Abstract
The absolute and relative abundance of major histocompatibility complex class I-presented viral epitopes is important in the induction and maintenance of antiviral cytotoxic-T-lymphocyte (CTL) responses. We demonstrate that the supra-abundant HLA-A*0201-restricted peptide KLWESPQEI of the measles virus nonstructural C protein induces strong gamma interferon CD8+-T-cell responses in children with acute measles. However, longitudinal analysis indicates that these responses are only short-lived. Thus, some viral epitopes that can be immunodominant during primary infection may fail to establish memory CTL responses.
Measles is a highly contagious respiratory infectious disease with a high mortality rate among children in sub-Saharan Africa, and outbreaks continue to occur in many communities despite extensive vaccination coverage (4). There is an urgent need to develop an alternative measles virus (MV) vaccine that can be given to younger infants. The induction of cell-mediated immunity is imperative for the rapid clearance of infection and for protection from subsequent disease (6, 9). However, paradoxically, the induction of intense immune system activation in measles does occur simultaneously with clinically relevant immune suppression (13), a phenomenon that is not yet clearly understood. Hence, for the understanding of the pathogenesis of measles and for the development of new vaccines, attention is now being focused on the role of cytotoxic-T-cell (CTL) responses during MV infection (4, 5, 11). For this, knowledge about major histocompatibility complex class I-presented viral epitopes, which are targets of these cells, needs to be enlarged (16). Recently, mass spectrometry was used to identify a supra-abundant naturally occurring HLA-A*0201-presented peptide (KLWESPQEI) derived from the MV nonstructural C protein (MV-C84-92), which is expressed on end-stage infected antigen-presenting cells at the unprecedented level of approximately 150,000 copies per cell (15). The MV-C protein is a presumed regulatory protein, encoded by the second of the six consecutive transcript units of the MV RNA genome. Although immunogenic in HLA-A2/Kb transgenic mice, the MV-C84-92 peptide does not elicit CTL memory responses in peripheral blood mononuclear cells (PBMC) from HLA-A*0201-positive adult individuals with a history of measles in childhood (15).
To analyze the importance of this supra-abundant MV-C84-92 epitope as a CTL target in natural infection, heparinized peripheral blood was obtained from Gambian measles patients at the time of rash (acute phase) and on recovery. PBMC were isolated by centrifugation over Ficoll-Hypaque (Nycomed), and cryopreserved buffy coats were used for DNA extraction and molecular HLA typing (2). PBMC from 10 patients with the HLA-A*02 subtype (mean age, 7.2 years) in the acute phase of infection were tested for peptide-induced gamma interferon (IFN-γ) responses in a direct IFN-γ enzyme-linked immunospot (ELISpot) assay (8). Cells from seven of these patients were also tested 41 to 231 days later in a stimulated IFN-γ ELISpot assay, which measures memory responses. Briefly, acute-phase PBMC were pulsed with 10 μM MV-C84-92 or with a control peptide, ESAT-6 (also HLA A2 restricted) derived from Mycobacterium tuberculosis (8) for 1 h before the cells were added to duplicate wells of polyvinylidene difluoride-backed plates (Millipore) that were coated with anti-IFN-γ antibody (Mabtech). PBMC (1 × 104 to 5 × 104/well) were incubated overnight at 37°C and 5% CO2. Plates were washed and developed with chromogenic alkaline phosphatase substrate after a two-step incubation with biotinylated anti-IFN-γ and streptavidin antibodies. Responses were measured as the number of spot-forming cells (SFC) per well and considered significant if a minimum of five SFC were present and the number of SFC was at least twice that in wells containing the control peptide. In stimulated cultures, cells obtained in the recovery phase were grown for 10 days in complete RPMI 1640-10% fetal calf serum (4), after pulsing with 10 μM peptide, in the presence of 10% interleukin 2 (IL-2; Lymphocult T; Biotest, Germany) and 5 ng of recombinanat human IL-7 (R&D Systems)/ml. Subsequently, 10% IL-2 was added at days 3 and 7 and cells were pulsed with 10 μM peptide on the final day before they were added to ELISpot plates overnight.
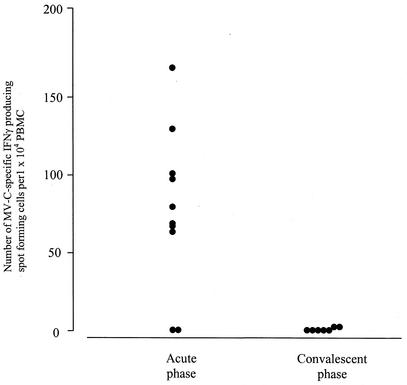
Effector cells producing IFN-γ in response to MV-C84-92, but not control peptide, were detected in 8 of 10 HLA-A*02 patients in the acute phase with effector frequencies that ranged from 1 in 63 to 1 in 300 PBMC (Fig. 1). Two measles patients who were not HLA-A*02 positive gave no MV-C84-92-specific IFN-γ SFCs (data not shown), indicating the HLA restriction of the response. Since only half of the HLA-A*02-positive individuals in West African populations are of the subtype A*0201 (7), it is important to study cross-presentation among allelic subtypes if epitopes are to be used in vaccines. Therefore, two patients with HLA-A*0201-related subtypes, HLA-A*0202 and HLA-A*0205, were included in the study. These patients responded as well as the HLA-A*0201 patients. Subtle differences in the binding and presentation properties of the closely related HLA-A*02 suballeles have been reported (1, 3, 14). It does not appear, however, that the very similar HLA-A*02 subtypes HLA-A*0201, -A*0202, and -A*0205 behave as functionally distinct HLA allotypes for MV-C84-92. The only two patients who failed to show MV-C84-92-specific IFN-γ responses in the acute phase in this study were HLA-A*0201 positive. Both had severe measles; one of them died. Taken together, these data indicate that in general, vigorous anti-MV-C84-92 reactivity is directly detected in PBMC obtained from acutely infected HLA-A*02-positive measles patients, suggesting an important role for this abundant epitope in vivo.
FIG. 1.
ELISpot assay of functional IFN-γ responses to synthetic MV-C84-92 in PBMC from measles patients in the acute phase and at convalescence. Numbers of background SFC in the presence of control peptide were subtracted. Each data point represents a patient, and all the responders were of the HLA-A*0201 subtype except two patients, who were HLA-A*0202 and HLA-A*0205. The data points for the convalescent phase represent six subjects who responded in the acute phase and one who did not respond.
We also used the MV-C84-92 peptide to restimulate PBMC obtained from seven of these patients (including six responders) when they were fully recovered from the disease. We were unable to stimulate IFN-γ production in five of the seven individuals tested (Fig. 1). However, there was a response in two patients, albeit at considerably lower effector frequencies (1 in 1,200 to 1,500) than that found in the acute phase. Two of the patients who showed a direct response in the acute phase were retested in the direct assay at convalescence; neither showed a response. We further tested expanded cells from the recovered patients in a standard 51Cr release assay for cytotoxicity against MV-C84-92-loaded HLA-A*0201-matched Epstein-Barr virus-transformed B cells, and only one patient who had still responded weakly in the ELISpot assay to MV-C84-92 after recovery showed specific cytolysis of 26% at an effector-target ratio of 30:1 (data not shown). This poor ability to stimulate MV-C84-92-specific IFN-γ responses at convalescence supports earlier experiments in which this peptide failed to boost CTL activity in HLA-A*0201 adults with a history of MV infection in childhood (15).
We then analyzed the phenotype of the MV-C84-92-responsive IFN-γ-producing cells from four HLA-A*0201-positive patients in the acute phase. Briefly, 106 PBMC were incubated with 106 HLA-A*02-matched B cells that were pulsed with 5 μM MV-C84-92 or control peptide for 6 h. During the last 4 h, brefeldin A (Sigma, Poole, United Kingdom) was added (10 μg/ml). Cells were then harvested, washed with phosphate-buffered saline-0.3% (wt/wt) bovine serum albumin, and stained for intracellular IFN-γ and cell surface markers, using standard reagents and protocols (Becton-Dickinson Immunocytometry Systems, San Jose, Calif.). Data were acquired by flow cytometry (FACSCalibur) and analyzed with CellQuest software. The frequency of positive cells producing IFN-γ is given as the percent gated CD8+ T cells. The cells of all four patients showed intracellular IFN-γ production in response to MV-C84-92 (Table 1), which was restricted to activated (CD69+) T cells of the CD8+ phenotype. In one patient (M1019), 7.4% of CD8+ T cells specifically produced IFN-γ in response to MV-C84-92 (versus 0.45% in response to control peptide). All four patients gave high ELISpot IFN-γ SFC values; M1019 had 110 SFC/104 PBMC. Little or no IFN-γ production by CD69+ CD4+ T cells was observed in these patients after stimulation with MV-C84-92 (data not shown).
TABLE 1.
Intracellular IFN-γ production after ex vivo stimulation with the MV-C84-92 peptide during the acute phase, determined by flow cytometry
| Patient | Peptide | Frequencya of:
|
|
|---|---|---|---|
| CD8+ CD69+ cells | CD8+ IFN-γ cells | ||
| MTS-3 | MV-C84-92 | 2 | 0.3 |
| Control | 0.07 | 0 | |
| M1020 | MV-C84-92 | 1.6 | 0.7 |
| Control | 0.3 | 0.01 | |
| MTS-2 | MV-C84-92 | 3.4 | 0.5 |
| Control | 0.6 | 0.02 | |
| M1019 | MV-C84-92 | 4.5 | 7.4 |
| Control | 0.1 | 0.45 | |
Quadrant markers for CD69 and IFN-γ were set with the proper isotype control; frequencies of positive cells are given as the percent gated CD8bright cells.
In this study, we identified an interesting MV CTL epitope derived from an MV regulatory protein that was not known as a potential target of MV immunity. Our findings indicate for the first time that expression of a human viral epitope at such extreme levels as MV-C84-92 (approximately 150,000 copies/cell) (15) does not preclude biological activity per se. In contrast, the abundant MV-C84-92 epitope elicits a massive IFN-γ T-cell response in acutely infected individuals, reaching effector cell frequencies as high as 1 in 63 PBMC (implicating that at the peak of the response, 7.5 to 15% of the CD8+ T cells may reconstitute anti-MV-C84-92 specificity). However, MV-C84-92-specific IFN-γ-producing cells were not detected, or were detected only at very low frequencies, in the recovery phase. Whether this indicates that after the disappearance of the initial wave of MV-C84-92-specific CD8+ effector T cells the epitope fails to establish a pool of memory T cells or that MV-C84-92-specific memory T cells are physically present but functionally unresponsive after reencounter with peptide in vitro remains to be established by using HLA-peptide tetramers. Overexpansion and consequent exhaustion of CTL responses have been observed in mice infected with a high load of lymphocytic choriomeningitis virus (10, 12). The MV-C84-92 epitope may serve as an example for studying the relevance of supra-abundance of human viral CTL epitopes for the establishment of CTL memory.
Through its immunodominance, the MV-C84-92 epitope may target the rapid clearance of respiratory epithelial cells or lymphoid cells, including dendritic cells, after being infected themselves or after passive uptake of the abundant epitope from the extracellular milieu. Early and massive destruction of dendritic cells during the induction of specific MV immune responses could, in fact, partly explain the severe immunosuppression observed after MV infection (13). In this respect, it will be interesting to determine whether HLA types other than those belonging to the HLA-A*02 group are able to present the dominant MV-C84-92 epitope. Preliminary data suggest that HLA-A68, another HLA-A*02-like allele which is common in the West African black population, may do so (A. Jaye and C. van Els, unpublished data).
Whether the acute anti-MV-C84-92 response is associated with a good or bad prognosis is not known. However, as it is strongly immunogenic and an abundant, naturally presented peptide, the epitope should prove to be a valuable tool to help understand the immunopathogenesis of measles and to monitor immune responses in vaccinated subjects.
Acknowledgments
A.J. and C.A.H. contributed equally to this work.
We thank our field nurses, Mansour Nyang and Awa Kinteh, and our chief physician, T. Corrah, and his team for clinical assistance.
This work was supported by the Medical Research Council, United Kingdom.
REFERENCES
- 1.Barouch, D., T. Friede, S. Stevanovic, L. Tussey, K. Smith, S. Rowland-Jones, V. Braud, A. McMichael, and H. G. Rammensee. 1995. HLA-A2 subtypes are functionally distinct in peptide binding and presentation. J. Exp. Med. 182:1847-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunce, M., C. M. O'Neill, M. C. Barnardo, P. Krausa, M. J. Browning, P. J. Morris, and K. I. Welsh. 1995. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 and DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP). Tissue Antigens 46:355-367. [DOI] [PubMed] [Google Scholar]
- 3.del Guercio, M. F., J. Sidney, G. Hermanson, C. Perez, H. M. Grey, R. T. Kubo, and A. Sette. 1995. Binding of a peptide antigen to multiple HLA alleles allows definition of an A2-like supertype. J. Immunol. 154:685-693. [PubMed] [Google Scholar]
- 4.Jaye, A., A. F. Magnusen, A. D. Sadiq, T. Corrah, and H. C. Whittle. 1998. Ex vivo analysis of cytotoxic T lymphocytes to measles antigens during infection and after vaccination in Gambian children. J. Clin. Investig. 102:1969-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaye, A., A. F. Magnusen, and H. C. Whittle. 1998. Human leukocyte antigen class I- and class II-restricted cytotoxic T lymphocyte responses to measles antigens in immune adults. J. Infect. Dis. 177:1282-1289. [DOI] [PubMed] [Google Scholar]
- 6.Kernahan, J., J. McQuillin, and A. W. Craft. 1987. Measles in children who have malignant disease. Br. Med. J. (Clin. Res. Ed.) 295:15-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krausa, P., M. Brywka III, D. Savage, K. M. Hui, M. Bunce, J. L. Ngai, D. L. Teo, Y. W. Ong, D. Barouch, C. E. Allsop, et al. 1995. Genetic polymorphism within HLA-A*02: significant allelic variation revealed in different populations. Tissue Antigens 45:223-231. [DOI] [PubMed] [Google Scholar]
- 8.Lalvani, A., R. Brookes, R. J. Wilkinson, A. S. Malin, A. A. Pathan, P. Andersen, H. Dockrell, G. Pasvol, and A. V. Hill. 1998. Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 95:270-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markowitz, L. E., F. W. Chandler, E. O. Roldan, M. J. Saldana, K. C. Roach, S. S. Hutchins, S. R. Preblud, C. D. Mitchell, and G. B. Scott. 1988. Fatal measles pneumonia without rash in a child with AIDS. J. Infect. Dis. 158:480-483. [DOI] [PubMed] [Google Scholar]
- 10.Moskophidis, D., F. Lechner, H. Pircher, and R. M. Zinkernagel. 1993. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362:758-761. [DOI] [PubMed] [Google Scholar]
- 11.Nanan, R., A. Rauch, E. Kampgen, S. Niewiesk, and H. W. Kreth. 2000. A novel sensitive approach for frequency analysis of measles virus-specific memory T-lymphocytes in healthy adults with a childhood history of natural measles. J. Gen. Virol. 81:1313-1319. [DOI] [PubMed] [Google Scholar]
- 12.Oxenius, A., R. M. Zinkernagel, and H. Hengartner. 1998. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity 9:449-457. [DOI] [PubMed] [Google Scholar]
- 13.Schneider-Schaulies, S., S. Niewiesk, J. Schneider-Schaulies, and V. ter Meulen. 2001. Measles virus induced immunosuppression: targets and effector mechanisms. Curr. Mol. Med. 1:163-181. [DOI] [PubMed] [Google Scholar]
- 14.Tanigaki, N., D. Fruci, A. Chersi, G. Falasca, R. Tosi, and R. H. Butler. 1994. HLA-A2-binding peptides cross-react not only within the A2 subgroup but also with other HLA-A-locus allelic products. Hum. Immunol. 39:155-162. [DOI] [PubMed] [Google Scholar]
- 15.van Els, C. A., C. A. Herberts, E. van der Heeft, M. C. Poelen, J. A. van Gaans-van den Brink, A. van der Kooi, P. Hoogerhout, G. Jan ten Hove, H. D. Meiring, and A. P. de Jong. 2000. A single naturally processed measles virus peptide fully dominates the HLA-A*0201-associated peptide display and is mutated at its anchor position in persistent viral strains. Eur. J. Immunol. 30:1172-1181. [DOI] [PubMed] [Google Scholar]
- 16.van Els, C. A., and R. Nanan. 2002. T cell responses in acute measles. Viral Immunol. 15:435-450. [DOI] [PubMed] [Google Scholar]