Abstract
The gene for gelsolin (an actin-binding, cytoskeletal regulatory protein) was shown earlier to be specialized for high corneal expression in adult zebrafish. We show here that zebrafish gelsolin is required for proper dorsalization during embryogenesis. Inhibition of gelsolin expression by injecting fertilized eggs with a specific morpholino oligonucleotide resulted in a range of concentration-dependent ventralized phenotypes, including those lacking a brain and eyes. These were rescued by coinjection of zebrafish gelsolin or chordin (a known dorsalizing agent) mRNAs, or human gelsolin protein. Moreover, injection of gelsolin mRNA or human gelsolin protein by itself dorsalized the developing embryos, often resulting in axis duplication. Injection of the gelsolin-specific morpholino oligonucleotide enhanced the expression of Vent mRNA, a ventral marker downstream of bone morphogenetic proteins, whereas injection of gelsolin mRNA enhanced the expression of chordin and goosecoid mRNAs, both dorsal markers. Our results indicate that gelsolin also modulates embryonic dorsal/ventral pattern formation in zebrafish.
Gelsolin comprises ≈50% of the water-soluble protein of the adult zebrafish cornea and has been considered as a corneal “crystallin” (1). More typically, gelsolin, an actin-severing cytoskeleton regulatory protein modulated by calcium and polyphosphoinositolphospholipids (2–5), is expressed in many tissues in lower amounts and has been implicated in multiple roles such as cell motility, signaling, apoptosis, and cancer (see ref. 3). Various developmental functions of gelsolin include morphogenesis in ascidians (6), gelation and contractility of early embryonic cells in Xenopus (7), retinal and neuronal morphogenesis (8, 9), skeletogenesis (10), mammary gland ductal morphogenesis (11), and erythropoiesis (12) in mammals. A gelsolin-like protein in Dictyostelium is essential in phototactic migration (13).
In humans, alternative splicing of a single gene accounts for a cytoplasmic and a secreted plasma gelsolin that carries an additional amino-terminal extension of 23 aa. Both forms of gelsolin are expressed in most adult tissues (14). Nucleotide substitution of G654 to A654 (15) gives rise to Finnish type familial amyloidosis (FAF), an autosomal-dominant disease characterized by corneal lattice dystrophy, skin changes, renal complications, and a cranial neuropathy that affects the cranial nerves in particular (16). In the developing rat brain, initial low levels of gelsolin precede increased expression around day 10 followed by a subsequent decrease near day 30, suggesting a functional role for gelsolin in early brain development (17). Cultured cells lacking gelsolin show reduced motility, whereas overexpression of gelsolin increases cell movement (18, 19).
In the present study, we show that gelsolin is differentially expressed during zebrafish development, already starting by the two-cell stage, before accumulating in the mature cornea. Furthermore, microinjection experiments using a gelsolin morpholino oligonucleotide (MO), gelsolin and chordin mRNAs, and human gelsolin protein indicated that gelsolin is required for dorsoventral patterning in zebrafish embryos. The morphological results were supported by in situ hybridization showing altered expression of dorsal [chordin (20) and goosecoid (21)] and ventral [Vent (22, 23)] markers in the microinjected embryos. Our findings provide evidence for a signaling role for gelsolin during embryogenesis and are consistent with the idea that abundant corneal proteins, like lens crystallins, may have multiple functions depending on their expression (24, 25).
Materials and Methods
Zebrafish.
WT zebrafish were maintained as described by Westerfield (26). Embryos were obtained by natural matings.
Antisense MOs.
Gelsolin MO (5′-CTGGAACTCCTTGTGAAAAACCATG-3′), an antisense sequence spanning −1 to +24 of the translational start site, control MO (5′-TACCAAAAAGTGTTCCTCAAGGTC-3′), the reverse of the gelsolin MO, and chordin MO (custom-made by the manufacturer) were purchased from Gene Tools LLC (Philomath, OR). The MOs were dissolved in water at a concentration of 4 mM and were diluted in 1× Danieu's buffer (27) before injection.
In Vitro Synthesis of mRNAs for Microinjection.
Gelsolin cDNA was constructed in pCS2 vector (BamHI/EcoRI sites). Zebrafish chordin in pCS2 vector was a gift from Marnie Halpern (Carnegie Institute of Washington, Baltimore). Capped synthetic mRNAs were prepared by using the SP6 mMESSAGE mMACHINE kit (Ambion, Austin, TX).
Microinjection.
MOs and mRNAs were injected into the yolk mass of the one- to two-cell embryos. Postinjection (4 h), damaged embryos were sorted out and the rest were allowed to grow at 28°C for further observation.
SDS/PAGE.
Embryo extracts were first immunoprecipitated with a rabbit polyclonal antibody raised against zebrafish gelsolin (1). The immunoblot of the proteins separated by SDS/10% PAGE was then probed with the same antibody. Immunoprecipitation was necessary because of the presence of an excessive amount of yolk lipovitellin polypeptide, which has a mobility (≈80 kDa) similar to that of gelsolin and crossreacts nonspecifically with the gelsolin antibody. Each lane was loaded with the immunoprecipitates of 20 embryos. The antibody was raised against a synthetic peptide composed of the amino-terminal 17 aa of the zebrafish-derived gelsolin-related protein (Research Genetics, Huntsville, AL), and was affinity-purified by standard methods. The rabbit horseradish peroxidase-coupled secondary antibody was obtained from Jackson ImmunoResearch. After SDS/PAGE, the separated proteins were blotted onto nitrocellulose membranes (NOVEX, San Diego), blocked with 3% (wt/vol) BSA in TBS, and were incubated with the primary antibody (1:1,000 in TBS) for 1 h at room temperature. After three washes in TBS, pH 7.5, for 5 min each time, the membranes were incubated with the second antibody (1:10,000 in TBS) at room temperature and were developed by using a chemiluminescence kit (Roche Molecular Biochemicals). Densitometric analyses of the Western blot signals were performed by using the CHEMIIMAGER Version 5.5 program (Alpha Innotech, San Leandro, CA).
In Situ Hybridization of Zebrafish Embryos.
In situ hybridization of whole embryos by using the in vitro-transcribed, digoxigenin-labeled riboprobe (Roche Molecular Biochemicals) was performed by following the protocol described by Westerfield (26). The plasmid, pPCR-Script Amp (Stratagene) containing the 5′ region of the gelsolin cDNA (1), was used as the template for T3 RNA polymerase-mediated sense probe and T7 RNA polymerase-mediated antisense probe synthesis. For histological analysis, the hatching-stage embryos were embedded in OCT compound, quick-frozen on dry ice, sectioned (8 μm), placed on poly-l-lysine-coated slides, and processed for color detection following the manufacturer's (Roche Molecular Biochemicals) protocol.
Results
Differential Expression of Gelsolin mRNA During Embryogenesis.
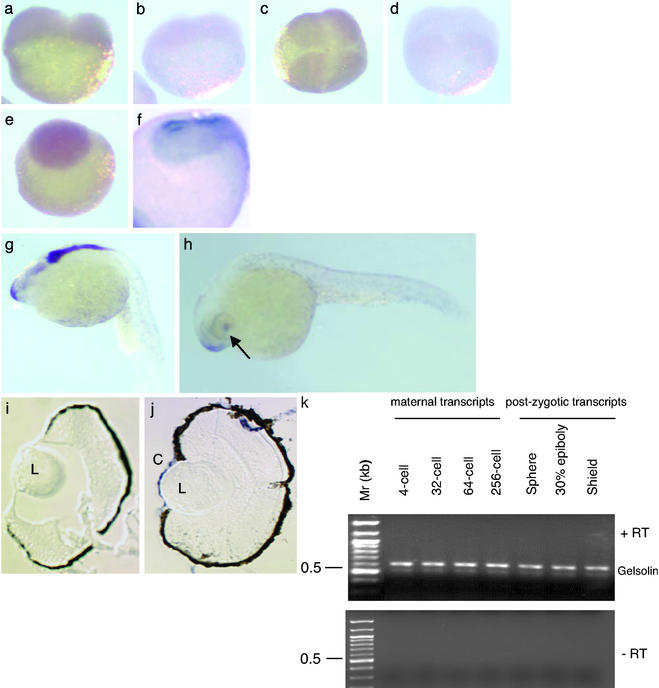
Gelsolin in adult zebrafish is highly expressed in the adult cornea. Here we explore the possibility that gelsolin plays a role in zebrafish development. The developmental spatiotemporal expression pattern of gelsolin mRNA was examined by in situ hybridization using a riboprobe derived from a 1.5-kb 5′ fragment of the gelsolin cDNA (ref. 1; Fig. 1). A ubiquitous hybridization signal was obtained at the two-cell (Fig. 1a), four-cell (Fig. 1c), and blastula (Fig. 1e) stages by using the antisense gelsolin riboprobe. A sense probe was used as the control to show hybridization signals in the two-cell (Fig. 1b) and four-cell (Fig. 1d) stages. At the late-gastrula stage of development, the neural plate stained positive (Fig. 1f), and at 22 h postfertilization (hpf), there was a transient signal in the forebrain and hindbrain (Fig. 1g). Strong expression was confined to the eyes, the rostral epithelium, and the olfactory regions at hatching (Fig. 1h). Although gelsolin mRNA was not detected in lens by in situ hybridization (Fig. 1j), our previous study demonstrated weak gelsolin protein expression in lens (1). The cornea of the hatching-stage embryo showed abundant gelsolin mRNA expression (Fig. 1j). The sense probe did not show any positive hybridization signal in the cornea or lens (Fig. 1i). RT-PCR tests confirmed the presence of gelsolin transcripts before (512-cell stage, maternal transcripts) and after the onset of postzygotic transcription (Fig. 1k). Although Western blot analysis did not detect gelsolin in unfertilized eggs, fertilized eggs, and four-cell stage embryos (data not shown), it was detected in the gastrulae 8 hpf (Fig. 2e).
Figure 1.
Developmental expression of gelsolin mRNA. Gelsolin mRNA expression is shown in four stages of embryo by using an antisense probe: two-cell (a), four-cell (c), blastula (e), and late-gastrula (f). A sense probe was used to hybridize in the two-cell (b) and four-cell (d) embryos. The gastrula (e) is photographed, magnified ×2 to show the anterior part of the embryo. Gelsolin expression in the forebrain and hindbrain is shown in a 22-h-old embryo (g), in the snout and cornea of a hatching-stage embryo (h), and in the sections of eyes from a hatching-stage embryo probed with the sense (i) and antisense (j) riboprobes. Arrow indicates the location of the eye. Locations of the lens (L) and cornea (C) are indicated. (k) RT-PCR for gelsolin by using cDNA synthesized from embryonic RNA obtained from the indicated developmental stages. (Upper) Reaction products with reverse transcriptase (+RT). (Lower) Products without the enzyme (−RT).
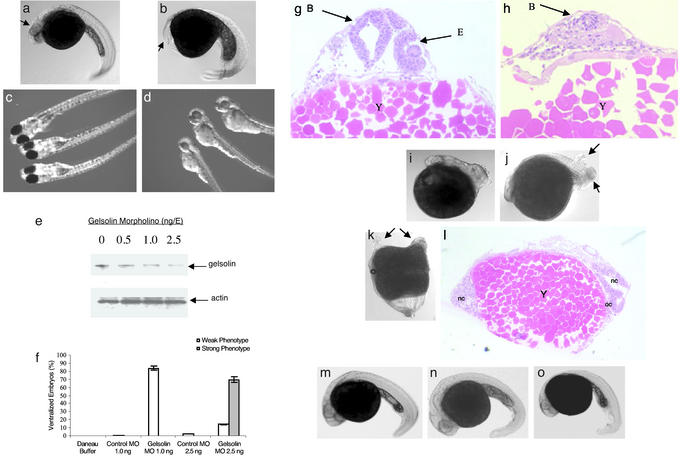
Figure 2.
MO knockdown of gelsolin ventralizes embryos. Embryos 30 hpf after microinjection of control MO (2.5 ng/E; a) and gelsolin MO (b) are shown. Arrows indicate the location of the eyes. Embryos injected with control (c) or gelsolin MO (1 ng/E; d) are shown 72 hpf. Gelsolin MO resulted in ventralized embryos (b) and hatchlings with smaller and less pigmented eyes (d). The effect of different doses of gelsolin MO on the gelsolin and actin levels was determined by immunoblotting of embryo extracts prepared 8 h after MO injection (e; ref. 14). A representative immunoblot of four separate experiments is shown. The phenotypic effect of different doses of the gelsolin MO is summarized (f). Histological analyses using hematoxylin/eosin staining compare the normal and reduced development of anterior cephalic structures in control (g) and gelsolin MO-injected (h) embryos at 30 hpf. Locations of brain (B), eye (E), and yolk mass (Y) are indicated. Gelsolin mRNA (50 pg/E) injection results in dorsalized embryos (i and j). Arrows indicate duplicated posterior axial regions. Microinjection of human plasma gelsolin protein (4 ng/E) resulted in axis duplication (k). Arrows indicate duplicated anterior axial region including head and eyes. Histological section (l) of the embryo with duplicated axes showing notochord (nc) and optic cup (oc). Coinjection of gelsolin MO (2.5 ng/E) with 4 ng of human gelsolin protein (m), or with gelsolin mRNA (50 pg/E; n), or with chordin mRNA (50 pg/E; o) rescued the gelsolin MO-injected phenotypes. Embryos at 30 hpf are shown.
Gelsolin Knockdown and Overexpression Experiments.
We next carried out loss-of-function experiments by injecting a MO (27) designed specifically against the 5′ end of the corneal gelsolin mRNA into the one- to two-cell embryos (Fig. 2). Injection of MO at 2.5 ng per embryo (ng/E) resulted in ventralized embryos (85%, n = 250). Seventy percent of the embryos had severely reduced head structures, including the brain and eyes (Fig. 2 b and h), and were categorized as strong phenotypes. An additional 15% of the hatched embryos developed slowly, were small, and resembled the ones that received 1 ng of gelsolin MO (Fig. 2d); these were categorized as weak phenotypes. Embryos injected with only 1 ng/E hatched (84%, n = 160) but were weakly ventralized, showed poor eye development, and were less pigmented in the body and eyes (Fig. 2d) than their control counterparts (Fig. 2c). On average, in the different tests, ≈64% of the affected embryos were rescued from the ventralized characteristics after coinjection with 2.5 ng of gelsolin MO and 4 ng of human plasma gelsolin protein (Cystoskeleton, Denver; Fig. 2m), or 50 pg of gelsolin mRNA (Fig. 2n; n = 80 for each experiment). The criteria for rescue were morphology of the embryos. Control injections included the vehicle, the Daneau buffer (n = 200), BSA at 4 ng/E (n = 75), and control MO at 1.0 ng/E (n = 142) and 2.5 ng/E (n = 120). All controls showed normal development.
Scans of the immunoblots indicated that MO at 1 and 2.5 ng/E decreased gelsolin protein expression 3- and 5-fold, respectively, 8 h after injection (Fig. 2e). By contrast, immunoblots using an antibody against human β-actin (catalog no. sc 1616, Santa Cruz Biotechnology) that crossreacts with zebrafish actin showed no change in actin content in the crude extracts of the embryos injected with gelsolin MO. A summary of our results on the effect of the different concentrations of injected gelsolin MO on embryonic phenotypes is given in Fig. 2f.
Injection of gelsolin mRNA by itself resulted in dorsalized phenotypes (Fig. 2 i and j). At 5 pg/E gelsolin mRNA, the embryos were phenotypically indistinguishable from the control MO- or control transcript-injected embryos (Fig. 2 a and g). Unexpectedly, at 50 pg/E gelsolin mRNA, many embryos (60%, n = 240) were dorsalized (Fig. 2i) and posterior axial duplication was observed in 20% (48 of 240) of the cases (Fig. 2j). Embryos injected with 4 ng/E human plasma gelsolin protein were also dorsalized (80%, n = 77) and 30% of the dorsalized embryos showed anterior axis duplication (Fig. 2k). Histological staining revealed the dual axes with separate notochords; an optic cup was distinct in one of the heads in the section shown in Fig. 2l. The small differences in the histological appearance of the two axes may be accounted for by the plane of sectioning and slight differences in the developmental rate of the two axes.
Evidence That Gelsolin Modulates the Bone Morphogenetic Protein (BMP) Signaling Pathway.
Our results suggest that gelsolin modulates certain BMP signaling pathways that are known to affect development of the dorsal/ventral axis (see refs. 28–30 and Fig. 3k). To provide evidence for this interpretation, we coinjected the gelsolin MO with the mRNA for chordin, an antagonist of BMP4 of the transforming growth factor-β (TGF-β) superfamily (31). Chordin mRNA, as gelsolin mRNA, rescued 70% of the gelsolin MO-injected embryos (n = 110; Fig. 2o), consistent with the possibility that the effect of gelsolin is similar to that of chordin, i.e., both can antagonize ventralization of the embryos.
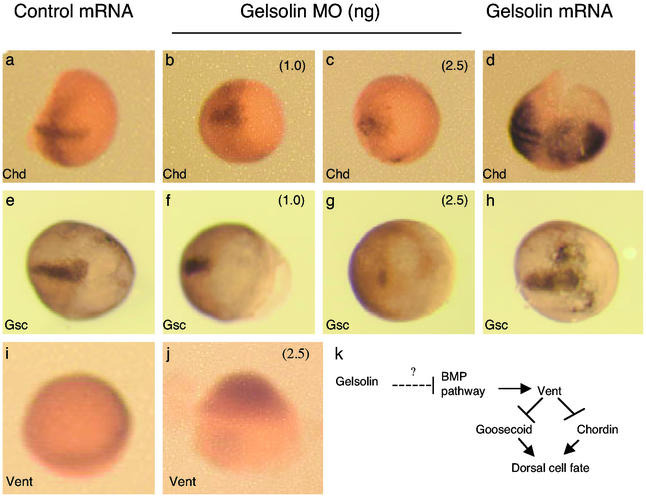
Figure 3.
In situ hybridization demonstrates the expression of chordin (a–d), goosecoid (e–h), and Vent (i and j) in embryos harvested 8 h postinjection with control mRNA synthesized from PCS2 vector control or gelsolin mRNA, as indicated. Control (a and e), gelsolin MO-injected (1 ng/E; b and f), gelsolin MO-injected (2.5 ng/E; c and g), and gelsolin mRNA-injected (50 pg/E; d and h) embryos are shown, with the anterior side to the right. Vent mRNA expression is shown in embryos injected with control MO (i) and gelsolin MO (j) at 2.5 ng/E. The animal pole of the embryos is oriented upward. (k) A schematic of how gelsolin might regulate cell fates in zebrafish.
Mutations that delete (20) or inactivate (28) chordin ventralize zebrafish embryos. Members of the BMP family, a subclass of the TGF-β superfamily (29, 30), induce ventral fates. Vent and Vox, two downstream components of the BMP signaling pathway, repress the transcription of dorsal markers, chordin and goosecoid, in zebrafish (refs. 22, 23, 32, and 33; see Fig. 3k). We thus explored the effect of gelsolin on the expression of chordin mRNA and goosecoid mRNA (Fig. 3). Whether gelsolin knockdown also enhances BMP signaling to ventralize the embryos was examined by monitoring Vent expression (Fig. 3). In situ hybridization showed that embryos injected with gelsolin mRNA up-regulated chordin (Fig. 3d) and goosecoid (Fig. 3h) transcripts. Conversely, gelsolin MO-injected embryos had lower intensity hybridization signals for chordin (Fig. 3 b and c) and goosecoid (Fig. 3 f and g) transcripts than did the control MO-injected embryos (Fig. 3 a and e). Moreover, Vent mRNA was increased on the dorsal side of the gelsolin MO-injected embryo (Fig. 3j) compared with the control embryo (Fig. 3i). Although not clear in Fig. 3 i and j, no change was observed in Vent expression in the ventral region of the gelsolin MO-injected embryos. These data suggest that gelsolin is upstream of chordin regulating the dorsal/ventral pattern of development (Fig. 3). This interpretation is supported by the fact that coinjection of chordin mRNA rescued the ventral phenotype induced by gelsolin MO (Fig. 2o), whereas coinjection of gelsolin mRNA did not rescue the ventral phenotype induced by chordin MO (3.0 ng of chordin MO plus 50 pg of gelsolin mRNA; n = 70; data not shown).
Discussion
The present results implicate gelsolin in modulating dorsal/ventral patterning during zebrafish development and suggest that it operates through the BMP signaling pathway. We cannot, however, eliminate the possibility that the Wnt–β-catenin pathway is also affected by gelsolin (34). The present evidence favors the idea that the ventralized/dorsalized phenotypes we obtained are cell-autonomous for the following reasons. First, the gelsolin MO used was derived from the 5′ end of gelsolin cDNA obtained from the corneal mRNA (1), which encodes the intracellular, nonsecreted form of gelsolin (14). Secreted gelsolin has a different amino-terminal sequence and its expression would not be targeted by the MO we used. Thus, it is extremely unlikely that the signaling gelsolin leaves the injected cells. Second, we injected zebrafish gelsolin mRNA encoding the intracellular form of the protein. Finally, we did not obtain any phenotypes when gelsolin mRNA was injected into a single blastomere at the eight-cell or later stages. This result is consistent with a cell-autonomous effect.
In contrast to the developmental gelsolin phenotypes in zebrafish, gelsolin-null mice show reduced cell motility and wound-healing ability (19). One possible explanation for this difference between zebrafish and mice is that the former has at least two gelsolin genes rather than the single-copy gelsolin gene of mice (35). This duplication may permit the gelsolin that accumulates in the cornea to subspecialize for a developmental role in zebrafish. Another consideration is that a functionally redundant pathway rescues the gelsolin-null mice, a possibility consistent with the numerous known actin-binding proteins. Although further analysis is required, it is possible that the gelsolin-related phenotypes we have observed in the present study are connected to interference with cell motility during axis formation. Finally, despite the absence of a marked developmental phenotype, gelsolin-null mice and the gelsolin-reduced zebrafish reported here may show similarities in that excessive bone growth occurs in aging mice that are lacking the gelsolin gene (10), consistent with deregulation of BMP signaling.
Although the present results do not explain the reason for the extreme abundance of gelsolin in the cornea of adult zebrafish (≈50% of the water-soluble protein; ref. 1), they do suggest that zebrafish gelsolin has at least two separate functions, a structural role in the cornea, and a regulatory role during development; we cannot rule out that these apparently different biological roles have mechanistic similarities. Nonetheless, our data indicating a developmental signaling role for gelsolin in the zebrafish add to the similarity between this abundant corneal protein and the multifunctional crystallins in the lens (36, 37). Both accumulate to exceedingly high levels in their respective transparent tissues that cooperate for refraction in the eye, and both play nonrefractive roles at lower concentrations in nonocular tissues. Our data thus support the idea that the abundant intracellular water-soluble corneal proteins, such as gelsolin in the zebrafish, and lens crystallins are multifunctional proteins that have evolved by a gene-sharing strategy (24, 25).
Acknowledgments
We thank Drs. David Grunwald (University of Utah, Salt Lake City) and Marnie Halpern (Carnegie Institute of Washington, Baltimore) for the zebrafish goosecoid cDNA (in pBluescript) and chordin cDNA (in pBluescript and pCS2 vectors), respectively, which were used as probes for in situ hybridizations; Drs. Igor Dawid and Reiko Toyama (National Institute of Child Health and Human Development, National Institutes of Health) and Paul Liu (National Human Genome Research Institute, National Institutes of Health) for initial help with our zebrafish work; and R. Barry Hough, David Nees, Z.-P. Xu, Janine Davis, and Zdenek Kostrouch for critical comments on the manuscript.
Abbreviations
- MO
morpholino oligonucleotide
- hpf
h postfertilization
- BMP
bone morphogenetic protein
- ng or pg/E
ng or pg per embryo
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Xu Y S, Kantorow M, Davis J, Piatigorsky J. J Biol Chem. 2000;275:24645–24652. doi: 10.1074/jbc.M001159200. [DOI] [PubMed] [Google Scholar]
- 2.Feng L, Mejillano M, Yin H L, Chen J, Prestwich G D. Biochemistry. 2001;40:904–913. doi: 10.1021/bi000996q. [DOI] [PubMed] [Google Scholar]
- 3.Kwiatkowski D J. Curr Opin Cell Biol. 1999;11:103–108. doi: 10.1016/s0955-0674(99)80012-x. [DOI] [PubMed] [Google Scholar]
- 4.Pollard T D, Cooper J A. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- 5.Janmey P A, Stossel T P. Nature. 1987;325:362–364. doi: 10.1038/325362a0. [DOI] [PubMed] [Google Scholar]
- 6.Ohtsuka Y, Obinata T, Okamura Y. Dev Biol. 2001;239:107–117. doi: 10.1006/dbio.2001.0425. [DOI] [PubMed] [Google Scholar]
- 7.Ankenbauer T, Kleinschmidt J A, Vandekerckhove J, Franke W W. J Cell Biol. 1988;107:1489–1498. doi: 10.1083/jcb.107.4.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Legrand C, Ferraz C, Clavel M C, Rabie A. Cell Tissue Res. 1991;264:335–338. doi: 10.1007/BF00313971. [DOI] [PubMed] [Google Scholar]
- 9.Westberg J A, Zhang K Z, Andersson L C. FASEB J. 1999;13:1621–1626. doi: 10.1096/fasebj.13.12.1621. [DOI] [PubMed] [Google Scholar]
- 10.Chellaiah M, Kizer N, Silva M, Alvarez U, Kwiatkowski D, Hruska K A. J Cell Biol. 2000;148:665–678. doi: 10.1083/jcb.148.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowley M R, Head K L, Kwiatkowski D J, Asch H L, Asch B B. Dev Biol. 2000;225:407–423. doi: 10.1006/dbio.2000.9844. [DOI] [PubMed] [Google Scholar]
- 12.Hinssen H, Vandekerckhove J, Lazarides E. J Cell Biol. 1987;105:1425–1433. doi: 10.1083/jcb.105.3.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stocker S, Hiery M, Marriott G. Mol Biol Cell. 1999;10:161–178. doi: 10.1091/mbc.10.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paunio T, Kangas H, Kiuru S, Palo J, Peltonen L, Syvanen A C. FEBS Lett. 1997;406:49–55. doi: 10.1016/s0014-5793(97)00237-8. [DOI] [PubMed] [Google Scholar]
- 15.Maury C P, Alli K, Baumann M. FEBS Lett. 1990;276:75–77. doi: 10.1016/0014-5793(90)80072-q. [DOI] [PubMed] [Google Scholar]
- 16.Maury C P, Kere J, Tolvanen R, de la Chapelle A. Genomics. 1992;13:902–903. doi: 10.1016/0888-7543(92)90183-s. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka J, Sobue K. J Neurosci. 1994;14:1038–1052. doi: 10.1523/JNEUROSCI.14-03-01038.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunningham C C, Stossel T P, Kwiatkowski D J. Science. 1991;251:1233–1236. doi: 10.1126/science.1848726. [DOI] [PubMed] [Google Scholar]
- 19.Witke W, Sharpe A H, Hartwig J H, Azuma T, Stossel T P, Kwiatkowski D J. Cell. 1995;81:41–51. doi: 10.1016/0092-8674(95)90369-0. [DOI] [PubMed] [Google Scholar]
- 20.Fisher S, Amacher S L, Halpern M E. Development (Cambridge, UK) 1997;124:1301–1311. doi: 10.1242/dev.124.7.1301. [DOI] [PubMed] [Google Scholar]
- 21.Stachel S E, Grunwald D J, Myers P Z. Development (Cambridge, UK) 1993;117:1261–1274. doi: 10.1242/dev.117.4.1261. [DOI] [PubMed] [Google Scholar]
- 22.Melby A E, Beach C, Mullins M, Kimelman D. Dev Biol. 2000;224:275–285. doi: 10.1006/dbio.2000.9780. [DOI] [PubMed] [Google Scholar]
- 23.Imai Y, Gates M A, Melby A E, Kimelman D, Schier A F, Talbot W S. Development (Cambridge, UK) 2001;128:2407–2420. doi: 10.1242/dev.128.12.2407. [DOI] [PubMed] [Google Scholar]
- 24.Piatigorsky J. Ann NY Acad Sci. 1998;842:7–15. doi: 10.1111/j.1749-6632.1998.tb09626.x. [DOI] [PubMed] [Google Scholar]
- 25.Piatigorsky J. Prog Retin Eye Res. 1998;17:145–174. doi: 10.1016/s1350-9462(97)00004-9. [DOI] [PubMed] [Google Scholar]
- 26.Westerfield M. The Zebrafish Book. Eugene: Univ. of Oregon Press; 1995. [Google Scholar]
- 27.Nasevicius A, Ekker S C. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- 28.Schulte-Merker S, Lee K J, McMahon A P, Hammerschmidt M. Nature. 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- 29.Harland R, Gerhart J. Annu Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- 30.Dale L, Wardle F C. Semin Cell Dev Biol. 1999;10:319–326. doi: 10.1006/scdb.1999.0308. [DOI] [PubMed] [Google Scholar]
- 31.Blader P, Rastegar S, Fischer N, Strahle U. Science. 1997;278:1937–1940. doi: 10.1126/science.278.5345.1937. [DOI] [PubMed] [Google Scholar]
- 32.Kodjabachian L, Dawid I B, Toyama R. Dev Biol. 1999;213:231–245. doi: 10.1006/dbio.1999.9392. [DOI] [PubMed] [Google Scholar]
- 33.Schier A F, Talbot W S. Curr Opin Genet Dev. 1998;8:464–471. doi: 10.1016/s0959-437x(98)80119-6. [DOI] [PubMed] [Google Scholar]
- 34.Sumoy L, Kiefer J, Kimelman D. Dev Genes Evol. 1999;209:48–58. doi: 10.1007/s004270050226. [DOI] [PubMed] [Google Scholar]
- 35.Kwiatkowski D J, Mehl R, Yin H L. J Cell Biol. 1988;106:375–384. doi: 10.1083/jcb.106.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wistow G J, Piatigorsky J. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- 37.de Jong W W, Hendriks W, Mulders J W, Bloemendal H. Trends Biochem Sci. 1989;14:365–368. doi: 10.1016/0968-0004(89)90009-1. [DOI] [PubMed] [Google Scholar]