubMed] [Google Scholar]
- 42.Torres, M., T. Herrera, H. Villareal, E. A. Rich, and E. Sada. 1998. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect. Immun. 66:176-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zugel, U., and S. H. Kaufmann. 1997. Activation of CD8 T cells with specificity for mycobacterial heat shock protein 60 in Mycobacterium bovis bacillus Calmette-Guerin-vaccinated mice. Infect. Immun. 65:3947-3950. [DOI] [PMC free article] [PubMed] [Google Scholar]