Abstract
Bovine herpesvirus 1 (BHV-1) is an important pathogen of cattle and infection is usually initiated via the ocular or nasal cavity. After acute infection, the primary site for BHV-1 latency is sensory neurons in the trigeminal ganglia (TG). Reactivation from latency occurs sporadically, resulting in virus shedding and transmission to uninfected cattle. The only abundant viral transcript expressed during latency is the latency-related (LR) RNA. An LR mutant was constructed by inserting three stop codons near the beginning of the LR RNA. This mutant grows to wild-type (wt) efficiency in bovine kidney cells and in the nasal cavity of acutely infected calves. However, shedding of infectious virus from the eye and TG was dramatically reduced in calves infected with the LR mutant. Calves latently infected with the LR mutant do not reactivate after dexamethasone treatment. In contrast, all calves latently infected with wt BHV-1 or the LR rescued mutant reactivate from latency after dexamethasone treatment. In the present study, we compared the frequency of apoptosis in calves infected with the LR mutant to calves infected with wt BHV-1 because LR gene products inhibit apoptosis in transiently transfected cells. A sensitive TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) assay and an antibody that detects cleaved caspase-3 were used to identify apoptotic cells in TG. Both assays demonstrated that calves infected with the LR mutant for 14 days had higher levels of apoptosis in TG compared to calves infected with wt BHV-1 or to mock-infected calves. Viral gene expression, except for the LR gene, is extinguished by 14 days after infection, and thus this time frame is operationally defined as the establishment of latency. Real-time PCR analysis indicated that lower levels of viral DNA were present in the TG of calves infected with the LR mutant throughout acute infection. Taken together, these results suggest that the antiapoptotic properties of the LR gene play an important role during the establishment of latency.
Bovine herpesvirus 1 (BHV-1) is an important viral pathogen of cattle that can cause severe respiratory infection, conjunctivitis, abortions, vulvovaginitis, balanopostitis, and systemic infection in neonate calves (66). BHV-1 infection is also an important component of an upper respiratory tract infection referred to as “shipping fever” or bovine respiratory complex (60). BHV-1 is not the sole infectious agent associated with shipping fever, but it can initiate the disorder by immunosuppressing infected cattle, which leads to secondary bacterial infections (5, 15-17). CD8+-T-cell recognition of infected cells is impaired because the expression of major histocompatibility complex class I and antigen presentation are inhibited (21, 24, 41). CD4+-T-cell function is repressed because BHV-1 can infect CD4+ T cells and induces apoptosis (62). Although modified live vaccines are available, the vaccine strains can cause disease in young calves or abortions in cows, and the vaccine strains establish latency and reactivate from latency (34).
BHV-1 is a member of the Alphaherpesvirinae subfamily and shares certain biological properties with herpes simplex virus type 1 (HSV-1) and HSV-2 (33). BHV-1, like HSV-1 and HSV-2, establishes lifelong latency in ganglionic neurons of the peripheral nervous system after initial replication in the mucosal epithelium. Corticosteroid-induced stress consistently leads to reactivation from latency and virus transmission (50, 56). Although the primary site of BHV-1 latency is sensory neurons, there is evidence that long-term persistence and reactivation occurs within germinal centers of pharyngeal tonsil (65).
The latency-related (LR) RNA is the only abundant viral transcript detected in latently infected neurons (35, 50, 51). A fraction of the LR RNA is polyadenylated and alternatively spliced in the trigeminal ganglia (TG), suggesting this RNA is translated into more than one LR protein (9, 25). LR gene products promote cell survival after induction of apoptosis in transiently transfected cells (7) and inhibit S phase entry (54), and LR protein is associated with cyclin-dependent kinase 2-cyclin complexes (32). We recently constructed an LR mutant virus that contains a 25-bp deletion near the beginning of LR open reading frame 2 (ORF2) and an insertion of three stop codons near the beginning of the LR RNA (27). Calves infected with the LR mutant consistently had diminished clinical symptoms and ocular shedding of the virus compared to calves infected with wild-type (wt) or the LR rescued virus. Conversely, the LR mutant had similar growth properties in productively infected bovine kidney cells (MDBK) and the nasal cavity of calves during acute infection. Calves infected with the LR mutant were not able to reactivate from latency (shed infectious virus from ocular or nasal cavities) after dexamethasone treatment (26). Reduced levels of viral DNA were detected in TG of calves latently infected with the LR mutant, suggesting that the LR gene played a role in establishing and/or maintaining latency.
HSV-1 establishes latency in ganglionic sensory neurons (33, 61) and the latency-associated transcript (LAT) is abundantly transcribed in latently infected neurons (52, 58). Numerous mutants that do not express detectable levels of LAT have been constructed. LAT enhances establishment of latency in mice (53, 59) and rabbits (49) because certain LAT-null mutants contain lower levels of viral DNA in TG relative to wt virus (8, 38). LAT is also important for in vivo reactivation in two different rabbit eye infection models (reviewed in references 33 and 61). The McKrae strain of HSV-1 is frequently shed in the tears of infected rabbits as a result of spontaneous reactivation, and LAT is necessary for efficient spontaneous reactivation (44-48). LAT interferes with apoptosis in transiently transfected cells and in infected mice or rabbits (1, 28, 43). The antiapoptotic functions of LAT correlate with its ability to promote spontaneous reactivation (28). The LR gene of BHV-1 restored high levels of spontaneous reactivation from latency to a LAT-null mutant (42), suggesting that the ability of LAT and the LR gene to inhibit apoptosis is important for the latency reactivation cycle.
In the present study, we examined the frequency of apoptosis in TG of calves infected with the LR mutant or wt BHV-1 during the course of acute infection and the establishment of latency. Apoptosis was measured by using the TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) assay and an antibody that recognizes cleaved caspase-3. At 14 days after infection, but not 6 days after infection, calves infected with the LR mutant contained higher levels of TUNEL-positive (TUNEL+) cells and cleaved caspase-3-positive (caspase-3+) neurons in the TG compared to calves infected with wt BHV-1. Calves infected with wt BHV-1 consistently had higher levels of viral DNA in the TG during acute infection compared to calves infected with the LR mutant. These results indicate that the antiapoptotic properties of LR gene products inhibit neuronal death and consequently promote the establishment of latency.
MATERIALS AND METHODS
Virus and cells.
Bovine kidney cells (MDBK, ATCC CCL-22) were plated at a density of 5 × 105 cells per 100-mm2 plastic dish in Earle modified medium. The medium was supplemented with 5% fetal bovine serum, penicillin (10 U/ml), and streptomycin (100 μg/ml).
The Cooper strain of BHV-1 (wt virus) was obtained from the National Veterinary Services Laboratory, Animal and Plant Health Inspection Services, Ames, Iowa. The LR mutant virus was developed by replacing wt (Cooper strain) LR gene sequences (25 bp) with an oligonucleotide that contains a unique EcoRI restriction site and three stop codons. The three stop codons are designed to prevent protein expression from all three reading frames. A complete description of the LR mutant virus has been published (27). Viral stocks were prepared by infecting MBDK cells with a plaque-purified virus at a multiplicity of infection of 0.001. Virus was titrated on MDBK cells by using 10-fold dilutions and determining the 50% tissue culture infectious dose or PFU.
Animal experiments.
BHV-1-free cross-bred calves (∼250 kg) were randomly assigned and housed in isolation rooms to prevent cross-contamination. Calves were anesthetized with Rompun (ca. 50 mg/50 kg [body weight]; Bayer Corp., Shawnee Mission, Kans.). Calves were then inoculated with 107 PFU of the indicated virus into each nostril and eye, without scarification, for a total of 4 × 107 PFU per animal as described previously (55, 62, 64, 65). Experiments with animals were performed in accordance with the American Association of Laboratory Animal Care guidelines. Calves were housed under strict isolation containment and given antibiotics before and after BHV-1 infection to prevent secondary bacterial infection.
DNA extractions.
BHV-1 DNA from productively infected MDBK cells and total DNA from the TG were extracted as described previously (26, 27, 34, 55).
Real-time PCR.
The BHV-1 gC gene primers used for the present study were as follows: upstream (+445 bp), 5′-ATGTTAGCGCTCTGGAACC-3′, and downstream (+567 bp) 5′-CTTTACGGTCGACGACTCC-3′. The dual-labeled fluorogenic probe that is specific for gC was 5′-FAM-ACGGACGTGCGCGAAAAGA-BHQ-3′ (+526 bp). The numbers for the primers or probes are relative to the start site of transcription. The black hole quencher (BHQ) absorbs fluorescence until FAM is cleaved by Taq (36). Bovine growth hormone gene primers used for the present study were as follows: upstream (847 bp), 5′-GGCGAAGGGAAAATATAGTTGT-3′; downstream (917 bp), 5′-CGTGCTGCTGAGAACGG-3′; and the fluorogenic probe (874 bp), 5′-HEX-AGCTCCGCGCTACGGTACGC-BHQ-3′. FAM fluoresces at 518 nM, whereas HEX fluoresces at 556 nM. Primers and probes were synthesized by Integrated DNA Technologies (Coralview, Iowa). The PCR was performed by using the TaqMan PCR kit (PE Applied Biosystems [catalog no. 4324018]). One μg of total TG DNA was added to a PCR mixture containing a 300 nM concentration of each primer, a 150 nM concentration of the probe, and 25 μl of 2× TaqMan universal PCR master mix for a final volume of 50 μl. The cycle parameters were as follows: activation of the AmpliTaq at 95°C for 10 min, followed by 40 cycles of 15 s at 95°C, 20 s at 55, and 1 min at 60°C, carried out with a 7700 Sequence Detector (PE Applied Biosystems). The level of fluorescence was analyzed at each step of the cycle.
Determination of viral copy numbers in bovine TG.
The number of viral copies was determined by using a standard curve generated by serially diluting the viral genome and performing real-time PCR with the gC primers and probes. Starting with 680 pg of viral DNA that corresponds to a 4.5 × 106 viral copy number (55), the DNA was serially diluted 10-fold. Then, 1 μg of bovine DNA was added to each well of the diluted viral DNA. By using this method we were able to detect 0.45 viral copies in 1 μg of DNA. Sequence detector software (PE Applied Biosystems) was used to determine the number of viral copy numbers in 1 μg of bovine TG by the threshold cycle value (CT). A CT value for each sample was calculated by determining the point at which the fluorescence exceeded a threshold limit (10 times the standard deviation of the baseline), and there is a direct correlation between the CT value and the amount of starting material (23). A duplicate plate was run with the gH primers and probe to ensure that similar amounts of starting material were used.
Detection of apoptosis in fixed tissue.
The TUNEL assay for in situ apoptosis was used to detect apoptotic cells in tissue sections from TG as described previously (62, 65).
Immunohistochemistry to detect cleaved caspase-3.
Tissue sections were deparaffinized and rehydrated in graded ethanol. For antigen unmasking, sections were immersed in 10 mM sodium citrate buffer (pH 6.0) and heated for 10 min in a microwave. The sections were then washed in distilled water and incubated in 1% hydrogen peroxide for 10 min. Incubation with 5% normal goat serum for 1 h at room temperature blocked nonspecific binding of antibody to sections. The antibody directed against cleaved caspase-3 (catalog no. 9661; Cell Signaling, Beverly, Mass.) was diluted 1:50, added to the slide, and incubated at 4°C overnight. The secondary antibody was applied for 30 min at room temperature. The ABC system from Vector Laboratories, Inc. (Burlingame, Calif.), was used for secondary antibody binding. These steps were done according to the manufacturer's recommendations. Some sections were counterstained with hematoxylin-eosin, mounted, and observed under the microscope.
To detect differences between wt and LR mutant virus-induced apoptosis in TG sections, the number of neurons positive for cleaved caspase-3 was counted. Computer images were collected from seven nonoverlapping fields containing positive neurons on the sections for 6 days after infection and 11 for 14 days after infection. The criteria used to choose the fields and number of fields were the same for all sections, including mock-infected tissue. The number of positive neurons was counted and compared to the total number of neurons present in the respective fields.
Statistical analysis.
Analysis of variance and the Student t test were performed by using SAS software (SAS Institute, Inc., Cary, N.C.).
RESULTS
Analysis of bovine TG by TUNEL after infection with BHV-1.
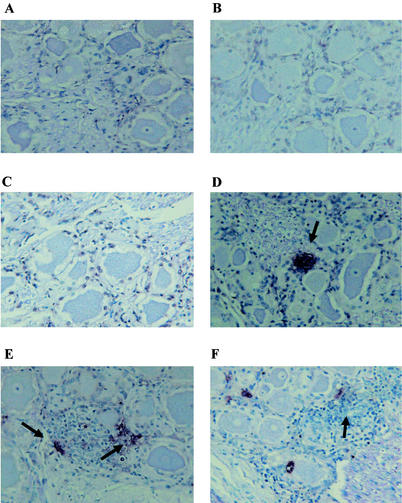
To compare the frequency of apoptosis in calves infected with wt BHV-1 or the LR mutant, TG was extracted at various times after infection, thin sections were prepared, and TUNEL analysis was performed. The LR gene protects cells from apoptosis in transiently transfected cells (7), suggesting that it may promote neuronal survival in infected calves. Previous studies demonstrated that infection with BHV-1 induces apoptosis in productively infected cells (10) and the TG of infected calves (63). Representative data from TUNEL studies of calves infected with wt BHV-1 or the LR mutant is shown in Fig. 1 and 2, respectively. TUNEL+ cells were rarely detected in TG sections from mock-infected calves (Fig. 1A and 2A), at 2 days after infection with wt BHV-1 (Fig. 1B), or at 4 days after infection (Fig. 1C). At 6 days after infection, TUNEL+ cells were occasionally detected in TG sections prepared from calves infected with wt BHV-1 (Fig. 1D). At 10 (Fig. 1E) or 14 (Fig. 1F) days after infection, the frequency of TUNEL+ cells appeared to be lower than at 6 days after infection (summarized in Table 1).
FIG. 1.
TUNEL analysis of calves infected with wt BHV-1. Thin sections were prepared from the TG of calves infected with wt BHV-1. TUNEL assays were performed as described in Materials and Methods. Shown are a section from a mock-infected calf (A) and sections obtained 2 days (B), 4 days (C), 6 days (D), 10 days (E), and 14 days (F) after infection. These sections are representative of the results from many sections. The TG from two calves were examined for each time point. The arrows denote foci of TUNEL+ cells.
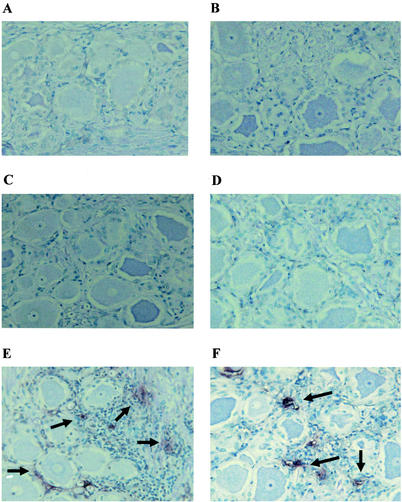
FIG. 2.
TUNEL analysis of calves infected with the LR mutant. Thin sections were prepared from the TG of calves infected with the LR mutant. TUNEL assays were performed as described in Materials and Methods. Shown are a section from a mock-infected calf (A) and sections obtained 2 days (B), 4 days (C), 6 days (D), 10 days (E), and 14 days (F) after infection. These sections are representative of the results from many sections. The TG from two calves were examined for each time point. The arrows denote foci of TUNEL+ cells.
TABLE 1.
Summary of TUNEL results
| Day (dpi)a | TUNEL resultb
|
||||
|---|---|---|---|---|---|
| Mock | LR mutant 1 | LR mutant 2 | wt 1 | wt 2 | |
| 2 | − | − | − | − | − |
| 4 | − | − | − | − | − |
| 6 | − | − | − | ++ | ++ |
| 10 | − | ++++ | ++++ | ++++ | ++++ |
| 14 | − | +++++ | +++ | ++ | ++ |
TG sections from two mock-infected calves (Mock), two calves infected with the LR mutant (LR mutant 1 and LR mutant 2), or two calves infected with wt BHV-1 (wt 1 and wt 2) were tested for each time point. dpi, days postinfection.
-, Sections lacking TUNEL+ cells. The relative numbers of TUNEL+ foci present in each section were scored as follows: ++, 5-8 foci of TUNEL+ cells; +++, 9-12 foci of TUNEL+ cells; ++++, 13-16 foci of TUNEL+ cells; +++++, >16 foci of TUNEL+ cells.
Calves infected with the LR mutant did not contain many TUNEL+ cells at 2 days (Fig. 2B) or 4 days (Fig. 2C) after infection. At 6 days after infection, fewer foci of TUNEL+ cells were detected in TG sections prepared from calves infected with the LR mutant (Fig. 2D) compared to calves infected with wt BHV-1 (Fig. 1D and summarized in Table 1). Calves infected with the LR mutant for 10 or 14 days contained frequent foci of TUNEL+ cells (Fig. 2E and F, respectively). At 14 days after infection, the LR mutant clearly contained more TUNEL+ cells in the TG relative to calves infected with wt BHV-1 (Table 1). In many instances, it was difficult to determine whether the TUNEL+ cells were satellite cells, neurons, or infiltrating lymphocytes. Because of the widespread nature of the foci of TUNEL+ cells at 10 or 14 days after infection, it was also difficult to accurately count the TUNEL+ cells. In summary, these results suggested that calves infected with the LR mutant contained higher levels of TUNEL+ cells in the TG at 10 and 14 days after infection.
Comparison of caspase-3+ neurons in TG of calves infected with the LR mutant versus wt BHV-1.
Although TUNEL+ cells were routinely detected in infected calves, it was not always clear whether these cells were neurons or were comprised of several apoptotic nonneural cells that were in close proximity to each other (Fig. 1 and 2). It is also likely that TUNEL+ neurons are prone to phagocytosis by satellite cells or infiltrating lymphocytes and thus the integrity and cellular morphology of apoptotic neurons is rapidly lost after they become TUNEL+.
To confirm the TUNEL results, we chose to examine cleavage of caspase-3 in sections of TG. Since DNA fragmentation that is detectable by TUNEL is a relatively late event in the apoptosis pathway compared to the cleavage of caspase-3 (6, 14), we felt that use of an antibody that detected cleavage of caspase-3 might yield readily identifiable neurons. Caspase-3 is present in the cell as an inactive procaspase that is activated by proteolytic cleavage and is a key executioner of apoptosis (6). A cleaved caspase-3 antibody (Cell Signaling [catalogue no. 9661]) recognizes the large fragment (17 to 20 kDa) of activated caspase-3 that results from cleavage after Asp175. The antibody was produced in rabbits by using human caspase-3 as the antigen. This antibody reacts with cleaved caspase-3, but not the procaspase, in human and rodent cells, suggesting this antibody could be used to estimate the number of apoptotic neurons in TG after infection.
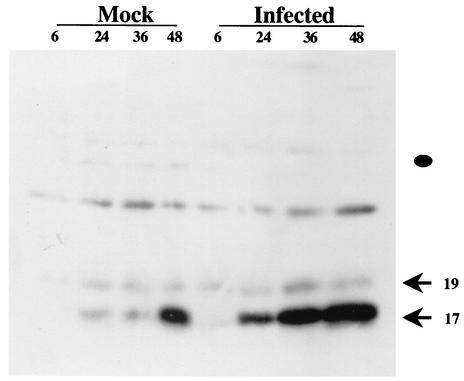
Before the cleaved caspase-3 antibody could be used on bovine TG sections, it was necessary to test whether the antibody specifically recognizes cleaved caspase-3 from bovine cells. MDBK cells (bovine kidney) contained higher levels of cleaved caspase-3 after infection compared to mock-infected cells (Fig. 3). The sizes of the cleaved bands were the same as those detected in mouse cells treated with UV light to induce apoptosis (22). This result was expected because BHV-1 infection induces apoptosis during productive infection and caspase-3 is activated (10). The finding that cleaved caspase-3 levels in mock-infected cells increased as a function of time was likely the result of depleting growth factors in the medium. The cleaved caspase-3 antibody did not appear to recognize procaspase-3 (the position is denoted by closed circle), but it did react with an unknown band migrating at ca. 25 kDa.
FIG. 3.
Western blot analysis of cleaved caspase-3 after productive infection. Bovine kidney cells (MDBK) were infected with wt BHV-1 (4 PFU/cell). At the designated times (hours postinfection), cell lysate was prepared from infected or mock-infected cells. Western blot analysis with 50 μg of protein was performed with the antibody that recognizes cleaved caspase-3. To further ensure that similar levels of total protein were added to each lane, a Western blot was performed with an antibody that recognizes β-actin (data not shown). The arrows denote the two major cleaved products of caspase-3. The closed circle denotes the position of uncleaved caspase-3.
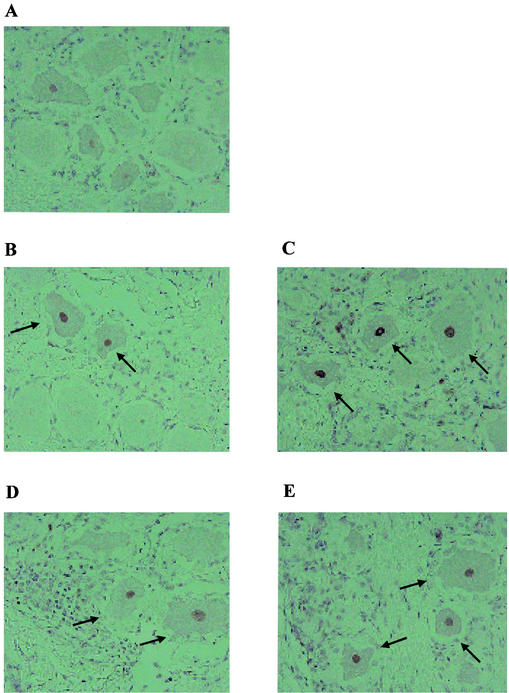
Caspase-3+ neurons were occasionally detected in TG prepared from mock-infected calves (Fig. 4A). At 6 days after infection, caspase-3+ neurons were readily detected in TG prepared from calves infected with wt BHV-1 (Fig. 4B) or the LR mutant (Fig. 4C). At 14 days after infection, caspase-3+ neurons were also present in calves infected with wt BHV-1 (Fig. 4D) or the LR mutant (Fig. 4E).
FIG. 4.
Staining of TG sections with the cleaved caspase-3 antibody. Thin sections were prepared from TG of calves infected with wt BHV-1 or the LR mutant. Immunohistochemistry was performed with the cleaved caspase-3 antibody. Shown are a section from a mock-infected calf (A), a TG section prepared from a calf infected with wt BHV-1 for 6 days (B), a TG section prepared from a calf infected with LR mutant for 6 days (C), a TG section prepared from a calf infected with wt BHV-1 for 14 days (D), and a TG section prepared from a calf infected with the LR mutant for 14 days (E). These sections are representative of the results from many sections. The TG from two calves were examined for each time point. The arrows denote caspase-3+ neurons.
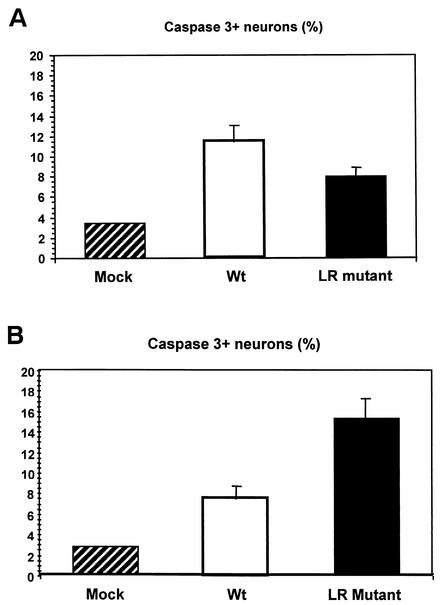
To quantify possible differences in caspase-3+ neurons in calves infected with the LR mutant versus wt BHV-1, the frequency of positive neurons were counted at 6 and 14 days after infection (Fig. 5). Because bovine TG are considerably larger than murine or rabbit TG, it is not possible to serially section the entire TG and count all of the neurons. Consequently, bovine TG were cut into smaller pieces and embedded in paraffin, and sections cut from the respective pieces in an attempt to sample all areas of the TG. No statistically significant difference (P = 0.12) was observed in the number of caspase-3+ neurons of calves infected with wt or the LR mutant virus at 6 days after infection (Fig. 5A). However, there was a significant difference between the frequency of caspase-3+ neurons in mock-infected calves versus infected calves.
FIG. 5.
Quantification of TG neurons that were stained with cleaved caspase-3 antibody. The number of neurons that stained positive for the cleaved caspase-3 antibody was counted as described in Materials and Methods. The results shown are the mean of two different experiments. (A) Data were obtained from calves infected for 6 days. Approximately 1,000 neurons were counted for each TG section (a total of 12 sections were counted/animal). TG sections were prepared from two calves infected with the LR mutant, two calves infected with wt BHV-1, and one mock-infected calf. Approximately 8% of the TG neurons were stained with the cleaved caspase-3 antibody in calves infected with the LR mutant virus. Approximately 11% of the TG neurons were stained with the cleaved caspase-3 antibody in calves infected with wt BHV-1, and 3.4% of the TG neurons were stained with the cleaved caspase-3 antibody from the mock-infected calf. There was no significant difference between the LR mutant and wt BHV-1 (P = 0.12). The difference between mock-infected TG and both virus-infected TG was significant (P value = 0.049). (B) Data were from obtained from calves infected for 14 days. Neurons were processed and counted as described in panel A and in Materials and Methods. Approximately 15% of the TG neurons were stained by the cleaved caspase-3 antibody in the TG of calves infected with the LR mutant for 14 days. Approximately 7.6% of the neurons were stained with the cleaved caspase-3 antibody in calves infected with wt BHV-1 for 14 days. Approximately 3.2% of the TG neurons were stained by the cleaved caspase-3 antibody in the TG of a mock-infected calf. The difference between the LR mutant and wt BHV-1 was significant (P = 0.048). The difference between the mock-infected TG and both virus-infected TG samples was also significant (P = 0.050).
At 14 days after infection, calves infected with the LR mutant contained higher levels of caspase-3+ neurons than wt-infected calves (Fig. 5B). The difference between the LR mutant group and the wt group was significantly different (P = 0.048). Calves infected with either virus contained more caspase-3+ neurons compared to TG sections prepared from mock-infected calves. In summary, these studies demonstrated that higher levels of caspase-3+ neurons were detected in calves infected with the LR mutant virus at 14 days after infection.
Analysis of viral DNA levels in TG by real-time PCR.
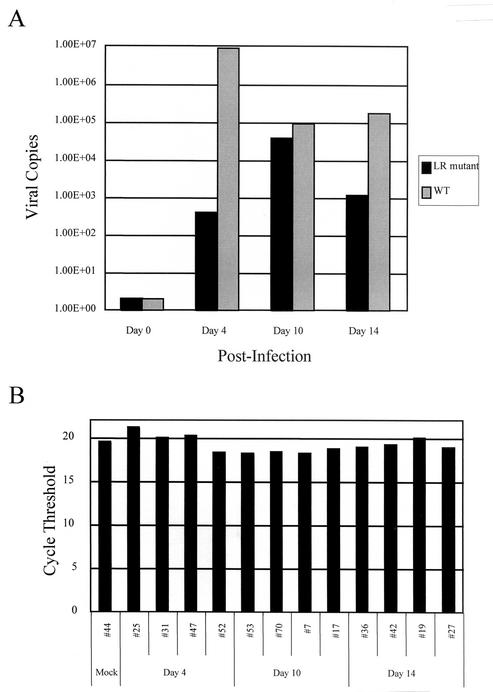
The finding that increased apoptosis was detected in the TG of calves infected with the LR mutant for 14 days suggested that the LR mutant did not establish latency efficiently. A previous study demonstrated that calves infected with the LR mutant contained 10 to 100 times less infectious virus in the TG during acute infection by using a semiquantitative assay and that less viral DNA was present in the TG of latently infected calves (26). However, the present study did not compare DNA levels in the TG of acutely infected calves. At 4 days after infection, calves infected with the LR mutant contained more than 400 times less viral DNA in TG compared to calves infected with wt BHV-1, but by 10 days after infection there was <10-fold fewer copies of the viral genome (Fig. 6). At 14 days after infection, the LR mutant contained ca. 100 times less viral genome equivalents in TG compared to TG of calves infected with wt BHV-1.
FIG. 6.
Real-time PCR to quantitate the number of viral copies in bovine TG. The TG were subjected to real-time PCR by using the TaqMan protocol with either virus-encoded gC or bovine growth hormone (gH) primers and probes. A standard curve was generated as described in Materials and Methods for which the number of viral copies was estimated. (A) Primers that detect BHV-1 gC DNA were used to determine the number of viral copies in 1 μg of total TG DNA. The values presented are the averages of two animals in each group at each day after infection. (B) Primers that detect gH DNA were used in a duplicate plate to demonstrate that similar amounts of starting material were used. The relationship between the CT value and the initial DNA amount in the PCR is linear (23). A CT value for each sample was calculated by determining the point at which the fluorescence exceeded a threshold limit (10 times the standard deviation of the baseline). The CT value presents data collected during the logarithmic phase of the PCR, and this phase is most accurate when DNA levels are measured (23). The numbers for the respective groups are the animal identification numbers.
These studies suggest that there is an initial delay in the accumulation of viral genomes in the TG of calves infected with the LR mutant. Although there appeared to be an increase in the numbers of viral genomes at 10 days after infection in calves infected with the LR mutant, fewer viral genomes were present at 14 days after infection (Fig. 6) and during latency (26). When the same experiment was performed with primers directed against the bovine growth hormone gene, it was evident that similar levels of DNA were included in the assays (Fig. 6B). In summary, we demonstrated here that reduced levels of viral DNA were present in the TG of calves that were acutely infected with the LR mutant.
DISCUSSION
We compared here the apoptotic frequencies in the TG of calves infected with the LR mutant versus the apoptotic frequencies in the TG of calves infected with wt BHV-1. Two independent techniques, TUNEL assay and the use of an antibody that specifically recognizes cleaved caspase-3, demonstrated that calves infected with the LR mutant had higher levels of apoptosis in calves infected with wt BHV-1. Considering that reduced levels of viral DNA were detected in TG of calves latently infected with the LR mutant (26) and during acute infection (Fig. 6), it was somewhat surprising to see increased levels of apoptosis at 14 days after infection. If one considers the level of apoptosis per unit of viral DNA in the TG, calves infected with the LR mutant had much higher frequencies of apoptosis at 6 and 14 days after infection relative to calves infected with wt BHV-1. Consequently, calves infected with the LR mutant demonstrated a reduced establishment of latency, which, in part, would explain why the LR mutant does not reactivate from latency after dexamethasone treatment (26).
These studies indicated that it was easier to morphologically identify cleaved caspase-3+ neurons than TUNEL+ neurons. Since caspase-3 cleavage is an earlier event in the apoptotic cascade relative to the extensive DNA cleavage that is required for a TUNEL+ cell, this result should be expected. Many neurons that were stained with the cleaved caspase-3 antibody had a strong nuclear signal. Prior to apoptosis, caspase-3 is localized in the cytoplasm and mitochondrial fraction in Jurkat cells (human T cells) (67). After induction of apoptosis, caspase-3 can be readily detected in the nucleus. When caspase-3 is expressed as a green fluorescent protein fusion protein, strong nuclear localization is observed in HeLa cells, but weak nuclear localization is observed in 293 (human epithelial cell-like cells) or Jurkat cells (57), indicating that the localization of caspase-3 can be cell type dependent. Preventing nuclear localization of activated caspases correlates with inhibiting apoptosis (11), indicating that detection of cleaved caspase-3 in the nucleus has biological relevance.
The LR mutant contains three stop codons near the beginning of the LR RNA that are designed to prevent protein expression from all three reading frames, and the LR mutant also lacks 25 bp from wt sequence to prevent reversion to wt (27). This mutation would likely interfere with expression of LR ORF2 because the first stop codon is eight bases downstream of the first in-frame ATG of LR ORF2. A peptide antibody that is directed against amino acid sequences within LR ORF2 recognizes a protein of ca. 40 kDa in cells transfected with a wt LR gene construct (7, 25, 32), but not when cells are transfected with a plasmid containing the mutation used to make the LR mutant virus (7). Because the LR RNA is alternatively spliced (9), there is a possibility that more than one protein is expressed. Although we believe that the expression of a protein encoded by the LR gene regulates the latency reactivation cycle, it is possible that differences in LR RNA expression in the LR mutant is responsible for the attenuated phenotype. Regardless of whether an LR protein or changes in LR RNA mediate the altered phenotype of the LR mutant, it is clear that wt expression of LR gene products is crucial for the latency reactivation cycle in cattle.
Plasmids expressing LR gene products (7) and LAT (1, 22, 28, 43) can inhibit apoptosis in transiently transfected cells. Two independent studies have also concluded that rabbits (43) or mice (1) infected with a LAT mutant have more apoptotic neurons during acute infection than do animals infected with the respective LAT-positive HSV-1 strains. The present study provides evidence that the antiapoptotic properties of the LR gene play a crucial role in the latency reactivation cycle of BHV-1. Taken together, these three studies strongly suggest that the HSV-1 gene encoding LAT has a similar role in the latency reactivation cycle in humans. Since the LR gene is required for dexamethasone-induced reactivation from latency in cattle (26) and promotes virus shedding in the ocular cavity of infected calves (27), it is also tempting to speculate that the importance of LAT has been underestimated with small animal models.
HSV-1 (2, 3, 12, 13, 37) and BHV-1 (10, 18-20) can induce or inhibit apoptosis in a cell type-dependent manner after infection of cultured cells. Several antiapoptotic genes within the HSV-1 genome have been identified (1-4, 12, 28-31, 39, 40, 42, 43), suggesting that BHV-1 contains a battery of antiapoptotic genes that promote efficient productive infection. Extensive BHV-1 gene expression is detectable in the TG of calves at 4 and 6 days after infection, but not at 15 days after infection (55). Thus, at 6 days after infection we predict that all of the BHV-1 antiapoptotic genes would be expressed, which is one reason why similar levels of apoptosis were detected in calves infected with the LR mutant or wt virus. The only abundant viral gene expressed at 14 days after infection is the LR gene, and thus the antiapoptotic properties of LR gene products (26) would be crucial for the survival of an infected neuron. During the maintenance of latency, the LR gene would also play a key role in promoting neuronal survival because it is the only viral gene expressed. Finally, we predict that the antiapoptotic properties of LR gene products stimulate reactivation from latency by (i) enhancing the establishment and maintenance of latency, (ii) enhancing productive infection by keeping infected neurons alive during reactivation, and (iii) promoting neuronal survival and reestablishment of latency of infected neurons that do not complete productive infection. Since the LR gene can restore high levels of spontaneous reactivation to an HSV-1 LAT-null mutant (42), LAT would be expected to have similar functions in the latency reactivation cycle in human beings.
Acknowledgments
This research was supported by grants from the U.S. Department of Agriculture (2000-02060) and the National Institutes of Health (P20RR15635). L.L. was supported by a scholarship from CAPES (Brazil).
We thank B. Clowser for assistance with cattle experiments.
REFERENCES
- 1.Ahmed, M., M. Lock, C. G. Miller, and N. W. Fraser. 2002. Regions of the herpes simplex virus type 1 latency-associated transcript that protect cells from apoptosis in vitro and protect neuronal cells in vivo. J. Virol. 76:717-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asano, S., T. Honda, F. Goshima, D. Watanabe, Y. Miyake, Y. Sugiura, and Y. Nishiyama. 1999. US3 protein kinase of herpes simplex virus type 2 plays a role in protecting corneal epithelial cells from apoptosis in infected mice. J. Gen. Virol. 80(Pt. 1):51-56. [DOI] [PubMed] [Google Scholar]
- 3.Aubert, M., and J. A. Blaho. 1999. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J. Virol. 73:2803-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaho, J. A., and M. Aubert. 2001. Modulation of apoptosis during herpes simplex virus infection in human cells. Microbes Infect. 3:1-8. [DOI] [PubMed] [Google Scholar]
- 5.Carter, J. J., A. D. Weinberg, A. Pollard, R. Reeves, J. A. Magnuson, and N. S. Magnuson. 1989. Inhibition of T-lymphocyte mitogenic responses and effects on cell functions by bovine herpesvirus 1. J. Virol. 63:1525-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, H. Y., and X. Yang. 2000. Proteases for cell suicide: functions and regulation of caspases. Microbiol. Mol. Biol. Rev. 64:821-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciacci-Zanella, J., M. Stone, G. Henderson, and C. Jones. 1999. The latency-related gene of bovine herpesvirus 1 inhibits programmed cell death. J. Virol. 73:9734-9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devi-Rao, G. B., D. C. Bloom, J. G. Stevens, and E. K. Wagner. 1994. Herpes simplex virus type 1 DNA replication and gene expression during explant-induced reactivation of latently infected murine sensory ganglia. J. Virol. 68:1271-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devireddy, L. R., and C. Jones. 1998. Alternative splicing of the latency-related transcript of bovine herpesvirus 1 yields RNAs containing unique open reading frames. J. Virol. 72:7294-7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devireddy, L. R., and C. J. Jones. 1999. Activation of caspases and p53 by bovine herpesvirus 1 infection results in programmed cell death and efficient virus release. J. Virol. 73:3778-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fankhauser, C., R. M. Friedlander, and V. Gagliardini. 2000. Prevention of nuclear localization of activated caspases correlates with inhibition of apoptosis. Apoptosis 5:117-132. [DOI] [PubMed] [Google Scholar]
- 12.Galvan, V., R. Brandimarti, and B. Roizman. 1999. Herpes simplex virus 1 blocks caspase-3-independent and caspase-dependent pathways to cell death. J. Virol. 73:3219-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galvan, V., and B. Roizman. 1998. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc. Natl. Acad. Sci. USA 95:3931-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granville, D. J., C. M. Carthy, H. Jiang, G. C. Shore, B. M. McManus, and D. W. Hunt. 1998. Rapid cytochrome c release, activation of caspases 3, 6, 7, and 8 followed by Bap31 cleavage in HeLa cells treated with photodynamic therapy. FEBS Lett. 437:5-10. [DOI] [PubMed] [Google Scholar]
- 15.Griebel, P. J., H. B. Ohmann, M. J. Lawman, and L. A. Babiuk. 1990. The interaction between bovine herpesvirus type 1 and activated bovine T lymphocytes. J. Gen. Virol. 71(Pt. 2):369-377. [DOI] [PubMed] [Google Scholar]
- 16.Griebel, P. J., L. Qualtiere, W. C. Davis, A. Gee, H. Bielefeldt Ohmann, M. J. Lawman, and L. A. Babiuk. 1987. T lymphocyte population dynamics and function following a primary bovine herpesvirus type-1 infection. Viral Immunol. 1:287-304. [DOI] [PubMed] [Google Scholar]
- 17.Griebel, P. J., L. Qualtiere, W. C. Davis, M. J. Lawman, and L. A. Babiuk. 1987. Bovine peripheral blood leukocyte subpopulation dynamics following a primary bovine herpesvirus-1 infection. Viral Immunol. 1:267-286. [DOI] [PubMed] [Google Scholar]
- 18.Hanon, E., S. Hoornaert, F. Dequiedt, A. Vanderplasschen, J. Lyaku, L. Willems, and P. P. Pastoret. 1997. Bovine herpesvirus 1-induced apoptosis occurs at the G0/G1 phase of the cell cycle. Virology 232:351-358. [DOI] [PubMed] [Google Scholar]
- 19.Hanon, E., G. Meyer, A. Vanderplasschen, C. Dessy-Doize, E. Thiry, and P. P. Pastoret. 1998. Attachment but not penetration of bovine herpesvirus 1 is necessary to induce apoptosis in target cells. J. Virol. 72:7638-7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanon, E., A. Vanderplasschen, S. Lyaku, G. Keil, M. Denis, and P. P. Pastoret. 1996. Inactivated bovine herpesvirus 1 induces apoptotic cell death of mitogen-stimulated bovine peripheral blood mononuclear cells. J. Virol. 70:4116-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hariharan, M. J., C. Nataraj, and S. Srikumaran. 1993. Downregulation of murine MHC class I expression by bovine herpesvirus 1. Viral Immunol. 6:273-284. [DOI] [PubMed] [Google Scholar]
- 22.Henderson, G., W. Peng, L. Jin, G.-C. Perng, A. B. Nesburn, S. L. Wechsler, and C. Jones. 2002. Suppression of caspase 8- and caspase 9-induced apoptosis by the herpes simplex virus (HSV-1) encoded latency-associated transcript (LAT). J. Neurovirol., 8(Suppl. 2):103-111. [DOI] [PubMed]
- 23.Higuchi, R., C. Fockler, G. Dollinger, and R. Watson. 1993. Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Bio/Technology 11:1026-1030. [DOI] [PubMed] [Google Scholar]
- 24.Hinkley, S., A. B. Hill, and S. Srikumaran. 1998. Bovine herpesvirus-1 infection affects the peptide transport activity in bovine cells. Virus Res. 53:91-96. [DOI] [PubMed] [Google Scholar]
- 25.Hossain, A., L. M. Schang, and C. Jones. 1995. Identification of gene products encoded by the latency-related gene of bovine herpesvirus 1. J. Virol. 69:5345-5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inman, M., L. Lovato, A. Doster, and C. Jones. 2002. A mutation in the latency related gene of bovine herpesvirus 1 interferes with the latency-reactivation cycle of latency in calves. J. Virol. 76:6771-6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inman, M., L. Lovato, A. Doster, and C. Jones. 2001. A mutation in the latency-related gene of bovine herpesvirus 1 leads to impaired ocular shedding in acutely infected calves. J. Virol. 75:8507-8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inman, M., G. C. Perng, G. Henderson, H. Ghiasi, A. B. Nesburn, S. L. Wechsler, and C. Jones. 2001. Region of herpes simplex virus type 1 latency-associated transcript sufficient for wild-type spontaneous reactivation promotes cell survival in tissue culture. J. Virol. 75:3636-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jerome, K. R., Z. Chen, R. Lang, M. R. Torres, J. Hofmeister, S. Smith, R. Fox, C. J. Froelich, and L. Corey. 2001. HSV and glycoprotein J inhibit caspase activation and apoptosis induced by granzyme B or Fas. J. Immunol. 167:3928-3935. [DOI] [PubMed] [Google Scholar]
- 30.Jerome, K. R., R. Fox, Z. Chen, A. E. Sears, H. Lee, and L. Corey. 1999. Herpes simplex virus inhibits apoptosis through the action of two genes, Us5 and Us3. J. Virol. 73:8950-8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jerome, K. R., J. F. Tait, D. M. Koelle, and L. Corey. 1998. Herpes simplex virus type 1 renders infected cells resistant to cytotoxic T-lymphocyte-induced apoptosis. J. Virol. 72:436-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang, Y., A. Hossain, M. T. Winkler, T. Holt, A. Doster, and C. Jones. 1998. A protein encoded by the latency-related gene of bovine herpesvirus 1 is expressed in trigeminal ganglionic neurons of latently infected cattle and interacts with cyclin-dependent kinase 2 during productive infection. J. Virol. 72:8133-8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones, C. 1998. Alphaherpesvirus latency: its role in disease and survival of the virus in nature. Adv. Virus Res. 51:81-133. [DOI] [PubMed] [Google Scholar]
- 34.Jones, C., T. J. Newby, T. Holt, A. Doster, M. Stone, J. Ciacci-Zanella, C. J. Webster, and M. W. Jackwood. 2000. Analysis of latency in cattle after inoculation with a temperature sensitive mutant of bovine herpesvirus 1 (RLB106). Vaccine 18:3185-3195. [DOI] [PubMed] [Google Scholar]
- 35.Kutish, G., T. Mainprize, and D. Rock. 1990. Characterization of the latency-related transcriptionally active region of the bovine herpesvirus 1 genome. J. Virol. 64:5730-5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, L. G., C. R. Connell, and W. Bloch. 1993. Allelic discrimination by nick-translation PCR with fluorogenic probes. Nucleic Acids Res. 21:3761-3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leopardi, R., and B. Roizman. 1996. The herpes simplex virus major regulatory protein ICP4 blocks apoptosis induced by the virus or by hyperthermia. Proc. Natl. Acad. Sci. USA 93:9583-9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maggioncalda, J., A. Mehta, Y. H. Su, N. W. Fraser, and T. M. Block. 1996. Correlation between herpes simplex virus type 1 rate of reactivation from latent infection and the number of infected neurons in trigeminal ganglia. Virology 225:72-81. [DOI] [PubMed] [Google Scholar]
- 39.Munger, J., A. V. Chee, and B. Roizman. 2001. The U(S)3 protein kinase blocks apoptosis induced by the d120 mutant of herpes simplex virus 1 at a premitochondrial stage. J. Virol. 75:5491-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munger, J., and B. Roizman. 2001. The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc. Natl. Acad. Sci. USA 98:10410-10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nataraj, C., S. Eidmann, M. J. Hariharan, J. H. Sur, G. A. Perry, and S. Srikumaran. 1997. Bovine herpesvirus 1 downregulates the expression of bovine MHC class I molecules. Viral Immunol. 10:21-34. [DOI] [PubMed] [Google Scholar]
- 42.Perng, G.-C., B. Maguen, L. Jin, K. R. Mott, N. Osorio, S. M. Slanina, A. Yukht, H. Ghiasi, A. B. Nesburn, M. Inman, G. Henderson, C. Jones, S. L. Wechsler. 2002. A gene capable of blocking apoptosis can substitute for the herpes simplex virus type 1 latency-associated transcript gene and restore wild-type reactivation levels. J. Virol. 76:1224-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perng, G.-C., C. Jones, J. Ciacci-Zanella, M. Stone, G. Henderson, A. Yukht, S. M. Slanina, F. M. Hoffman, H. Ghiasi, A. B. Nesburn, S. Wechsler. 2000. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript (LAT). Science 287:1500-1503. [DOI] [PubMed] [Google Scholar]
- 44.Perng, G. C., K. Chokephaibulkit, R. L. Thompson, N. M. Sawtell, S. M. Slanina, H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 1996. The region of the herpes simplex virus type 1 LAT gene that is colinear with the ICP34.5 gene is not involved in spontaneous reactivation. J. Virol. 70:282-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perng, G. C., E. C. Dunkel, P. A. Geary, S. M. Slanina, H. Ghiasi, R. Kaiwar, A. B. Nesburn, and S. L. Wechsler. 1994. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J. Virol. 68:8045-8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perng, G. C., H. Ghiasi, S. M. Slanina, A. B. Nesburn, and S. L. Wechsler. 1996. The spontaneous reactivation function of the herpes simplex virus type 1 LAT gene resides completely within the first 1.5 kilobases of the 8.3-kilobase primary transcript. J. Virol. 70:976-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perng, G. C., S. M. Slanina, H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 1996. A 371-nucleotide region between the herpes simplex virus type 1 (HSV-1) LAT promoter and the 2-kilobase LAT is not essential for efficient spontaneous reactivation of latent HSV-1. J. Virol. 70:2014-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perng, G. C., S. M. Slanina, A. Yukht, B. S. Drolet, W. Keleher, Jr., H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 1999. A herpes simplex virus type 1 latency-associated transcript mutant with increased virulence and reduced spontaneous reactivation. J. Virol. 73:920-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perng, G. C., S. M. Slanina, A. Yukht, H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 2000. The latency-associated transcript gene enhances establishment of herpes simplex virus type 1 latency in rabbits. J. Virol. 74:1885-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rock, D., J. Lokensgard, T. Lewis, and G. Kutish. 1992. Characterization of dexamethasone-induced reactivation of latent bovine herpesvirus 1. J. Virol. 66:2484-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rock, D. L., S. L. Beam, and J. E. Mayfield. 1987. Mapping bovine herpesvirus type 1 latency-related RNA in trigeminal ganglia of latently infected rabbits. J. Virol. 61:3827-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rock, D. L., A. B. Nesburn, H. Ghiasi, J. Ong, T. L. Lewis, J. R. Lokensgard, and S. L. Wechsler. 1987. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J. Virol. 61:3820-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sawtell, N. M., and R. L. Thompson. 1992. Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishment and reactivation from latency. J. Virol. 66:2157-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schang, L. M., A. Hossain, and C. Jones. 1996. The latency-related gene of bovine herpesvirus 1 encodes a product which inhibits cell cycle progression. J. Virol. 70:3807-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schang, L. M., and C. Jones. 1997. Analysis of bovine herpesvirus 1 transcripts during a primary infection of trigeminal ganglia of cattle. J. Virol. 71:6786-6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sheffy, B. E., and D. H. Davies. 1972. Reactivation of a bovine herpesvirus after corticosteroid treatment. Proc. Soc. Exp. Biol. Med. 140:974-976. [DOI] [PubMed] [Google Scholar]
- 57.Shikama, Y., M. U, T. Miyashita, and M. Yamada. 2001. Comprehensive studies on subcellular localizations and cell death-inducing activities of eight GFP-tagged apoptosis-related caspases. Exp. Cell Res. 264:315-325. [DOI] [PubMed] [Google Scholar]
- 58.Stevens, J. G., E. K. Wagner, G. B. Devi-Rao, M. L. Cook, and L. T. Feldman. 1987. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science 235:1056-1059. [DOI] [PubMed] [Google Scholar]
- 59.Thompson, R. L., and N. M. Sawtell. 1997. The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J. Virol. 71:5432-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tikoo, S. K., M. Campos, and L. A. Babiuk. 1995. Bovine herpesvirus 1 (BHV-1): biology, pathogenesis, and control. Adv. Virus Res. 45:191-223. [DOI] [PubMed] [Google Scholar]
- 61.Wagner, E. K., and D. C. Bloom. 1997. Experimental investigation of herpes simplex virus latency. Clin. Microbiol. Rev. 10:419-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Winkler, M. T., A. Doster, and C. Jones. 1999. Bovine herpesvirus 1 can infect CD4+ T lymphocytes and induce programmed cell death during acute infection of cattle. J. Virol. 73:8657-8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winkler, M. T., A. Doster, J. H. Sur, and C. Jones. 2002. Analysis of bovine trigeminal ganglia following infection with bovine herpesvirus 1. Vet. Microbiol. 86:139-155. [DOI] [PubMed] [Google Scholar]
- 64.Winkler, M. T., L. S. Schang, A. Doster, T. Holt, and C. Jones. 2000. Analysis of cyclins in trigeminal ganglia of calves infected with bovine herpesvirus-1. J. Gen. Virol. 81(Pt. 12):2993-2998. [DOI] [PubMed] [Google Scholar]
- 65.Winkler, M. T. C., A. Doster, and C. Jones. Bovine herpesvirus-1 can infect CD4+ T lymphocytes and induce programmed cell death during acute infection of cattle. 2000. Persistence and reactivation of bovine herpesvirus 1 in the tonsil of latently infected calves. J. Virol. 74:5337-5346.10799611 [Google Scholar]
- 66.Wyler, R., M. Engels, and M. Schwyzer. 1989. Infectious bovine rhinotracheitis/vulvovaginitis (BHV-1), p. 1-72. In G. Witman (ed.), Herpesvirus diseases of cattle, horses, and pigs. Kluwer Academic Publishers, Boston, Mass.
- 67.Zhivotovsky, B., A. Samali, A. Gahm, and S. Orrenius. 1999. Caspases: their intracellular localization and translocation during apoptosis. Cell Death Differ. 6:644-651. [DOI] [PubMed] [Google Scholar]